Lyotropic ordering for high proton conductivity in sulfonated semialiphatic polyimide thin films
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The influence of the semialiphatic backbone on molecular ordering and proton conductivity was investigated in comparison to the rigid aromatic backbone in highly proton-conductive
organized polyimide thin films. We newly synthesized two alkyl-sulfonated semialiphatic polyimides (ASSPIs) with different molecular weights and investigated their molecular organized
structure, proton conductivity, water uptake, and the dissociation state of protons from sulfonic acid groups in thin films by in situ measurements for grazing incidence small-angle X-ray
scattering (GISAXS), quartz crystal microbalance (QCM), fourier transform infrared (FT-IR) spectra, and impedance spectra. Declining planarity in the semialiphatic backbone reduced the
aggregative character and molecular ordering in the lyotropic liquid-crystalline (LC) structure. However, the higher-molecular-weight ASSPI exhibited the oriented lamellar structure despite
the lower planarity of the main chain. The proton conductivity of the oriented lamellar thin film had more than half an order of magnitude higher value of 1.5 × 10-1 S cm-1 than did the
nonoriented lamellar thin film (3.0 × 10-2 S cm-1) at 25 °C and 95% RH. These results indicate that in sulfonated polyimide thin films, the lamellar orientation greatly contributes to the
high proton conductivity in ASSPI thin films. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS ORDERED ARRANGEMENT OF F4TCNQ
ANIONS IN THREE-DIMENSIONALLY ORIENTED P3HT THIN FILMS Article Open access 18 November 2020 EFFECT OF BISPHENOLS ON THE ELECTRICAL CONDUCTIVITY AND STRUCTURE OF
POLY(3,4-ETHYLENEDIOXYTHIOPHENE): POLY(STYRENE SULFONATE) Article 08 March 2022 QUANTITATIVE ANALYSIS OF STEREOSCOPIC MOLECULAR ORIENTATIONS IN THERMALLY REACTIVE AND HETEROGENEOUS
NONCRYSTALLINE THIN FILMS VIA VARIABLE-TEMPERATURE INFRARED PMAIRS AND GI-XRD Article 01 February 2021 INTRODUCTION Structural control of highly ion-conductive channels is a notable strategy
for energy conversion system, water treatment, and biotechnology [1,2,3,4,5]. Nanostructured liquid crystals that self-organize into dimensionally ordered states represent a promising
approach to the development of structural control of ion-conductive materials [6, 7]. Thermotropic and lyotropic liquid crystal properties can drive organized structure of various kinds.
Kato et al. demonstrated switchable ionic conductivities induced by a rectangular–hexagonal phase transition in wedge-shaped liquid-crystalline (LC) ammonium salts [8]. Several groups have
also reported relationships between ionic conductivity and LC structure with thermotropic and lyotropic liquid crystal properties [9,10,11,12]. Designing phase-separated conductive channels
composed of hydrophobic backbones and hydrophilic parts is a fundamentally important strategy to create highly proton-conductive materials [13,14,15,16]. Typical proton exchange membranes
(PEMs) have sulfonic acid groups attached to the hydrophobic polymer as a side chain [17]. State-of-the-art membranes are based on perfluorosulfonic acid (PFSA) ionomers such as Nafion [13,
18, 19]. They exhibit high levels of proton conductivity, water transport, and durability. Protons are transported through water-swollen conductive channels. Understanding the relationship
between the structure and proton transport property is fundamentally important, but such attempts have often been hampered in many highly proton-conductive polymers because less structural
information can be derived from an amorphous or amorphous-like nature. Our recent studies have elucidated that proton conductivity enhancement originates from the improvement of molecular
ordering and the main chain orientation of LC domains in alkyl-sulfonated polyimide (ASPI) thin films [20,21,22,23]. In fact, ASPI thin films with rigid backbones exhibit lamellar-organized
structure by water uptake using a lyotropic LC property. However, no reports have described the influence of the semialiphatic backbones on the organized structure and proton conductivity of
alkyl-sulfonated polyimide thin films. This study elucidated the relationship between the organized structure and proton conductivity by introduction of a semialiphatic structure in the
main chain. Lower planarity of backbones can change the organized molecular structure and ordering in the lyotropic LC structure. For polyimides without sulfonic acid groups, Ando and
coworkers [24] reported the intermolecular aggregation structures in thin films of fully aromatic polyimides and semialiphatic polyimides by grazing incidence X-ray scattering measurements.
In our work, the lyotropic LC structure and proton conductivity were assessed in two alkyl-sulfonated semialiphatic polyimide (ASSPI) films with different molecular weights by in situ
studies of grazing incidence small-angle X-ray scattering (GISAXS), quartz crystal microbalance (QCM), Fourier transform infrared spectra (FT-IR) and impedance analyses. Based on those
results, we conducted systematic evaluations of the molecular structure, proton conductivity, water uptake, and dissociation state of protons from sulfonic acid groups in thin films. Two
ASSPI thin films exhibited different orientations of the lamellar structure and proton conductivity. In contrast, water uptake isotherms and the proton dissociation properties from sulfonic
acid groups were almost identical in the two thin films. Therefore, the orientation of the lyotropic lamellar structure largely contributed to the proton conductivity enhancement. Our
results indicate that not only main chain planarity but also the increment of molecular weight affects the lyotropic LC structure and, moreover, the oriented lamellar structure enhanced
proton conductivity. MATERIALS AND METHODS MATERIALS The 3,3′-dihydroxybenzidine, 1,3−propanesultone, 9,10-dihydro-9-oxa-10-phosphaphenanthrene 10-oxide (DOPO) and
1,2,4,5-cyclohexanetetracarboxylic dianhydride (HPMDA) were purchased from Tokyo Chemical Industry Co. Ltd., Japan and were used without further purification. The
3,3′-bis(sulfopropoxy)-4,4′-diaminobiphenyl (3,3′-BSPA) was synthesized from 3,3’-dihydroxybendizine and 1,3−propanesultone as described in the literature [22]. Acetone and m-cresol were
purchased from Wako Pure Chemical Ind. Ltd., Japan. Triethylamine (TEA) was used as received from Kanto Chemical Co. Inc., Japan. SYNTHESIS OF ASSPI The ASSPIs of different molecular weights
were synthesized using a similar polymerization scheme. Scheme 1 represents the synthesis of the higher-molecular-weight (HMw) ASSPIs. For HMw ASSPIs, DOPO was used as the catalyst, whereas
it was not used in the case of lower-molecular-weight (LMw) ASSPIs. Polymerization was conducted using 3,3′-BSPA (0.46 g), HPMDA (0.22 g), DOPO (0.86 g), m-cresol (5 ml), and TEA (0.3 ml)
in a 50-mL three-necked round-bottomed flask with a mechanical stirrer under an argon atmosphere. m-Cresol was used as the solvent. After reaction for 25 h at 180 °C, the polymerized mixture
was poured dropwise into cooled acetone and washed by centrifugation. The final product was dried under a vacuum and again subjected to an ion-exchange process using Amberlyst. According to
the previous literature [25], molecular weight and polydispersity were examined by gel permeation chromatography (GPC), and the results are shown in Supplementary Table S1. In this scheme,
we obtained the ASSPI polymer with a high-molecular-weight (HMw, 4.0 × 104). We also obtained the ASSPI polymer with a lower-molecular weight (LMw, 2.5 × 104), which was polymerized
according to the same scheme without DOPO (Scheme S1). The obtained product was characterized based on 1H NMR and FT-IR spectra (Supplementary Figs. S1 and S2). We selected it for
comparison. (Supplementary Fig. S3 shows the GPC results). The degree of sulfonation for the final product was estimated from the 1H NMR data as more than 98%. The calculated ion-exchange
capacity (IEC) was 2.9 meq g-1. PREPARATION OF THIN FILMS ASSPI thin films onto Si, SiO2 substrates and SiO2-coated 9 MHz quartz crystals (Seiko EG&G Co. Ltd.) were prepared from 5 wt%
of ASSPI solution with a mixed solvent of Milli-Q water and tetrahydrofuran using a spincast method with a spin-coater (ACT−200D; Active Co. Ltd.). Before film deposition, the substrates
were washed by soaking in 2-propanol and plasma treatment with a vacuum plasma system (Cute-MP; Femto Science, Korea) to improve the surface hydrophilicity. The thicknesses of the thin films
were measured using a white interference microscope (BW-S506; Nikon Corp.) and an atomic force microscope (AFM, VN-8000; Keyence Co.). GRAZING INCIDENCE SMALL-ANGLE X-RAY SCATTERING
(GISAXS) GISAXS measurements were taken with a FR-E X-ray diffractometer equipped with R-AXIS IV two-dimensional (2D) detector (FR-E; Rigaku Corp.). Thin-film samples were placed into a
humidity-controlled cell with X-ray transparent polyester film (Lumirror) windows. To control the humidity, nitrogen carrier gas was used as received from the gas cylinder without further
dehumidification. A voltage of 45 kV, current of 45 mA, and irradiation time of 1 h were applied to create copper Cu Kα radiation (_λ_ = 0.1542 nm) with a beam size of approximately 300 ×
300 μm. The camera length was 300 mm. X-ray scattering patterns were recorded on an imaging plate (Fujifilm Corp.). The incident angle was chosen as 0.20°–0.22°. For 1D out-of-plane and
in-plane patterns, the integrated regions were taken, respectively, between –0.5° to + 0.5° as 2_θ_ from the center (0°) and a width of 1° as 2_θ_, respectively. THIN-FILM CONDUCTIVITY
MEASUREMENTS For conductivity measurements of ASSPI thin films, impedance spectroscopy measurements were performed using a two-probe method to determine the proton conductivity parallel to
the film surface with a frequency response analyzer and high-frequency dielectric interface (SI1260 and SI1296; Solartron Analytical). Gold paste was used as thin-film electrodes for the
conductivity measurements. The relative humidity (RH) and temperature were monitored by a computer-controlled environmental test chamber (SH-221; Espec Corp.). Impedance data were collected
by application of an alternating potential of 50 mV over frequencies ranging from 10 MHz to 1 Hz. The thin-film conductivity (_σ_) was calculated from the resistance value (_R_) obtained
directly from the impedance measurements using $$\sigma = \frac{d}{{Rlt}}$$ where _d_ represents the space between the gold electrodes, _t_ stands for the film thickness, and _l_ expresses
the contact electrode length. WATER UPTAKE MEASUREMENTS OF THIN FILMS Water uptake of ASSPI thin films was measured by an in situ QCM system. The relative humidity (RH) was controlled by a
dry N2 gas and humidified streams using a humidity controller (BEL Flow; BEL Japan Inc.). QCM substrates were connected to an oscillation circuit with a DC power supply and frequency counter
(53131 A; Agilent Technologies Japan Ltd.). The QCM substrate was placed in an in-house constructed humidity chamber with a high-resolution RH sensor. The frequencies found before and after
spin-coating of the QCM substrate were confirmed at the dry N2 stream to determine the mass of dry film by the Sauerbrey equation as $$\Delta m = \frac{{S \times \sqrt {\rho \mu } }}{{2
\times F^2}} \times ( - \Delta F)$$ where _S_ denotes the electrode surface area, _ρ_ stands for the quartz density, _µ_ expresses the shear modulus of quartz, and _F_ signifies the
fundamental frequency of the QCM substrate. The water content _λ_, the number of water molecules per sulfonic acid, was calculated as shown below: $$\lambda = \left(\frac{m}{{m_0}} -
1\right) \times \frac{{{\mathrm{E }}{\mathrm{W }}}}{{M_{{\mathrm{H }}_2{\mathrm{O }}}}}$$ where _m_ represents the film mass at each RH, _m_0 stands for the film mass at 0% RH,\(M_{H_2O}\)
is the molecular mass of the water molecular, and EW expresses the equivalent of each ASSPI. IN SITU FT-IR The dissociation state of protons from sulfonic acid groups was examined by in situ
FT-IR measurements. ASSPI thin films on Si wafers were placed in a homemade cell. CaF2 windows were used for the humidity-controlled cell. Transmission in situ FT-IR measurements were then
collected using an FT-IR spectrometer (Nicolet 6700; Thermo Fisher Scientific Inc.) equipped with a deuterium triglycine sulfate (DTGS) detector. The relative humidity (RH) was controlled by
a humidity generator (me-40DP-2PW; Micro equipment). The thicknesses of the films prepared on oxidized Si substrates for this measurement were approximately 500 nm. RESULTS AND DISCUSSION
IN SITU GISAXS GISAXS is a powerful tool to reveal molecular packings and molecular orderings in molecular organized thin films [26,27,28]. Matsui and coworkers [29] showed anisotropic
proton conductivity in a polymer multilayer thin film with a well-defined lamellar structure that was confirmed based on GISAXS measurements. Proton conductivity was enhanced by the
formation of 2D hydrogen-bonding networks in multilayer nanosheets [30, 31]. To investigate the influence of the semialiphatic main chain on the lyotropic organized structure, RH-dependent
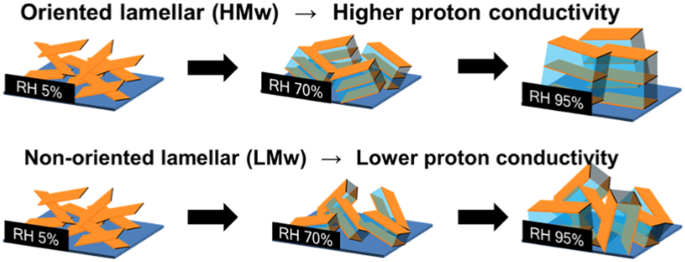
in situ GISAXS measurements were conducted in ASSPI thin films. The 2D scattering images are shown in Fig. 1a–d and Fig. 2a–d for HMw and LMw ASSPI thin films, respectively. A series of
in-plane and out-of-plane 1D profiles for HMw and LMw thin films with various RH values are depicted in Fig. 1e, f and Fig. 2e, f, respectively. In the in situ GISAXS measurements of the HMw
thin film, scattering peaks in the out-of-plane position were observed around the small-angle region of 2_θ_ = 4–5° (_d_ = 2.1–2.7 nm) at more than 70% RH (Fig. 1c, d). The out-of-plane
peaks in the HMw thin film implied that the repeating ordered structure was formed perpendicularly to the substrate plane in the thin film. Our earlier work demonstrated that the
out-of-plane scatterings were attributed to the lamellar structure in the in-plane stacked hydrophobic polyimide backbone with hydrophilic sulfonated side chains [21]. Under high RH
conditions, this out-of-plane scattering peak was enhanced and shifted to the smaller angle region (Fig. 1f). At 95% RH, the out-of-plane peak reached 3° (_d_ = 2.7 nm); it was markedly
enhanced relative to the X-ray specular peak. These results reflect that the lamellar structure is constructed by containing water through the thin film, which is incorporated selectively
into the interlamellar space. The scattering peak shift and intensity enhancement with humidity represented the lamellar expansion and ordering of the amphiphilic lamellar structure to the
out-of-plane direction, respectively. Molecular ordering is enhanced as the lamellar spacing expands to the out-of-plane direction as humidity further increases. In contrast, the LMw thin
film exhibited semicircle scattering under conditions of high humidity of more than 70% RH (Fig. 2d–f), unlike the anisotropic scattering in the HMw thin film. In the in-plane profile, the
scattering shifted to the small-angle region as humidity increased (Fig. 2e), indicating the formation of the same lamellar structure as the HMw thin film. In the out-of-plane direction,
however, peak shifts were not clearly observed due to the weak scattering intensity. The semicircle scattering suggested an isotropic structure, and, therefore, the LMw thin film formed a
nonoriented lamellar structure. Ando and coworkers [24] have discussed the details of main chain aggregates by ch-pack as intermolecular main chain packing and π-stacks as aromatic ring
stacking in both aromatic and semialiphatic polyimides with no sulfonated alkyl side chains. These polyimides consisting of diaminocyclohexylmethane and pyromellitic dianhydride form a
smectic LC-like ordered structure based on the main chain aggregations. Alkyl-sulfonated aromatic polyimides exhibit the scattering attributed to the periodic monomer unit length and ch-pack
for the polyimide main chain in the in-plane and out-of-plane positions, respectively [20,21,22,23, 25]. The scatterings imply smectic LC ordering of the intermolecular aggregation in the
lyotropic lamellar structure. However, in the present both cases of ASSPI thin films, scattering due to the main chain aggregations was not observed. The nonplanar aliphatic rings in the
ASSPI thin films probably inhibited the intermolecular aggregation of the main chain in the lyotropic lamellar structure so that the main chain smectic ordering was not formed. Thereby,
ASSPI thin films only exhibited scattering due to the lyotropic lamellar structure under high humidity conditions in comparison to aromatic polyimide thin films. In contrast, the HMw thin
film formed an oriented lamellar structure, although the LMw thin film exhibited a nonoriented one. The reason for this structural difference might derive from the number of shorter main
chains in ASSPI (Supplementary Fig. S3). Polarized optical microscopic observation clearly revealed the differences in the long range ordering and LC domain size between HMw and LMw thick
films (Supplementary Fig. S4). Birefringence textures were clearly observed in the HMw film (Supplementary Fig. S4a, b), whereas the LMw film only exhibited an image close to the dark field
(Supplementary Fig. S4c, d). These findings clearly indicated that the LMw film had a reduced long range order and small domain size. Hereinafter, we respectively designate HMw thin film as
oriented lamellar thin films and LMw thin films as nonoriented lamellar thin films. PROTON CONDUCTIVITY Figure 3 depicts RH-dependent proton conductivity plots for both oriented lamellar and
nonoriented lamellar thin films. A linear increase in conductivity was observed by the increase in RH, which was similar for both the oriented lamellar and nonoriented lamellar thin films.
However, the proton conductivity of the oriented lamellar film displayed more than half an order of magnitude higher value of 1.5 × 10-1 S cm-1 (at 25 °C and 95% RH) than the nonoriented
lamellar thin film (3.0 × 10-2 S cm-1 at 25 °C and 95% RH). In the high RH region, this increased proton conductivity was probably due to improved molecular ordering and an oriented lamellar
structure arising from an increased molecular weight. Generally, the IEC value is responsible for proton conductivity under high RH conditions [32, 33]. In the present work, however, the
IEC value was fixed because of the same chemical structure of ASSPI. From the lamellar structure observed in the GISAXS measurement, the hydrophilic sulfonic acid side chains and hydrophobic
polyimide main chains were segregated to form the lamellar structure parallel to the substrate. Under high humidity conditions, the hydrophilic sulfonic layers were selectively hydrated and
expanded upon the adsorption of water molecules. Such continuously formed hydrophilic layers in the highly ordered structure promoted the marked enhancement of conductivity. To elucidate
whether this increment of proton conductivity was a result of structural effects or water uptake, in situ QCM measurements were conducted. WATER UPTAKE Water uptake for the PEM affects
proton conductivity because water facilitates the transport of protons through the membrane [34]. Figure 4 shows RH-dependent water uptake plots for both the oriented lamellar and
nonoriented lamellar thin films. The water uptake for both films increased concomitantly with increasing RH, reaching a value of _λ_ = 16 in the oriented lamellar thin films and _λ_ = 14 in
the nonoriented films. Although no difference was detected in water uptake at low humidity (30–80% RH), the oriented lamellar thin film absorbed somewhat more water molecules than the
nonoriented film. The slightly higher water uptake in the oriented film resulted from the anisotropic expansion normal to the substrate plane under high RH conditions, as observed in the
GISAXS results. To elucidate the relationship between conductivity and water uptake, plots of conductivity vs. _λ_ are shown in Fig. 5. When the proton conductivity was compared at the same
_λ_ value, the oriented lamellar thin-film showed more than half an order of magnitude higher proton conductivity than the nonoriented films. This result clearly indicated that the water
adsorption was not responsibility for the enhancement of proton conductivity. Hence, we inferred that the oriented lamellar structure facilitated proton conductivity and engendered higher
proton conductivity in the high RH region. DISSOCIATION STATE OF PROTONS FROM SULFONIC ACID GROUPS USING IN SITU FT-IR To evaluate the dissociation of protons at sulfonic acid groups during
the conductivity change, in situ FT-IR measurements under controlled humidity were conducted. Figure 6 shows the humidity-dependent FT-IR spectra for the oriented lamellar and nonoriented
lamellar thin films. The peaks were clearly observed at bands of 1715, 1640, 1200, and 1030 cm-1 corresponding to the νas(C = O) symmetric stretching vibrations of the imide groups,
δ(H–O–H), νas(SO3−), and νs(SO3−), respectively. With increases in humidity, the absorbance of the peaks was enhanced. The number of dissociated protons could be estimated by the absorbance
due to the νs(SO3−) band. Figure 7 depicts the absorbance of the νs(SO3−) band at 1030 cm-1 as a function of the _λ_ value. The absorbance of the νs(SO3-) bands increased with the _λ_ value
and was saturated for _λ_ > 6 in both the oriented lamellar and nonoriented lamellar thin films. Saturation of the νs(SO3-) band absorbance suggested an almost complete dissociation of
the protons at sulfonic acid groups with relatively low water uptake at approximately _λ_ = 6. However, the proton conductivity increased considerably to more than _λ_ = 6, as shown in Fig.
5. Miyatake and coworkers [33] reported a similar tendency to correlate the proton dissociation of sulfonic acid groups and proton conductivity during the hydration process in a sulfonated
block poly(arylene ether sulfone ketone) membrane [35]. Additionally, both oriented and nonoriented films exhibited essentially the same behaviors. Therefore, the dissociation state of
protons at sulfonic acid groups was also not responsible for the enhancement of proton conductivity in oriented lamellar thin film. In our earlier work, the highly proton-conductive
organized structure was obtained using the lyotropic LC property derived from rigid aromatic main chains. In the present work, the influence of the semialiphatic structure is discussed. The
molecular ordering weakened as a result of the reduced planarity of the main chain due to suppressed (_π_-stack) aggregation of the main chain in the lyotropic lamellar structure. The degree
of molecular ordering improved with increasing molecular weight, and an oriented lamellar-organized structure was obtained. Although the IEC values, water uptake and dissociation behavior
of proton were identical in both films, the oriented lamellar film exhibited half an order of magnitude higher proton conductivity compared with the nonoriented film. Therefore, we inferred
that the oriented lamellar structure enhanced proton conductivity more than the nonoriented lamellar structure. CONCLUSION As described herein, the influence of the semialiphatic backbone on
molecular ordering and proton conductivity was discussed for sulfonated polyimide thin films. A new alkyl-sulfonated polyimide with less planarity for the main chain was synthesized. The
lower-molecular weight of the sulfonated polyimide had a nonoriented lamellar structure under conditions of 70–95% RH. However, the higher-molecular weight enhanced the degree of molecular
ordering and resulted in the oriented lamellar-organized structure based on the lyotropic LC property. The reason for the structural difference might derive from the number of shorter main
chains in ASSPIs. Moreover, proton conductivity was enhanced in the case of the lamellar-organized structure with the same water amounts and dissociation states of protons. We concluded that
higher proton conductivity was achieved with the oriented lamellar-organized structure. REFERENCES * Nagao Y. Proton-conductivity enhancement in polymer thin films. Langmuir.
2017;33:12547–58. Article CAS Google Scholar * Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Mariñas BJ, Mayes AM. Science and technology for water purification in the coming decades.
Nature. 2008;452:301. Article CAS Google Scholar * Peckham TJ, Holdcroft S. Structure-morphology-property relationships of non-perfluorinated proton-conducting membranes. Adv Mater.
2010;22:4667–90. Article CAS Google Scholar * Nagao Y, Matsui J, Abe T, Hiramatsu H, Yamamoto H, Miyashita T, et al. Enhancement of proton transport in an oriented polypeptide thin film.
Langmuir. 2013;29:6798–804. Article CAS Google Scholar * Amiri H, Shepard KL, Nuckolls C, Hernández Sánchez R. Single-walled carbon nanotubes: mimics of biological ion channels. Nano
Lett. 2017;17:1204–11. Article CAS Google Scholar * Kato T, Mizoshita N, Kishimoto K. Functional liquid-crystalline assemblies: Self-organized soft materials. Angew Chem Int Ed.
2006;45:38–68. Article CAS Google Scholar * Kato T, Yoshio M, Ichikawa T, Soberats B, Ohno H, Funahashi M. Transport of ions and electrons in nanostructured liquid crystals. Nat Rev
Mater. 2017;2:17001. Article Google Scholar * Soberats B, Yoshio M, Ichikawa T, Zeng X, Ohno H, Ungar G, et al. Ionic switch induced by a rectangular–hexagonal phase transition in
benzenammonium columnar liquid crystals. J Am Chem Soc. 2015;137:13212–5. Article CAS Google Scholar * Chen Y, Lingwood MD, Goswami M, Kidd BE, Hernandez JJ, Rosenthal M, et al.
Humidity-modulated phase control and nanoscopic transport in supramolecular assemblies. J Phys Chem B. 2014;118:3207–17. Article CAS Google Scholar * Hernandez JJ, Zhang H, Chen Y,
Rosenthal M, Lingwood MD, Goswami M, et al. Bottom-up fabrication of nanostructured bicontinuous and hexagonal ion-conducting polymer membranes. Macromolecules. 2017;50:5392–401. Article
CAS Google Scholar * Tonozuka I, Yoshida M, Kaneko K, Takeoka Y, Rikukawa M. Considerations of polymerization method and molecular weight for proton-conducting poly(p-phenylene)
derivatives. Polym (Guildf). 2011;52:6020–8. Article CAS Google Scholar * Lee JH, Han KS, Lee JS, Lee AS, Park SK, Hong SY, et al. Facilitated ion transport in smectic ordered ionic
liquid crystals. Adv Mater. 2016;28:9301–7. Article CAS Google Scholar * Mauritz KA, Moore RB. State of understanding of Nafion. Chem Rev. 2004;104:4535–85. Article CAS Google Scholar
* Li N, Guiver MD. Ion transport by nanochannels in ion-containing aromatic copolymers. Macromolecules. 2014;47:2175–98. Article CAS Google Scholar * He G, Li Z, Zhao J, Wang S, Wu H,
Guiver MD, et al. Nanostructured ion-exchange membranes for fuel cells: Recent advances and perspectives. Adv Mater. 2015;27:5280–95. Article CAS Google Scholar * Kreuer KD. On the
development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J Membr Sci. 2001;185:29–39. Article CAS Google Scholar * Hickner MA, Ghassemi H, Kim YS, Einsla
BR, McGrath JE. Alternative polymer systems for proton exchange membranes (PEMs). Chem Rev. 2004;104:4587–611. Article CAS Google Scholar * Kusoglu A, Weber AZ. New insights into
perfluorinated sulfonic-acid lonomers. Chem Rev. 2017;117:987–1104. Article CAS Google Scholar * Karan K. PEFC catalyst layer: Recent advances in materials, microstructural
characterization, and modeling. Curr Opin Electrochem. 2017;5:27–35. Article CAS Google Scholar * Krishnan K, Yamada T, Iwatsuki H, Hara M, Nagano S, Otsubo K, et al. Influence of
confined polymer structure on proton transport property in sulfonated polyimide thin films. Electrochemistry. 2014;82:865–9. Article CAS Google Scholar * Krishnan K, Iwatsuki H, Hara M,
Nagano S, Nagao Y. Proton conductivity enhancement in oriented, sulfonated polyimide thin films. J Mater Chem A. 2014;2:6895–903. Article CAS Google Scholar * Krishnan K, Iwatsuki H, Hara
M, Nagano S, Nagao Y. Influence of molecular weight on molecular ordering and proton transport in organized sulfonated polyimide thin films. J Phys Chem C. 2015;119:21767–74. Article CAS
Google Scholar * Nagao Y, Krishnan K, Goto R, Hara M, Nagano S. Effect of casting solvent on interfacial molecular structure and proton transport characteristics of sulfonated polyimide
thin films. Anal Sci. 2017;33:35–9. Article CAS Google Scholar * Wakita J, Jin S, Shin TJ, Ree M, Ando S. Analysis of molecular aggregation structures of fully aromatic and semialiphatic
polyimide films with synchrotron grazing incidence wide-angle X-ray scattering. Macromolecules. 2010;43:1930–41. Article CAS Google Scholar * Ono Y, Goto R, Hara M, Nagano S, Abe T, Nagao
Y. High proton conduction of organized sulfonated polyimide thin films with planar and bent backbones. Macromolecules. 2018;51:3351–9. Article CAS Google Scholar * Sirringhaus H, Brown
PJ, Friend RH, Nielsen MM, Bechgaard K, Langeveld-Voss BMW, et al. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature. 1999;401:685. Article CAS
Google Scholar * Nagano S, Kodama S, Seki T. Ideal spread monolayer and multilayer formation of fully hydrophobic polythiophenes via liquid crystal hybridization on water. Langmuir.
2008;24:10498–504. Article CAS Google Scholar * Nagano S. Inducing planar orientation in side‐chain liquid‐crystalline polymer systems via interfacial control. Chem Rec. 2016;16:378–92.
Article CAS Google Scholar * Sato T, Hayasaka Y, Mitsuishi M, Miyashita T, Nagano S, Matsui J. High proton conductivity in the molecular interlayer of a polymer nanosheet multilayer film.
Langmuir. 2015;31:5174–80. Article CAS Google Scholar * Sato T, Tsukamoto M, Yamamoto S, Mitsuishi M, Miyashita T, Nagano S, et al. Acid-group-content-dependent proton conductivity
mechanisms at the interlayer of poly(N-dodecylacrylamide-co-acrylic acid) copolymer multilayer nanosheet films. Langmuir. 2017;33:12897–902. Article CAS Google Scholar * Matsui J, Miyata
H, Hanaoka Y, Miyashita T. Layered ultrathin proton conductive film based on polymer nanosheet assembly. ACS Appl Mater Inter. 2011;3:1394–7. Article CAS Google Scholar * Saito J,
Miyatake K, Watanabe M. Synthesis and properties of polyimide ionomers containing 1H-1,2,4-triazole groups. Macromolecules. 2008;41:2415–20. Article CAS Google Scholar * Miyahara T,
Miyake J, Matsuno S, Watanabe M, Miyatake K. A sulfonated polybenzophenone/polyimide copolymer as a novel proton exchange membrane. RSC Adv. 2015;5:50082–6. Article CAS Google Scholar *
Chang Y, Brunello GF, Fuller J, Hawley M, Kim YS, Disabb-Miller M, et al. Aromatic Ionomers with highly acidic sulfonate groups: Acidity, hydration, and proton conductivity. Macromolecules.
2011;44:8458–69. Article CAS Google Scholar * Kunimatsu K, Yagi K, Bae B, Miyatake K, Uchida H, Watanabe M. ATR-FTIR Analysis of the state of water in a sulfonated block poly(arylene
ether sulfone ketone) membrane and proton conductivity measurement during the hydration/dehydration cycle. J Phys Chem C. 2013;117:3762–71. Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported in part by the Nanotechnology Platform Program (Molecule and Material Synthesis) of the Ministry of Education, Culture, Sports, Science and
Technology (MEXT), Japan. This work was partially supported by the Iketani Science and Technology Foundation (ISTF), JAPAN. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School of Materials
Science, Japan Advanced Institute of Science and Technology, 1-1 Asahidai, Nomi, Ishikawa, 923-1292, Japan Kensaku Takakura, Yutaro Ono & Yuki Nagao * Department of Molecular Design
& Engineering Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa, Nagoya, 464-8603, Japan Kota Suetsugu & Mitsuo Hara * Nagoya University Venture Business
Laboratory, Nagoya University, Furo-cho, Chikusa, Nagoya, 464-8603, Japan Shusaku Nagano * Graduate School of Science and Technology, Niigata University, 8050 Ikarashi 2-no-cho, Nishi-ku,
Niigata, 950-2181, Japan Takashi Abe Authors * Kensaku Takakura View author publications You can also search for this author inPubMed Google Scholar * Yutaro Ono View author publications You
can also search for this author inPubMed Google Scholar * Kota Suetsugu View author publications You can also search for this author inPubMed Google Scholar * Mitsuo Hara View author
publications You can also search for this author inPubMed Google Scholar * Shusaku Nagano View author publications You can also search for this author inPubMed Google Scholar * Takashi Abe
View author publications You can also search for this author inPubMed Google Scholar * Yuki Nagao View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHOR Correspondence to Yuki Nagao. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ELECTRONIC SUPPLEMENTARY MATERIAL
SUPPLEMENTARY MATERIAL RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Takakura, K., Ono, Y., Suetsugu, K. _et al._ Lyotropic ordering for high proton
conductivity in sulfonated semialiphatic polyimide thin films. _Polym J_ 51, 31–39 (2019). https://doi.org/10.1038/s41428-018-0111-1 Download citation * Received: 11 April 2018 * Revised: 05
June 2018 * Accepted: 04 July 2018 * Published: 03 August 2018 * Issue Date: January 2019 * DOI: https://doi.org/10.1038/s41428-018-0111-1 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative