Biodegradable, flexible silicon nanomembrane-based nox gas sensor system with record-high performance for transient environmental monitors and medical implants
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT A novel transient electronics technology that is capable of completely dissolving or decomposing in certain conditions after a period of operation offers unprecedented opportunities
for medical implants, environmental sensors, and other applications. Here, we describe a biodegradable, flexible silicon-based electronic system that detects NO species with a
record-breaking sensitivity of 136 Rs (5 ppm, NO2) and 100-fold selectivity for NO species over other substances with a fast response (~30 s) and recovery (~60 s). The exceptional features
primarily depend on not only materials, dimensions, and design layouts but also temperatures and electrical operations. Large-scale sensor arrays in a mechanically pliable configuration
exhibit negligible deterioration in performance under various modes of applied loads, consistent with mechanics modeling. In vitro evaluations demonstrate the capability and stability of
integrated NOx devices in severe wet environments for biomedical applications. SIMILAR CONTENT BEING VIEWED BY OTHERS A FLEXIBLE AND PHYSICALLY TRANSIENT ELECTROCHEMICAL SENSOR FOR REAL-TIME
WIRELESS NITRIC OXIDE MONITORING Article Open access 25 June 2020 ECORESORBABLE AND BIORESORBABLE MICROELECTROMECHANICAL SYSTEMS Article 21 July 2022 BIODEGRADABLE GERMANIUM ELECTRONICS FOR
INTEGRATED BIOSENSING OF PHYSIOLOGICAL SIGNALS Article Open access 21 July 2022 INTRODUCTION Electronic materials and devices that are biologically benign or mechanically elastic have been
utilized in versatile applications, including electronic skin (E-skin) and biomedicine, for the diagnosis and treatment of diseases due to their advantages of nontoxicity, bioresorption, and
mechanical properties similar to those of human skin and organs. Published examples involve sweat-based analysis1,2,3, epidermal virtual reality4,5, self-healing electronics6,7,
optoelectronic elements8,9, and neurorelated systems10,11,12,13. Although the technological advances in the corresponding areas delivered innovative output products, a relatively unexplored
research opportunity exists in detectable species indicative of occurrences in organisms that may affect the prevalence and severity of human health, such as infections, injuries, diseases,
or environmental exposures. One of the key signaling elements is nitric oxide (NO), which is produced naturally in the human body and plays an essential role in many aspects of maintaining
health by relaxing or widening blood vessels to enhance blood flow, allowing nutrients and oxygen to travel to every part of the human body14,15. In nonbiological cases, highly reactive
nitric oxide can be transformed into nitrogen dioxide (NO2) with other participants, contributing to acid rain deposition and harmful ozone generation16,17. Research approaches for NO
species in previous works have explored high-performance channel materials such as graphene, carbon nanotubes, polymers, and metal oxides18,19,20 or modifications to these structures for a
high surface-to-volume ratio21,22,23,24,25, while several issues, including rigidity, low sensitivity, poor selectivity, high power consumption, and nonbiodegradability, still need to be
considered for wearable and implantable electronic systems. Here, we report a flexible and bioresorbable single-crystal silicon nanomembrane (SC-Si NM)-based NOx sensing system operating at
room temperature with exceptional sensitivity and selectivity. The results focus on detailed studies of electrical responses of Si NMs under various conditions, and of the mechanical
properties of the electronic system with theoretical considerations. In vitro assessments that incorporate semipermeable membranes provide information on their potential applicability in
disposable environmental monitors and bioresorbable medical implants. EXPERIMENTAL PROCEDURE FABRICATION OF A TRANSIENT NOX (NO/NO2)-SENSING SYSTEM ON VARIOUS SUBSTRATES Device fabrication
began with thinning down SC-Si NMs on silicon-on-insulator (SOI, SOITEC, France) wafers (top silicon ~300 nm in thickness). Repeated oxidation of SC-Si NMs at an elevated temperature (1100
°C) and removal of the thermally grown SiO2 by wet etching with hydrofluoric acid (HF, 49%, J. T Baker, USA), allowed to reach a desired target thickness of Si NMs (~100 nm). Phosphorus
doping at 950 °C with spin-on dopant (SOD, Filmtronics, USA) formed highly doped regions for electrical contacts of a NOx sensing system. Undercut wet etching of box oxide released the
thinned SC-Si NMs from the carrier wafers, enabling transfer printing onto a PMMA/diluted PI-coated temporary substrate. Active areas of the SC-Si NMs for NOx gas, humidity, and temperature
sensors were defined by sulfur hexafluoride (SF6)-based reactive ion etching, followed by deposition of interdigitated electrodes (IDEs) with a layer of magnesium (Mg, ~300 nm) using an
electron-beam (e-beam) evaporator (VER5004, SNTEK, Korea). Formations of patterned dielectric layers and contact pads were generated by wet etching using buffered oxide etchant (BOE, 6:1, J.
T baker, USA) and e-beam evaporation. A geometry of mesh-typed bridges was employed to facilitate lifting off the device, enabling the release of the system from the temporary substrate.
After removal of the bottom PI layer, the device was transfer printed onto degradable substrates for a complete transient electronic system. CHARACTERIZATION OF SOFT, BIODEGRADABLE NOX
SENSORS The electrochemical properties of a silicon-based gas detector were measured using an electrometer (Keithley 2636B) under a DC bias voltage of 0.01 V. Experiments were conducted in a
temperature-adjustable chamber (RT ~50 °C, Lindberg, USA) with a constant flow rate of 1000 sccm controlled by mass flow controllers (MFCs). Electrical responses of sensors were determined
by measurements of baseline resistances in dry N2/air and saturated resistances after exposure to target gases for 500 s. The responses to reducing and oxidizing gases were defined as
_R_0/_R_gas and _R_gas/_R_0, where _R_gas and _R_0 denote the resistances of the target gases and dry N2/air, respectively. CELL CULTURE AND M1 ACTIVATION RAW 264.7 cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were incubated at 37 °C with 5% CO2 in a
humidified incubator. To induce M1 activation, 100 ng/ml LPS (lipopolysaccharide, Sigma) and 20 ng/ml IFN-γ (interferon-gamma, Peprotech) were added to the culture medium, and it was
incubated for 24 h. DAF-FM ASSAY FOR NO MEASUREMENT After M1 activation, cells were incubated in l-arginine-free DMEM (Thermo, 88364) for 30 min, collected using a cell scraper and replated
in 48-well plates. To measure NO production in cells, 10 μM DAF-FM DA (Sigma, D1946) was added to medium with or without 150 μM l-arginine, and the system was incubated for 30 min. The
fluorescence intensity of DAF-FM-stained cells was measured using a Cytation 3 Cell Imaging Multi-Mode Reader (Bio-Tek). IMMUNOFLUORESCENCE MICROSCOPY Cells were fixed using 4%
paraformaldehyde for 10 min at 4 °C and permeabilized with 0.05% Triton X-100 for 5 min. After blocking with FBS (10%, v/v)-supplemented PBS for 30 min, cells were incubated with anti-iNOS
antibody (1:500, Abcam, ab178945) for 60 min. Cells were then incubated with secondary antibodies (1:500, Bethyl, A120-100D4) for 60 min in the dark. The nucleus and F-actin were stained
with DAPI (Invitrogen) and Alexa Fluor phalloidin (1:50, Invitrogen, A12379), respectively. The fluorescence confocal images were captured using confocal laser microscopy (A1R, Nikon)
through a ×60 oil immersion objective (N.A. 1.4). DETECTION OF NOX PRODUCED IN M1-ACTIVATED CELLS M1-activated cells were transferred into a sterile cell-culture flask containing 2 ml
l-arginine-free DMEM. NO sensors were placed into the flask, and l-arginine was provided through needles embedded in stoppers. The NO sensors were connected with ACF cables to an
electrometer, and all measurements were recorded through LabVIEW with the GPIB interface. RESULTS AND DISCUSSION INTEGRATED BIORESORBABLE, FLEXIBLE NOX (NO/NO2) SENSING SYSTEM Figure 1a
illustrates examples of various adverse environmental and biological effects that may be caused by nitrogen oxides (NOx). NO has been well recognized as a biomarker for nervous, vascular,
and respiratory systems26,27,28, and a major air pollutant giving rise to acid rain and ozone generation after transformation into NO2 as a reaction intermediate. Figure 1b provides an
exploded view drawing of a soft, bioresorbable NOx sensing system that includes highly sensitive and selective silicon nanomembrane (Si NM)-based gas sensors at room temperature, and a
temperature and hydration monitor to adjust the properties of the gas sensors affected by those parameters. The electronic system consists of patterned single-crystal silicon nanomembranes
(SC-Si NMs, thickness ~100 nm) doped with phosphorous using spin-on dopant (SOD, Filmtronics) for reactive materials, magnesium (Mg, thickness ~300 nm) as electrodes, silicon dioxide (SiO2,
thickness ~100 nm) as interlayer dielectric, and an elastomeric polymer (PDMS, thickness ~20 μm) as a semipermeable membrane to maintain a stable gas sensing performance in humid and wet
conditions. Sample preparation involves thinning of SC-Si NMs to a targeted value (~100 nm), doping with phosphorous at selected regions on silicon-on-insulator (SOI) wafers and transfer
printing doped SC-Si NMs to a temporary foreign substrate. Electrodes and insulators were evaporated and patterned through deposition, photolithography and etching, and transfer printing of
the resulting components to a biodegradable substrate completed the whole process. In the case of measurements in humid or wet conditions, facile spin casting of PDMS was applied to the
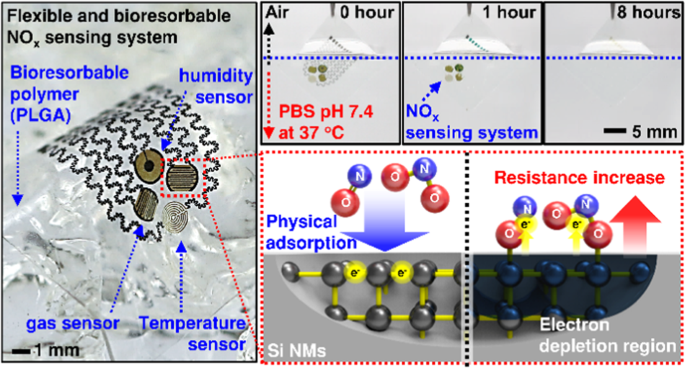
obtained sample to form a gas-permeable membrane. The bioresorbable, flexible NOx sensing system laminated on a wrist appears in Fig. 1c, with a magnified image of the individual gas,
temperature, and humidity sensors in the inset (more images in Fig. S1). Figure 1d shows a set of images of the temporal degradation behavior of a representative NOx sensing system assembled
on a poly(lactic-co-glycolic) acid (PLGA) substrate during half-immersion in a PBS (pH 7.4) at 37 °C for 0, 1, 4, and 8 h. All constituent materials dissolved via hydrolysis to form
nontoxic end products. Mg electrodes, with a relatively rapid dissolution rate, disappeared faster than other components via the reaction Mg+ 2H2O → Mg(OH)2 + H2, and SC-Si NMs, SiO2, and
PLGA gradually dissolved at rates similar to previously reported values while undergoing the reactions Si+ 4H2O ↔ Si(OH)4 + 2H2, SiO2+ 2H2O → Si(OH)4, and poly(lactic-co-glycolic acid) →
lactic acid + glycolic acid + H229,30,31. Additional dissolution images at different stages appear in Fig. S2. HIGH-PERFORMANCE BIODEGRADABLE NOX SENSING SYSTEM BASED ON SILICON
NANOMEMBRANES Figure 2a illustrates the fundamental mechanism of SC-Si NMs reacting with nitrogen oxides. Changes in the electrical properties of _n_-type semiconductor sensors resulted from
variations in a depletion layer on the surface through adsorption and reaction with the oxidizing gas molecules, i.e., electrons were extracted from the SC-Si NMs as the reaction NOx (gas)
+ e− → NOx− (ads) occurred. Figure 2b represents a measured response to 5 ppm of NO2 at room temperature (RT) with a superb sensitivity of 136 Rs (13,600%) as well as fast response (30 s)
and recovery (60 s) rates, which surpass those of previously reported room-temperature sensors based on various materials, including carbon nanotubes (1.14–1.2 Rs), graphene (1.12–1.6 Rs),
polymers (5 Rs) and metal oxides (2.2 Rs), and a commercial gas sensor (7 Rs, MICS-2714, Amphenol SGX Sensortech, Switzerland) (Fig. S3). Investigations at a wide range of gas concentrations
indicated that the detection had linear electrical responses, and the inset (Fig. 2c) provides a detection limit (DL) of ~20 ppb, which was estimated by a linear least squares fit for data
collected at room temperature32. The excellent sensitivity sufficiently detects concentrations required in potential applications, such as concentrations in exhalation by asthma and
halitosis patients (>100 ppb) and the atmosphere (0.6–5 ppm)33,34,35. The rationale that crystallinity, thickness, device layout, and electrical operation would affect the exceptional
performance of the bioresorbable silicon devices motivated various types of examinations. Figure 2d shows the measured responses using different kinds of silicon, such as SC-Si NMs (black),
polycrystalline silicon (red, p-Si), and amorphous silicon (blue, α-Si), with identical dimensions (1.45 mm × 1.2 mm) and thicknesses (~100 nm), providing evidence of a dependence on Si
crystallinity (Fig. S4). Analyses of other factors, e.g., film thickness, electrode layout, and applied voltage, are shown in Fig. S5, and dissolvable metals for coplanar interdigitated
electrodes (IDEs) did not affect the functional properties (Fig. S6). The selective response to specific target gases is also an important criterion. We performed selectivity assays for
well-known gases: oxidizing gases such as NO2 (black) and NO (red) and reducing gases such as NH3 (blue), CH3COOH3 (green, acetone), CH2H3OH (magenta, ethanol), CO2 (cyan), CO (navy), and
H2S (purple). The resulting properties in Fig. 2e indicate that the responsiveness to NO and NO2 was at least 100-fold greater than that to other substances (detailed data in Fig. S7), which
was achieved without additional physical and chemical treatments. We note that the variation in the responses between NO and NO2 gases was negligible (see Fig. S8). The temperature
dependence of the sensitivity shown in Fig. 2f was evaluated to consider potential utility as medical implants and environmental monitors (black, RT; red, 37 °C; blue, 50 °C). The results
were opposite to the typical behavior of conventional gas sensors, assuming that the electrical resistance/conductance of the silicon membranes would somewhat deviate from the optimum value
at increased temperatures. Integration with temperature and humidity sensors can adjust the output values of gas detectors susceptible to ambient conditions for system-level functionality.
The electrical characteristics of the resistive temperature device showed a sensitivity of ~9.6 nS/°C and a temperature coefficient of ~0.006 /°C (Fig. 2g), and changes in the capacitance of
the humidity component were well resolved over a wide range of relative humidities (Fig. 2h). MECHANICAL STABILITY OF LARGE-SCALE, FLEXIBLE, AND STRETCHABLE GAS SENSOR ARRAYS As introduced
in previous reports on deformable electronics, structures that involved device islands connected by serpentine bridges allowed devices to accommodate mechanical loads without deterioration
in electrical performance36,37,38. Figure 3a represents a large-scale, biodegradable sensor array (5 × 5, 1.7 cm × 1.9 cm) deployed in a mechanically soft geometry, consisting of patterned
SC-Si NMs as active components, Mg conductors for contact pads and interconnects, patterned SiO2 layers for interlayer dielectrics, and polycaprolactone (PCL) as substrate/encapsulant. The
inset shows a magnified optical micrograph of an individual unit. Figure 3b, c show a set of images under mechanical tests using several bending radii (left, flat; middle, 5 mm; right, 3 mm)
and uniaxial tensile strains up to 40%, respectively. An experimental setup combined with a stretching system is shown in Fig. S9. The resulting values under those deformed conditions
remained within effective ranges of 90% (Fig. 3d, bending) and 95% (Fig. 3e, stretching). In addition, the mechanical robustness and durability of the electronic system were investigated
under cyclic bending with a radius of 5 mm (black) and stretching with 35% (red), as shown in Fig. 3f, and the decreases in the relative response rate in both cases after 1000 cycles were
negligible. Three-dimensional finite element analysis was implemented in the commercial ABAQUS package to study the mechanical deformation of the biodegradable Si-based gas sensor upon
bending and stretching. Without losing generality, a 2 × 2 array of gas sensors was considered in the simulation, and the substrate (100 µm) and encapsulation (50 µm) layers were modeled by
an 8-node 3D element (C3D8R). PCL (Young’s modulus, 0.25 GPa; Poisson ratio, 0.44) and semipermeable membranes (PDMS, 2.61 MPa; 0.49) were explored for the substrate and encapsulation in the
bending test (Fig. 3d), whereas PDMS was used for the substrate in the tensile test (Fig. 3e). Because of their thinness, gas-sensitive Si nanomembranes (188 GPa; 0.28), Mg electrodes and
interconnects (45 GPa; 0.29), and SiO2 interlayer dielectrics (66.3 GPa; 0.15) could be modeled as skin layers, as shown in Fig. S10. For bending with a radius of curvature of 3 mm in Fig.
3g, the maximum principal strains in the layers of Mg, SiO2, and Si were obtained at 1.66, 1.81, and 0.24% (Fig. S11), far below their corresponding fracture strains, which explains the
mechanical stability. Upon stretching, the maximum principal strain in the skin layer occurred in the serpentine interconnect near the Si nanomembrane (Fig. 3h), and detailed strain
distributions for each material are shown in Fig. S11. When the total stretchability of the system is defined at a level, where the maximum principal strain of half the width of one section
exceeded the fracture strain of the metal interconnect (9–10% for Mg thin film39,40), as in a previous report37, the obtained total stretchability of ~40% corresponded well to the
experimental value of Fig. 3e. The strain distribution in the SiO2 layer was similar to that in the Mg layer, although the SiO2 layer would fracture before the Mg layer due to its slightly
smaller fracture strain of 8%41. In addition, the peak strain of 4.1% in the Si layer was immediately below its fracture strain of 4.25%42, which explained the fracture of the Si layer upon
further stretching. IN VITRO ASSESSMENTS OF DISSOLVABLE GAS SENSORS USING A SEMIPERMEABLE MEMBRANE Humidity-free gas meters enable the maintenance of robust performance even in harsh
environments in envisioned areas of interest. Figure 4a illustrates the fundamental principle that a PDMS-based semipermeable membrane can effectively deliver desired gas components to
active areas of an electronic system and repel water and/or aqueous components43,44. The PDMS membrane provides hydrophobicity and gas permeability since methyl groups (Si–CH3) on the
surface of PDMS reduce the surface energy, and siloxane backbones (Si–O) inside the polymer network form a pathway for NO diffusion. In comparative experiments without (Fig. 4b) or with
(Fig. 4c) membranes, absolute values of the responsiveness were slightly reduced, while the electrical characteristics still exhibited a high level of sensitivity compared to those in the
tests where there were nearly no responses with even a small amount of moisture in the absence of the membrane. Similar behaviors were found during immersion in aqueous media, in which the
membrane enabled stable detection in phosphate-buffered saline (PBS, pH 7.4) solution, whereas no signals were observed without the membrane (Fig. 4d and experimental setup appeared in Fig.
S12). Detailed studies of the correlation between membrane thickness and system performance could enhance the sensitivity under optimized conditions (Fig. S13). In vitro assessment of the NO
detection capability indicated the feasibility of using silicon-based bioresorbable devices for applications in temporary implants. Figure 4e briefly describes the overall procedure of NO
production that is recognized as a regulator of inflammatory responses in living macrophages26. Macrophages undergo M1 activation in response to pathogen-specific molecules,
lipopolysaccharide (LPS) or proinflammatory cytokines such as TNF-α and interferon-γ (INF-γ)45. As a result of inflammatory activation, macrophages produce inducible nitric oxide synthase
(iNOS), one of the key enzymes that acts as a catalyst to produce NO by consuming l-arginine46. A set of fluorescence confocal images verified the expression of iNOS, cellular morphological
changes (Fig. 4f) and NO production in the presence of l-arginine (Fig. 4g). Quantitative analysis of immunofluorescence intensity is shown in Fig. S14. The produced NO reacted with DAF-FM
to increase the fluorescence intensity in Fig. 4h, suggesting that both iNOS expression and the presence of l-arginine were essential factors. Figure 4i shows the measured responses after an
injection of l-arginine (150 μM) into control (black) and M1-activated macrophages (red) in medium (l-arginine-free DMEM (2 ml)). Details of the experimental setup are shown in Fig. S15.
The results showed that there was no response in the control group without M1 activation, while a linear response was clearly seen in the activated group, as expected. Evaluation of the
dynamic response over time in Fig. 4j was performed through periodic l-arginine injections and ventilations, verifying the capability of the system for reliable and repeatable measurements
of produced NO concentrations. CONCLUSION The concepts introduced here provide materials, a manufacturing strategy and device layouts for a soft, transient NOx gas detector integrated with
temperature and hydration meters. Electrical measurements reveal that the thickness, crystallinity, and operation voltage have a strong influence on the record-high sensitivity and
selectivity with rapid response and recovery rates. Upon mechanical deformations by diverse modalities, the performance of large-scale arrays change negligibly, consistent with those
determined by theoretical calculations. In vitro studies provide the feasibility of stable operation in harsh conditions, suggesting practical uses in environmental monitoring and biomedical
implants. REFERENCES * Koh, A. et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. _Sci. Transl. Med._ 8, 366ra165–366ra165 (2016).
Google Scholar * Gao, W. et al. A fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. _Nature_ 529, 509–514 (2016). CAS Google Scholar * Yang, Y. et al.
A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. _Nat. Biotechnol._ 38, 217–224 (2020). CAS Google Scholar * Kaltenbrunner, M. et al. An
ultra-lightweight design for imperceptible plastic electronics. _Nature_ 499, 458–463 (2013). CAS Google Scholar * Yu, X. et al. Skin-integrated wireless haptic interfaces for virtual and
augmented reality. _Nature_ 575, 473–479 (2019). CAS Google Scholar * Tee, B. C.-K., Wang, C., Allen, R. & Bao, Z. An electrically and mechanically self-healing composite with
pressure-and flexion-sensitive properties for electronic skin applications. _Nat. Nanotechnol._ 7, 825 (2012). CAS Google Scholar * Huang, Y. et al. A self-healable and highly stretchable
supercapacitor based on a dual crosslinked polyelectrolyte. _Nat. commun._ 6, 1–8 (2015). Google Scholar * Kim, T. I. et al. Injectable, celluar-scale optoelectronics with applications for
wireless optogenetics. _Science_ 340, 211–216 (2013). CAS Google Scholar * Jeong, J.-W. et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. _Cell_
162, 662–674 (2015). CAS Google Scholar * Yu, K. J. et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. _Nat.
Mater._ 15, 782–791 (2016). CAS Google Scholar * Kang, S.-K. et al. Bioresorbable silicon electronic sensors for the brain. _Nature_ 530, 71–76 (2016). CAS Google Scholar * Koo, J. et
al. Wireless bioresorbable electronic system enables sustained non-pharmacological neuroregenerative therapy. _Nat. Med._ 24, 1830–1836 (2018). CAS Google Scholar * Kim, H.-S. et al.
Bioresorbable silicon nanomembranes and iron catalyst nanoparticles for flexible, transient electrochemical dopamine monitors. _Adv. Healthc. Mater._ 7, 1801071 (2018). Google Scholar *
Guzik, T. J., West, N. E. J., Pillai, R., Taggart, D. P. & Channon, K. M. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. _Hypertension_ 39,
1088–1094 (2002). CAS Google Scholar * Farah, C., Michel, L. Y. M. & Balligand, J.-L. Nitric oxide signalling in cardiovascular health and disease. _Nat. Rev. Cardiol._ 15, 292–316
(2018). CAS Google Scholar * Wennberg, P. O. & Dabdub, D. Rethinking ozone production. _Science_ 319, 1624–1625 (2008). CAS Google Scholar * Li, S., Matthews, J. & Sinha, A.
Atmospheric hydroxyl radical Production from electronically excited NO2 and H2O. _Science_ 319, 1657–1660 (2009). Google Scholar * Liu, S., Wang, Z., Zhang, Y., Zhang, C. & Zhang, T.
High performance room temperature NO2 sensors based on reduced graphene oxide-multiwalled carbon nanotubes-tin oxide nanoparticles hybrids. _Sens. Actuators B_ 211, 318–324 (2015). CAS
Google Scholar * Wang, Z., Huang, L., Zhu, X., Zhou, X. & Chi, L. An ultra-sensitive organic semiconductor NO2 sensor based on crystalline TIPS‐pentacene films. _Adv. Mater._ 29,
1703192 (2017). Google Scholar * Sahu, P. K., Pandey, R. K., Dwivedi, E., Mishra, V. N. & Prakash, R. Polymer/Graphene oxide nanocomposite thin film for NO2 sensor: an in situ
investigation of electronic, morphological, structural, and spectroscopic properties. _Sci. Rep._ 10, 2981 (2020). CAS Google Scholar * Peng, K.-Q., Wang, X. & Lee, S.-T. Gas sensing
properties of single crystalline porous silicon nanowires. _Appl. Phys. Lett._ 95, 243112 (2009). Google Scholar * Yan, W., Hu, M., Wang, D. & Li, C. Room temperature gas sensing
properties of porous silicon/V2O5 nanorods composite. _Appl. Surf. Sci._ 346, 216–222 (2015). CAS Google Scholar * Moon, H. G. et al. Glancing angle deposited WO3 nanostructures for
enhanced sensitivity and selectivity to NO2 in gas mixture. _Sens. Actuators B Chem._ 229, 92–99 (2016). CAS Google Scholar * Zheng, X. & Cheng, H. Y. Flexible and stretchable metal
oxide gas sensors for healthcare. _Sci. China Technol. Sci._ 62, 209–223 (2019). CAS Google Scholar * Yang, L. et al. Novel gas sensing platform based on a stretchable laser-induced
graphene pattern with self-heating capabilities. _J. Mater. Chem. A_ 8, 6487–6500 (2020). CAS Google Scholar * Bogdan, C. Nitric oxide and the immune response. _Nat. Rev. Immunol._ 2,
907–916 (2001). CAS Google Scholar * Calabrese, V. et al. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. _Nat. Rev. Neurosci._ 8, 767–775 (2007). Google
Scholar * Moon, H. G. et al. All villi-like metal oxide nanostructures-based chemiresistive electronic nose for an exhaled breath analyzer. _Sens. Actuators B_ 257, 295–302 (2018). CAS
Google Scholar * Hwang, S.-W. et al. A physically transient form of silicon electronics. _Science_ 337, 1640–1644 (2012). CAS Google Scholar * Kang, S.-K. et al. Dissolution behaviors and
applications of silicon oxides and nitrides in transient electronics. _Adv. Funct. Mater._ 24, 4427–4434 (2014). CAS Google Scholar * Hwang, S.-W. et al. High‐performance
biodegradable/transient electronics on biode-gradable polymers. _Adv. Mater._ 26, 3905–3911 (2014). CAS Google Scholar * Long, G. L. & Winefordner, J. D. Limit of detection. A closer
look at the IUPAC definition. _Anal. Chem._ 55, 712A–724A (1983). CAS Google Scholar * Ricciardolo, F. L. M., Sterk, P. J., Gaston, B. & Folkerts, G. Nitric oxide in health and disease
of the respiratory system. _Physiol. Rev._ 84, 731–765 (2004). CAS Google Scholar * Hesterberg, T. W. et al. Critical review of the human data on short-term nitrogen dioxide (NO2)
exposures: evidence for NO2 no-effect levels. _Crit. Rev. Toxicol._ 39, 743–781 (2009). CAS Google Scholar * Council, N. R. _Assessment of Exposure-Response Functions for Rocket-Emission
Toxicants_. (National Academies Press, Washington, 1998) 147–162. Google Scholar * Kim, D.-H. et al. Epidermal electronics. _Science_ 333, 838–843 (2011). CAS Google Scholar * Fan, J. A.
et al. Fractal design concepts for stretchable electronics. _Nat. Commun_ 5, 3266 (2014). Google Scholar * Hwang, S.-W. et al. Biodegradable elastomers and silicon nanomembranes/nanoribbons
for stretchable, transient electronics, and biosensors. _Nano Lett._ 15, 2801–2808 (2014). Google Scholar * Somekawa, H. & Mukai, T. Effect of grain refinement on fracture toughness in
extruded pure magnesium. _Scr. Mater._ 53, 1059–1064 (2005). CAS Google Scholar * Jessen, L. K., Zamponi, C. & Quandt, E. Mechanical properties of magnetron sputtered free standing
Mg-Ag alloy films. _Front. Mater._ 6, 236 (2019). Google Scholar * Mačović, M. et al. A novel approach for preparation and in situ tensile testing of silica glass membranes in the
transmission electron microscope. _Front. Mater._ 4, 10 (2017). Google Scholar * Ando, T., Shikida, M. & Sato, K. Tensile-mode fatigue testing of silicon films as structural materials
for MEMS. _Sens. Actuators A_ 93, 70–75 (2001). CAS Google Scholar * Kim, R.-H. et al. Waterproof AlInGaP optoelectronics on stretchable substrates with applications in biomedicine and
robotics. _Nat. Mater._ 9, 929–937 (2010). CAS Google Scholar * Wolf, M. P., Salieb-Beugelaar, G. B. & Hunziker, P. PDMS with designer functionalities properties, modifications
strategies, and applications. _Prog. Polym. Sci._ 83, 97–134 (2018). CAS Google Scholar * Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for
reassessment. _F1000Prime Rep._ 6, 13 (2014). Google Scholar * Aktan, F. iNOS-mediated nitric oxide production and its regulation. _Life Sci._ 75, 639–653 (2004). CAS Google Scholar
Download references ACKNOWLEDGEMENTS This work was supported by a Korea University grant; KU-KIST Graduate School of Converging Science and Technology Program, the National Research
Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (MSIP) (grant NRF-2017R1E1A1A01075027); and the Technology Innovation Program (20002974, Development
of biosensing function antibiosis wound dressing and instrument for the treatment) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea). Computations for this research were
performed on the Pennsylvania State University’s Institute for Computational and Data Sciences’ Roar supercomputer. J.Z. and H.C. also acknowledge support from the Doctoral New Investigator
grant from the American Chemical Society Petroleum Research Fund (59021-DNI7) and National Science Foundation (ECCS-1933072). J.Z. also acknowledge the Leighton Riess Graduate Fellowship and
the Diefenderfer Graduate Fellowship from the College of Engineering at Penn State university. AUTHOR INFORMATION Author notes * These authors contributed equally: Gwan-Jin Ko, Soo Deok Han
AUTHORS AND AFFILIATIONS * KU-KIST Graduate School of Converging Science and Technology, Korea University, 145 Anam-ro, Seongbuk-gu, Seoul, 02841, Republic of Korea Gwan-Jin Ko, Jeong-Ki
Kim, Won Bae Han, Jinmook Chung, Seung Min Yang, Dong-Hwee Kim, Chong-Yun Kang & Suk-Won Hwang * Electrical Engineering Division, Department of Engineering, University of Cambridge, 9 JJ
Thomson Avenue, Cambridge, CB3 0FA, UK Soo Deok Han * Department of Engineering Science and Mechanics, The Pennsylvania State University, University Park, PA, 16802, USA Jia Zhu &
Huanyu Cheng * Center for Electronic Materials, Korea Institute of Science and Technology, 5, Hwarang-ro 15-gil, Seongbuk-gu, Seoul, 02792, Republic of Korea Chong-Yun Kang Authors *
Gwan-Jin Ko View author publications You can also search for this author inPubMed Google Scholar * Soo Deok Han View author publications You can also search for this author inPubMed Google
Scholar * Jeong-Ki Kim View author publications You can also search for this author inPubMed Google Scholar * Jia Zhu View author publications You can also search for this author inPubMed
Google Scholar * Won Bae Han View author publications You can also search for this author inPubMed Google Scholar * Jinmook Chung View author publications You can also search for this author
inPubMed Google Scholar * Seung Min Yang View author publications You can also search for this author inPubMed Google Scholar * Huanyu Cheng View author publications You can also search for
this author inPubMed Google Scholar * Dong-Hwee Kim View author publications You can also search for this author inPubMed Google Scholar * Chong-Yun Kang View author publications You can
also search for this author inPubMed Google Scholar * Suk-Won Hwang View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence
to Chong-Yun Kang or Suk-Won Hwang. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN
ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format,
as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ko, GJ., Han, S.D., Kim, JK. _et
al._ Biodegradable, flexible silicon nanomembrane-based NOx gas sensor system with record-high performance for transient environmental monitors and medical implants. _NPG Asia Mater_ 12, 71
(2020). https://doi.org/10.1038/s41427-020-00253-0 Download citation * Received: 06 June 2020 * Revised: 03 September 2020 * Accepted: 15 September 2020 * Published: 06 November 2020 * DOI:
https://doi.org/10.1038/s41427-020-00253-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative