A new vulnerability to bet inhibition due to enhanced autophagy in brca2 deficient pancreatic cancer
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Pancreatic cancer is one of the deadliest diseases in human malignancies. Among total pancreatic cancer patients, ~10% of patients are categorized as familial pancreatic cancer
(FPC) patients, carrying germline mutations of the genes involved in DNA repair pathways (e.g., BRCA2). Personalized medicine approaches tailored toward patients’ mutations would improve
patients’ outcome. To identify novel vulnerabilities of _BRCA2_-deficient pancreatic cancer, we generated isogenic _Brca2_-deficient murine pancreatic cancer cell lines and performed
high-throughput drug screens. High-throughput drug screening revealed that _Brca2_-deficient cells are sensitive to Bromodomain and Extraterminal Motif (BET) inhibitors, suggesting that BET
inhibition might be a potential therapeutic approach. We found that _BRCA2_ deficiency increased autophagic flux, which was further enhanced by BET inhibition in _Brca2_-deficient pancreatic
cancer cells, resulting in autophagy-dependent cell death. Our data suggests that BET inhibition can be a novel therapeutic strategy for _BRCA2_-deficient pancreatic cancer. SIMILAR CONTENT
BEING VIEWED BY OTHERS LOSS OF TUMOR SUPPRESSOR WWOX ACCELERATES PANCREATIC CANCER DEVELOPMENT THROUGH PROMOTION OF TGFΒ/BMP2 SIGNALING Article Open access 27 December 2022 NLRP4 RENDERS
PANCREATIC CANCER RESISTANT TO OLAPARIB THROUGH PROMOTION OF THE DNA DAMAGE RESPONSE AND ROS-INDUCED AUTOPHAGY Article Open access 26 August 2024 CRISPR/CAS9-MEDIATED KNOCK-IN OF _BRCA1/_2
MUTATIONS RESTORES RESPONSE TO OLAPARIB IN PANCREATIC CANCER CELL LINES Article Open access 31 October 2023 INTRODUCTION Pancreatic cancer is the 3rd leading cause of cancer-related deaths
in the United States, with a dismal 5-year survival rate of 11% [1]. The low survival rate of pancreatic ductal adenocarcinoma (PDAC) is largely attributed to the late diagnosis of the
disease when cancer has metastasized, making surgical resection a viable option for less than 20% of PDAC patients [2]. In addition, the highly resistant and heterogeneous nature of PDAC
makes the benefits from the first-line chemotherapies rather modest. Therefore, there is an urgent need to develop more effective therapies for patients with PDAC. PDAC is driven by somatic
mutations in the oncogene _KRAS_ and the tumor suppressor _TP53_ [3]. In addition, around 10% of PDAC cases are hereditary and are categorized as familial pancreatic cancer (FPC), which
describes families with two or more first-degree relatives afflicted by PDAC [4]. Previously, sequencing of FPC patient samples has revealed that FPC patients harbor germline mutations in
genes related to DNA repair pathways (hereinafter referred to as ‘FPC genes’) which increase the likelihood of developing PDAC in their lifetime [4]. The most widely studied FPC gene is
_BRCA1/2_, which code for key proteins mediating the homologous recombination (HR) DNA repair pathway [5]. Defects in BRCA1/2 create a unique set of vulnerabilities, such as increased
genomic instability and defects in the HR pathway, which augments sensitivity to platinum-based chemotherapies and increases the reliance on alternative DNA repair pathways, respectively
[6]. In the past decades, studies have found that these unique vulnerabilities inherent in _BRCA1/2_ mutant cancers (e.g., breast, ovarian, and pancreatic cancers) could be utilized to
induce synthetic lethality [7]. Other FPC gene mutations which can cause a defect in HR pathway (termed ‘BRCAness’) are thought to get benefits from similar approaches. Indeed, Pancreas
Cancer Olaparib Ongoing (POLO) clinical trial has found that Poly ADP-ribose Polymerase inhibitor (PARPi) showed significant benefit in _BRCA1/2_ mutant PDAC patients [8]. The success of
PARPi as a targeted therapy for _BRCA1/2_ mutant PDAC patients highlighted the need to develop other forms of targeted therapies that could exploit the unique vulnerabilities within FPC
patients. This, in turn, will allow for the treatment of a wider range of patients with a larger range of FPC mutations. To identify novel targeted therapies that could induce synthetic
lethality in _BRCA2_-deficient PDAC, we established isogenic _BRCA2_-deficient murine PDAC cell lines, performed a high-throughput screening (HTS) of drugs, and validated the drug responses.
From this screening, we identified that _BRCA2_ deficiency confers increased sensitivity to Bromodomain and Extra terminal Motif Domain (BET) inhibitors (BETi). These BET proteins contain
two tandem bromodomains in N-terminal, which can bind to lysine-acetylated histones and regulate gene transcription [9]. Further genetic and pharmacological perturbation study revealed that
BET inhibition induces increased cell death in _BRCA2_-deficient PDAC, through promoting autophagy-dependent cell death. MATERIALS AND METHODS CELL LINES AND CELL CULTURE CONDITION Murine
KPC cell lines (KPC-mT3 and KPC-mT19) were maintained with DMEM (Corning, 10-013-CV) supplemented with 10% FBS (Gen Clone, 25-550H), 1% penicillin-streptomycin (P/S, Gibco, 15140-122) [10].
Human pancreatic cancer cell lines (CAPAN1, PANC1, CFPAC1, CAPAN2, and MiaPaCa-2) were obtained from ATCC. All human cell lines were cultured in RPMI 1640 (Corning, 10-040-CV) supplemented
with 10% FBS and 1% P/S. COMPOUND LIBRARY AND HTS A collection of ~3300 clinically approved drugs obtained from multiple vendors were assembled at the UF Scripps Biomedical Research
High-throughput Screening Center and reformatted into 1536-well source plates for automated robotics screening [11]. In addition, the NCI-approved oncology drug set of 133 compounds was
obtained directly from the NCI, described previously [12, 13]. CELL VIABILITY ASSAY AND CYTOTOXICITY ASSAY 1500 cells (for mouse KPC cells) or 3000 cells (for human PDAC cell lines) in 50 µL
of complete DMEM were plated per well of 96 well plates. 24 h post-plating, corresponding drugs were serially diluted in complete DMEM. 50 µL of diluted drugs were treated to cells and
cells were incubated. 72 h post drug treatment, media was carefully removed. Then, 100 µL of diluted AlamarBlue solution prepared with 180 µL of Resazurin (Fisher Scientific, AC418900010) in
50 mL of 1x PBS was carefully added to each well. Cells with AlamarBlue solution were incubated in the 37 °C 5% CO2 incubator for 2 h. Absorbance at 570 nm and 600 nm was measured with a
plate reader. Using the PRISM software, the cell viability measurements from each cell line were normalized to measurement from vehicle treated wells, then cell viability curves were
generated using nonlinear regression (curve fit). Drug combination effect was analyzed using the Combenefit software [14]. To measure the cytotoxicity, the CytoTox-Glo assay (Promega, G9291)
was used, and normalized by the cell viability based on CellTiter-Glo Luminescent cell viability assay. Luminescence signal was read by the SpectraMax iD5 plate reader (Molecular Device).
RNA-SEQ AND GSEA For RNA-seq with the FPC gene KO cells and control cells, cells were plated, then treated with 1 µM of JQ1 or DMSO for 72 h. Cells were lysed with 1 mL of TRIzol (Thermo
Fisher Scientific, 15596026) and 200 µL of Chloroform following the manufacturer’s instructions. The collected RNA was purified with PureLink RNA Mini Kit (Invitrogen, 12183018A) following
the manufacturer’s protocol. RNA concentration was determined with nanodrop. Library preparation and RNA-sequencing were performed by Novogene Co., LTD (Beijing, China). In brief, mRNA was
enriched using oligo(dT) beads, and rRNA was removed using the Ribo-Zero kit. The mRNA was fragmented, and cDNA was synthesized by using an mRNA template and random hexamers primer, after
which a second-strand synthesis buffer (Illumina), dNTPs, RNase H, and DNA polymerase I were added for the second-strand synthesis, followed by adapter ligation and size selection. The
library was sequenced by the Illumina Novaseq platform. Raw data were aligned to mm10 genome using HISAT2, read counts and normalized read counts were generated using the featureCounts, and
the differentially expressed genes were identified using DESeq2. Principal Component Analysis plots were generated utilizing packages “factoMineR” and “factoextra” in R utilizing normalized
counts of RNA-seq. Gene set enrichment analysis was performed with 7658 gene sets contained in C5: Gene Ontology Biological Processes database with normalized RNA-seq data. WESTERN BLOTTING
Cells were trypsinized into single cells to yield 106 cells. To prepare whole cell lysate, cell pellets were lysed with 1% TNET buffer (50 mM Tris pH 7.5, 1% Triton X-100, 150 mM NaCl, 5 mM
EDTA) with 1× protease inhibitor cocktails (Sigma-Aldrich, Sial-11836170001), and phosphatase inhibitor cocktails (Roche, 04906837001) on ice for 30 min. The lysate was clarified at 16,000
RCF for 10 min at 4 °C, and aliquoted into new tubes for storage at −80 °C. Total protein concentration was determined by DC Protein assay using DC Protein Assay Reagents A, B, S (Bio-Rad,
5000113, 5000114, 5000115). The cell extracts were mixed with 4X LDS sample buffer (Life Technologies, B0007), 10X NuPAGE Sample Reducing Agent (Invitrogen, NP0009) and heated at 70 °C for
10 min. The same amount of protein (20 μg) was loaded on NuPAGE gels (Life Technologies, NP0321). Protein lysates were separated on 4% to 12% Bis-Tris NuPAGE gels and run with MOPS Running
Buffer (Life Technologies, J62847), transferred to polyvinylidene difluoride membrane (Millipore, IPVH00010), and blocked in 5% milk powder in TBST for 1 h. The membranes were then incubated
in primary antibody overnight with rocking at 4 °C followed by incubation in appropriate secondary antibodies conjugated to horseradish peroxidase (1:5000) for 1 h and detection by Super
Signal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific, 34577). Primary antibodies used were: Beclin-1 (Cell Signaling Technology, D40C5), LC3A/B (Cell Signaling
Technology, D3U4C), Atg5 (Cell Signaling Technology, D5F5U), Atg12 (Cell Signaling Technology, D88H11), Atg16l1 (Cell Signaling Technology, D6D5), Atg7 (Cell Signaling Technology, D12B11),
SQSTM1/p62 (Cell Signaling Technology, 5114), Cleaved Caspase-3 (Cell Signaling Technology, 5A1E), Ku80 (Invitrogen, PA5-17454), Rad51 (Cell Signaling Technology, D4B10), cMyc (Abcam,
AB32072), Vinculin (Cell Signaling, 13901) and GAPDH (ProSci, 3783, 1:5000). 1:1000 dilution for primary antibodies was used unless otherwise indicated. ANIMAL EXPERIMENTS Female athymic
nude (Nu/Nu) mice with 6 weeks of age were purchased from Charles River Laboratory and used for subcutaneous engraftment. All the mice were housed in the Genome and Biomedical Sciences
Facility at University of California, Davis under specific pathogen free environment, ambient temperature and a standard light-dark cycle. All the animal experiments were conducted in
accordance with procedures approved by the Intuitional Animal Care and Use Committee (IACUC). For the subcutaneous engraftment, cells were trypsinized and resuspended in 50 µL of Matrigel
(Fisher Scientific, CB-40230C). All the mice were either subcutaneously injected with 0.5 ×106 cells of control or _Brca2_-KO KPC cells on the left and right flank, respectively. Following 3
days of tumor injection, the mice were randomized into 2 groups (8 mice per group). The mice were intraperitoneally administered with either vehicle or JQ1 (MedChemExpress, HY-13030) with a
dose of 50 mg/kg 5 times per week. To prepare drug treatment for in vivo experiment, the vehicle consisted of 5% DMSO and 95% (20% SBE-β-CD in saline), while JQ1 was first resuspended in
DMSO and then diluted with the vehicle to the final concentration of 5 mg/ml. Tumor size was measured with Vernier calipers three times a week, and tumor volumes were calculated according to
the formula: \(\frac{\pi \times {length}\times {{width}}^{2}}{6}\) [15]. After the course of drug administration, the mice were euthanized, and tumors were collected for histological
examination. Note that one mouse in the DMSO group was lost, which is unrelated to the experiment. Additional experimental details and methods can be found in the Supplementary information.
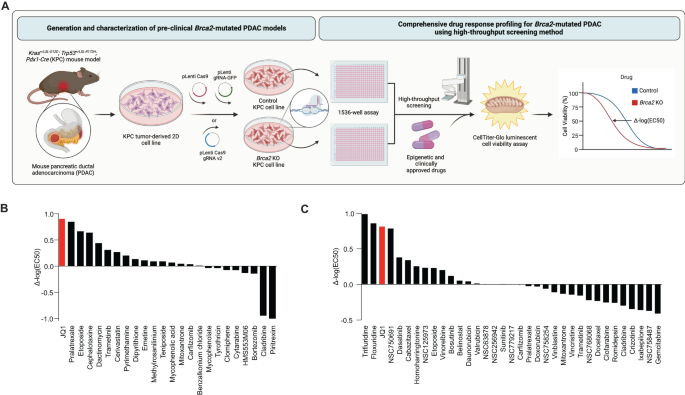
RESULTS HIGHTHROUGHPUT DRUG SCREENS IN _BRCA2_-DEFICIENT PDAC CELLS IDENTIFY BET INHIBITOR The rarity of FPC gene mutations in PDAC patients and commercially available PDAC cell lines
hampered the development of personalized medicine for FPC patients [16]. We reasoned that isogenic _Brca2_-deficient PDAC cells would enable us to identify unique vulnerabilities associated
with _BRCA2_ deficiency. To this end, we inactivated _Brca2_ utilizing CRISPR/Cas9 in the murine PDAC cell lines derived from _Kras__+/LSL-G12D__; Trp53__+/LSL-R172H__; Pdx1-Cre_ (KPC) mouse
(herein after referred to as ‘KPC-mT3’ and ‘KPC-mT19’). (Fig. 1A and Supplementary Fig. 1A, B). As a control, we used a gRNA targeting _Rosa26_ locus as described elsewhere [17]. From this
approach, we were able to establish the clonally derived isogenic _Brca2_ knock-out (KO) KPC-mT3 and -mT19 cell lines. _Brca2_-KO KPC-mT3 cells exhibited a defect in RAD51 foci formation
upon DNA damage and reduced HR efficiency in HR efficiency assay (Supplementary Fig. 1C–F). To determine if the isogenic _Brca2_-deficient KPC cell lines recapitulate the drug sensitivity
observed in the clinical setting, we subjected our _Brca2_-KO KPC-mT3 cells to platinum-based chemotherapies and PARP inhibitors, which have been shown to be clinically effective in
_BRCA1/2_ mutant PDAC patients [8, 18]. _Brca2_-KO KPC-mT3 cells displayed an increased sensitivity to oxaliplatin and PARPi, olaparib and talazoparib, compared to the control, without any
proliferation change (Supplementary Fig. 1G, H). However, we did not see any significant change for other common first-line chemotherapies gemcitabine and 5-FU, suggesting that the increased
sensitivity to PARPi is specifically due to _Brca2_ deficiency. To identify new vulnerabilities in _BRCA2_ deficient PDAC, we performed a HTS with a set of epigenetic drugs, clinically
approved drugs, and 133 National Cancer Institute (NCI) approved drugs (Fig. 1A) as described previously [13]. For screening a set of epigenetic drugs and 3300 approved drugs, we tested at a
single concentration (2 µM) for both control and _Brca2_-KO KPC-mT3 cells (Supplementary file 1). 110 compounds that exhibited more than 50% reduced viability in either control or
_Brca2_-KO KPC-mT3 cells were chosen for concentration response curve (CRC) experiments (Fig. 1B and Supplementary file 2). From the screening of a set of epigenetic drugs and 3300 approved
drugs, we identified JQ1 as a top hit (Fig. 1B and Supplementary Fig. 2A). JQ1 rendered the biggest difference in IC50, indicating that _Brca2_-KO KPC-mT3 cells are preferentially sensitive
to JQ1, a BET inhibitor. We further tested if JQ1 stood out in the second round of drug screening with the 133 NCI approved drug panel. This analysis confirmed that JQ1 is one of the top
hits compared to the hits from the 133 NCI drug panel (Fig. 1C, Supplementary Fig. 2B, C and Supplementary file 3). _BRCA2_-DEFICIENT PDAC CELLS ARE SENSITIVE TO BET INHIBITION To validate
our HTS results, we tested JQ1 as well as birabresib and molibresib, additional BET inhibitors with two independent clonally derived _Brca2_-KO KPC cells from two different KPC cell lines
each (KPC-mT3 and KPC-mT19-v2). We confirmed that _Brca2_ deficiency rendered increased sensitivity to BET inhibitors (Fig. 2A–C). To confirm our findings in human PDAC cells, we
additionally generated _BRCA2_-deficient MiaPaCa-2 human PDAC cell line and confirmed that _BRCA2_ deficiency conferred increased sensitivity to BET inhibitors and olaparib (Fig. 2D and
Supplementary Fig. 3A, B). This finding was further corroborated by the observation that CAPAN1, a _BRCA2_ mutated PDAC cell line showed lower IC50 than three other cell lines with no
mutation in _BRCA2_ (Fig. 2E). Consistent with our finding in PDAC, a publicly available database for Genomics of Drug Sensitivity in Cancer [19] revealed that PARP inhibitors and BET
inhibitors (e.g., JQ1, PFI-1, I-BET-762) are preferentially cytotoxic in mutant _BRCA2_ context in breast cancer and pan-cancer, respectively (Fig. 2F, G). We additionally validated two
other drugs, etoposide and afatinib from the HTS as these drugs have also been shown to be effective in _BRCA2_ deficient setting (Supplementary Fig. 3C) [20, 21]. Consistent with
pharmacological BET inhibition, depletion of Brd2, Brd3 and Brd4 by transfection of pooled siRNA against Brd2, Brd3 and Brd4 in _Brca2_-KO cells resulted in a significant decrease in cell
viability (Fig. 2H, I and Supplementary Fig. 3D, E). While BETi competitively blocks the bromodomain pocket of BET proteins, BETi has differential affinities to BRD2, BRD3, and BRD4 [22].
For instance, JQ1 is known to bind with the highest affinity to BRD4 [23]. To discern which BET protein is responsible for the increased sensitivity to BET inhibition in _Brca2_-KO KPC-mT3
cells, we individually knocked down Brd2, Brd3, and Brd4 using siRNA transfection. Brd4 depletion, but neither Brd2 nor Brd3, led to the reduced viability only in _Brca2_-KO KPC-mT3 cells,
indicating that the inhibition of BRD4 function is critical for BET inhibition in _Brca2_-KO KPC-mT3 cells (Supplementary Fig. 3F, G). Previously, BET inhibition has been shown to
down-regulate DDR genes and MYC expression, resulting in deleterious effects in PDAC [24, 25]. While we observed similar down-regulation of DDR genes and MYC expression, these did not seem
to be responsible for the differential response to BETi in _Brca2_-deficient KPC-mT3 cells (Supplementary Fig. 4A, B). In addition, BET inhibition was reported to induce BRCAness in
HR-proficient PDAC cells, leading to the increased sensitivity to PARP inhibitor [24]. The previously reported synergism between JQ1 and PARPi disappeared in _Brca2_-deficient KPC-mT3 cells,
likely due to the increased sensitivity to BETi in _Brca2_ deficiency (Supplementary Fig. 5). JQ1 SIGNIFICANTLY SUPPRESSES PROGRESSION OF _BRCA2_-DEFICIENT KPC TUMORS IN VIVO To evaluate if
BET inhibition is an effective therapeutic approach for _BRCA2_-deficient pancreatic cancer in vivo, we subcutaneously injected KPC-mT3 cells into immunocompromised athymic nu/nu mice, and
administered either with vehicle or JQ1 (50 mg/kg) via intraperitoneal injection (Fig. 3A). Over the 14-day treatment period, JQ1 treatment significantly reduced the tumor volume in both
control and _Brca2_-deficient tumors, but more significant reduction in _Brca2_-deficient tumors (Fig. 3B, C). To evaluate the acute effect of JQ1 administration in vivo, we collected tumors
after one-week treatment of JQ1 in separate cohorts of mice subcutaneously injected with control and _Brca2_-KO KPC-mT3 and mT19-v2 cells (one clone from KPC-mT3 and two clones from
KPC-mT19-v2 with their control cells) (Fig. 3D). Immunohistochemical (IHC) analyses revealed that BET inhibition reduced cell proliferation and increased cell death, characterized by Ki67
IHC and TUNEL staining, respectively (Fig. 3E–J, Supplementary Fig. 6A–C). We did not see obvious evidence of cleaved caspase 3 activation (Supplementary Fig. 6D, E), suggesting alternative
mechanisms of growth inhibition and cell death. Taken together, BET inhibition reduced the growth of PDAC tumors in vivo with more pronounced effect in _BRCA2_ deficiency. _BRCA2_ DEFICIENCY
AUGMENTS JQ1-MEDIATED UPREGULATION OF AUTOPHAGY-RELATED GENES IN PDAC To extend our finding in _Brca2_-deficient PDAC cells, we tested BET inhibitors in _Atm_- and _Bub1b_-KO KPC-mT3 cells.
Inactivation of these genes resulted in the increased sensitivity to BET inhibitors, suggesting that a defect in DNA damage response (DDR) or HR DNA repair pathway rendered a new
vulnerability to BET inhibitors (Supplementary Fig. 7A–D). To elucidate the common mechanism behind the increased sensitivity to JQ1 in _Brca2_-KO, _Atm_-KO, and _Bub1b_-KO (hereinafter
referred to as FPC-gene-KO) KPC-mT3 cells, we performed RNA-seq to compare the transcriptomic differences between FPC-gene-KO KPC-mT3 cells and the control upon JQ1 treatment. As expected,
JQ1 drastically changed the transcriptomic profiles of the PDAC cells (Fig. 4A). Gene set enrichment analysis (GSEA) revealed that the genes involved in the epigenetic regulatory pathway
were significantly upregulated upon JQ1 treatment (Fig. 4B). Interestingly, the gene sets related to macroautophagy and many autophagy-related pathways (e.g., phagosome acidification,
phagosome maturation, and regulation of macroautophagy) were also significantly upregulated upon JQ1 treatment. The analysis of publicly available transcriptomic data [26, 27] from four
different human PDAC cell lines with JQ1 treatment also confirmed the consistent up-regulation of the gene set involved in macroautophagy (Supplementary Fig. 8A, B). Macroautophagy is a
proteo-homeostatic mechanism that sequesters and traffics unwanted proteins and/or organelles to the lysosome for degradation [28]. Dysfunction in the autophagy process has been widely
implicated in cancer, both as a cancer-promoting mechanism in times of nutrient deprivation and as an anti-cancer mechanism through autophagy-dependent cell death [28]. When using GSEA to
compare the transcriptomic changes between the control and FPC-gene-KO KPC-mT3 cells upon JQ1, we found that the up-regulation of macroautophagy and autophagy-related pathways were even more
prominent in FPC-gene-KO KPC-mT3 cells (Fig. 4C–E), suggesting that the increased autophagic flux might play a role in the drug response of FPC-gene-KO KPC-mT3 cells. While the mechanism
behind BET inhibition-mediated gene up-regulation remains unclear, a proposed potential mechanism involves the re-organization of the associated chromatin complex upon BET inhibition [29].
To determine if the changes to the level of autophagy-related transcripts upon JQ1 were regulated at the epigenetic level, we performed CUT-and-RUN-seq for H3K27ac to identify the genomic
regions of the active promoters and enhancers in the control and _Brca2_-KO KPC-mT3 cells upon JQ1 treatment. The H3K27ac CUT-and-RUN-seq analysis showed that the autophagy-related genes
that were up-regulated upon JQ1 treatment had increased H3K27ac in the proximal transcriptional start site in the JQ1 treated _Brca2_-KO KPC-mT3 cells compared to the control (Fig. 4F, G),
suggesting that _Brca2_-KO KPC-mT3 cells might exhibit more autophagy and JQ1 might preferentially induce autophagy mediated cell death in _Brca2_-KO KPC-mT3 cells. Taken together, JQ1
transcriptionally upregulates the genes associated with autophagy in _Brca2_-deficient PDAC. _BRCA2_ DEFICIENCY INCREASES AUTOPHAGIC FLUX UPON JQ1 TREATMENT From the transcriptomic analyses,
we hypothesized that JQ1-mediated upregulation of autophagy-associated genes induces increased autophagic flux in _Brca2_-KO KPC cells. Recent reports suggested that JQ1 induces the
accumulation of autophagosomes and autolysosomes, and increased autophagy flux [30]. To test this hypothesis, we measured the expression of autophagy protein markers upon JQ1 treatment.
_Brca2_-KO KPC-mT3 cells exhibited higher base-line expression of autophagy markers LC3A/B II and BECLIN-1 compared to the control, and JQ1 treatment increased the expression LC3A/B II and
BECLIN-1 in both control and _Brca2_-KO KPC-mT3 cells (Fig. 5A). Particularly, JQ1-treated _Brca2_-KO KPC-mT3 cells had further increased expression of autophagy marker proteins along with
decreased expression of autophagy substrate SQSTM1/p62, indicative of increased autophagic flux. Despite an increase in autophagic flux in JQ1-treated _Brca2_-KO KPC-mT3 cells, there was no
significant difference in the expression of ATG family proteins (Supplementary Fig. 9). Immunofluorescence (IF) staining of LC3 showed more autophagosome formations depicted by the increased
number of LC3 puncta in _Brca2_-KO cells upon JQ1 treatment (Fig. 5B, C). To visualize the autophagy flux at the cellular level, we utilized an autophagy reporter construct (EGFP-LC3-RFP)
to observe autophagosome puncta formation and trafficking in the cell. This reporter allowed us to distinguish early autophagic vacuoles from autolysosomes using the differential pH
sensitivities as described elsewhere [31]. JQ1 treatment in control and _Brca2_-KO KPC-mT3-v2 cells increased LC3 puncta intensity per cell (Fig. 5D, E). Consistent with IF results,
_Brca2_-KO KPC-mT3-v2 cells also had higher baseline LC3 puncta intensity compared to control cells, and this intensity in the _Brca2_-KO KPC-mT3-v2 cells further increased upon JQ1
treatment. Using the recently developed quantitative method to measure dynamic autophagy rates in live cells [32], we measured the rates of three major steps in autophagy: the rate of
formation of autophagosomes (R1), the rate of fusion of autophagosomes with autolysosomes (R2), and the rate clearance of autolysosomes (R3) (Fig. 5F). First, we validated our system with
rapamycin, an autophagy inducer, resulting in the increased R1, R2, and R3 of murine KPC control cells (Supplementary Fig. 10A). The measurement of dynamic autophagy rates revealed that
_Brca2_-KO KPC-mT3-v2 cells have higher basal autophagy flux (Fig. 5G), and JQ1 treatment further increased autophagy flux in both control and _Brca2_-KO cells (Fig. 5H, Supplementary Fig.
10B). Collectively, we concluded that JQ1 induces increased autophagic flux in _Brca2_-deficient PDAC cells. BET INHIBITION IN _BRCA2_ DEFICIENCY CAUSES AUTOPHAGY-DEPENDENT CELL DEATH The
increased autophagy induced by JQ1 in _Brca2_-KO KPC-mT3 cells prompted us to investigate whether autophagy-dependent cell death is critical for _Brca2_-KO KPC-mT3 cells upon JQ1 treatment.
Previously, JQ1 has also been known to induce ferroptosis, an iron-dependent cell death [33]. However, the increased sensitivity to JQ1 in _Brca2_-KO KPC-mT3 cells was not due to increased
ferroptosis (Supplementary Fig. 11A, B). Furthermore, cleaved caspase 3 expression was not significantly increased in _Brca2_-KO KPC-mT3 cells upon JQ1 treatment, compared to the control
cells treated with JQ1 (Supplementary Fig. 11C). This indicated that the preferential cell death in _Brca2_ deficiency is not likely due to either ferroptosis or caspase3-dependent
apoptosis. To show JQ1-mediated cell death in _Brca2_-KO KPC-mT3 cells is autophagy-dependent, we asked whether hydroxychloroquine (HCQ), an autophagy inhibitor, can rescue JQ1-induced cell
death in _Brca2_-KO KPC-mT3 cells. As expected, HCQ treatment partially rescued the increased sensitivity to JQ1 in _Brca2_-KO-mT3 cells while the control cells did not show any antagonistic
effect upon JQ1 and HCQ treatment (Fig. 6A, B and Supplementary Fig. 11D). Likewise, we were able to rescue the increased sensitivity to JQ1 with autophagy inhibition in _Atm_- and
_Bub1b_-deficient KPC cells, suggesting that autophagy-dependent cell death might be a common mechanism in HR-deficiency (Fig. 6C, D). While HCQ is known to block the fusion of
autophagosomes with lysosomes, it also has other autophagy-independent effects. Therefore, we decided to genetically perturb the autophagy genes responsible for autophagosome formation such
as _Atg5, Atg12_ and _Atg16l1_ using RNA interference. In line with pharmacological inhibition of autophagy, depletion of autophagy genes also rescued the effect of JQ1 in _Brca2_-KO KPC-mT3
cells (Fig. 6E, F and Supplementary Fig. 11E). Taken together, our data with pharmacological and genetic perturbation of autophagy indicated that the induction of autophagy-dependent cell
death in _Brca2_ deficiency is in part responsible for the increased sensitivity to BET inhibition in _Brca2_-KO setting. DISCUSSION In this study, we identified JQ1, a small molecule BET
inhibitor from the high-throughput drug screen in _Brca2_-KO KPC cells. This finding was validated using isogenic human and mouse cell lines, human PDAC cell lines and publicly available
pan-cancer analysis. We propose that BET inhibition might be a potential therapeutic strategy for FPC patients with the mutated genes involved/implicated in HR DNA repair pathway. BET
proteins have recently emerged as therapeutic targets for human malignancies, and BRD4 among BET proteins appears to be a major target of BET inhibition [34]. In line with this, our genetic
perturbation experiments using siRNA confirmed that the increased sensitivity to BET inhibitors in _Brca2_-KO KPC cells was mainly mediated by BRD4. While the BET proteins share ~75%
identity between family members, it has been shown that they play distinct roles in gene transcription, chromatin remodeling, DNA replication, and DNA damage repair [9]. In particular, a
recent study further confirms that BRD4 is directly involved in DNA double strand break repair [35]. In addition, due to a poor pharmacokinetic profile, low oral bioavailability, and
unwanted toxicities of JQ1, there has been extensive effort to develop selective inhibitors of BET with reduced toxicity. Nonetheless, our findings highlight a novel vulnerability of FPC,
particularly in _BRCA2_ deficiency. Identification of BET inhibitors as a new vulnerability of FPC was an unexpected finding because many epigenetic drugs including BET inhibitors were used
to induce a “BRCAness” phenotype and exert a synergistic cytotoxicity effect in combination with olaparib in HR-proficient tumors, including breast, ovarian and prostate cancers [36]. This
phenomenon has also been observed in PDAC upon combination therapy with JQ1 and olaparib as JQ1 mitigates DNA double strand break repair pathway and further improves the efficacy of olaparib
[24]. However, in our study, we found that _BRCA2_-deficient PDAC is sensitive to BET inhibition alone, which sheds light on unexplored mechanisms between HR DNA repair pathway and
epigenetic regulations. Several PARP inhibitors have already been approved for _BRCA1/2_ mutated cancers. However, the development of PARPi resistance appears to be inevitable [37]. JQ1 has
been shown to re-sensitize _BRCA2_-mutated ovarian cancer cells that have developed resistance to olaparib [38]. It remains to be determined whether BETi can be still effective to
_BRCA2_-mutated PDAC cells that become resistant to PARPi. Therefore, BET inhibition as a therapeutic approach might be broadly applicable to both HR-proficient and -deficient as well as
PARPi-resistant cancers. There is ample evidence that autophagy and DNA repair pathways are interconnected. For instance, autophagy deficiency has been shown to induce genomic instability
through various mechanisms, reviewed in Vesonni et al. [39]. Here, we showed that the increased sensitivity to BETi in _Brca2_-deficient PDAC cells is in part due to autophagy-dependent cell
death. Autophagy is a conserved catabolic process that contributes to cellular homeostasis, through the degradation and recycling of cytoplasmic components and organelles in the lysosome
[28, 40, 41]. Recently, it has been reported that PDAC tumors exhibit high autophagy activity which confers resistance to chemotherapies [42]. Therefore, a significant effort to combine
autophagy inhibitor with other therapies are being pursued in both preclinical and clinical settings. For example, the combination of autophagy inhibitors with MAPK pathway inhibitors are
currently being tested in patients with PDAC [42,43,44]. We found that _Brca2_ deficiency resulted in increased autophagy activity. This is in line with recent observations that _BRCA1/2_
can negatively regulate autophagy [45,46,47]. Upon BET inhibition, the autophagy activity was further pronounced, resulting in autophagy-dependent cell death. Unlike the current therapeutic
strategy of combining autophagy inhibition with other chemo- or targeted therapies, autophagy inhibition confers antagonistic effect of JQ1 sensitivity in _Brca2_ deficiency. This raises a
potential concern that autophagy inhibition might exert antagonistic effect depending on the context. It should be noted that autophagy has been shown to have dual roles in cancer
progression: both pro- and anti-tumorigenic roles [48, 49]. For instance, suppression or deficiency of autophagy genes has been shown to enhance tumorigenesis. In pancreatic cancer, _Atg5_
or _Atg7_ deficiency resulted in increased PanIN lesions, albeit lack of progression to malignant disease [50]. In line with this, a monoallelic deletion of _BECLIN1_ is frequently found in
breast cancer samples [51]. It is tempting to speculate that the increased sensitivity to JQ1 might be associated with DDR defects as we showed the defects in other FPC genes (e.g., _ATM,
BUB1B_) resulted in increased sensitivity to BETi. Because of the scarcity of non-_BRCA_ mutations in FPC, the clinical benefit of PARPi and other therapeutic strategies have only been
focused on pancreatic cancer with _BRCA1/2_ mutations. It is urgently required to develop appropriate preclinical models to evaluate the PARPi therapies and identify novel vulnerabilities
tailored toward each FPC gene. Isogenic FPC gene KO of murine KPC or human PDAC cell lines can provide a platform to identify a new vulnerability of FPC and investigate the underlying
molecular mechanisms of synthetic lethality and potential resistance mechanisms in the future. DATA AVAILABILITY The accession number for the data for RNA-seq and CUT-and-RUN-seq is NCBI
GEO: GSE207298. REFERENCES * Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. Article PubMed Google Scholar * Collins A, Bloomston M.
Diagnosis and management of pancreatic cancer. Minerva Gastroenterol Dietol. 2009;55:445–54. CAS PubMed PubMed Central Google Scholar * Waddell N, Pajic M, Patch AM, Chang DK, Kassahn
KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. Article CAS PubMed PubMed Central Google Scholar * Roberts NJ,
Norris AL, Petersen GM, Bondy ML, Brand R, Gallinger S, et al. Whole genome sequencing defines the genetic heterogeneity of familial pancreatic cancer. Cancer Discov. 2016;6:166–75. Article
CAS PubMed Google Scholar * Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–72. Article CAS PubMed Google
Scholar * Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer.
2014;111:1132–8. Article CAS PubMed PubMed Central Google Scholar * Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355:1152–8. Article CAS
PubMed PubMed Central Google Scholar * Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N
Engl J Med. 2019;381:317–27. Article CAS PubMed PubMed Central Google Scholar * Cheung KL, Kim C, Zhou MM. The functions of BET proteins in gene transcription of biology and diseases.
Front Mol Biosci. 2021;8:728777. Article CAS PubMed PubMed Central Google Scholar * Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, et al. Organoid models of human and mouse
ductal pancreatic cancer. Cell. 2015;160:324–38. Article CAS PubMed Google Scholar * Baillargeon P, Fernandez-Vega V, Sridharan BP, Brown S, Griffin PR, Rosen H, et al. The Scripps
Molecular Screening Center and Translational Research Institute. SLAS Discov. 2019;24:386–97. Article CAS PubMed PubMed Central Google Scholar * Fernandez-Vega V, Hou S, Plenker D,
Tiriac H, Baillargeon P, Shumate J, et al. Lead identification using 3D models of pancreatic cancer. SLAS Discov. 2022;27:159–66. Article CAS PubMed PubMed Central Google Scholar * Hou
S, Tiriac H, Sridharan BP, Scampavia L, Madoux F, Seldin J, et al. Advanced development of primary pancreatic organoid tumor models for high-throughput phenotypic drug screening. SLAS
Discov. 2018;23:574–84. Article CAS PubMed PubMed Central Google Scholar * Di Veroli GY, Fornari C, Wang D, Mollard S, Bramhall JL, Richards FM, et al. Combenefit: an interactive
platform for the analysis and visualization of drug combinations. Bioinformatics. 2016;32:2866–8. Article PubMed PubMed Central Google Scholar * Kersemans V, Cornelissen B, Allen PD,
Beech JS, Smart SC. Subcutaneous tumor volume measurement in the awake, manually restrained mouse using MRI. J Magn Reson Imaging. 2013;37:1499–504. Article PubMed Google Scholar *
Casolino R, Corbo V, Beer P, Hwang C-I, Paiella S, Silvestri V, et al. Germline aberrations in pancreatic cancer: implications for clinical care. Cancers. 2022;14:3239. Article CAS PubMed
PubMed Central Google Scholar * Shi J, Wang E, Milazzo JP, Wang Z, Kinney JB, Vakoc CR. Discovery of cancer drug targets by CRISPR-Cas9 screening of protein domains. Nat Biotechnol.
2015;33:661–7. Article CAS PubMed PubMed Central Google Scholar * Cancer Genome Atlas Research Network. Electronic address aadhe, Cancer Genome Atlas Research N. Integrated Genomic
Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2017;32:185–203.e13. Article Google Scholar * Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, et al.
Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–5. Article CAS PubMed PubMed Central Google Scholar * Treszezamsky AD, Kachnic LA,
Feng Z, Zhang J, Tokadjian C, Powell SN. BRCA1- and BRCA2-deficient cells are sensitive to etoposide-induced DNA double-strand breaks via topoisomerase II. Cancer Res. 2007;67:7078–81.
Article CAS PubMed Google Scholar * Liu C, Zhao J, Lu W, Dai Y, Hockings J, Zhou Y, et al. Individualized genetic network analysis reveals new therapeutic vulnerabilities in 6,700 cancer
genomes. PLoS Comput Biol. 2020;16:e1007701. Article CAS PubMed PubMed Central Google Scholar * Shorstova T, Foulkes WD, Witcher M. Achieving clinical success with BET inhibitors as
anti-cancer agents. Br J Cancer. 2021;124:1478–90. Article CAS PubMed PubMed Central Google Scholar * Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective
inhibition of BET bromodomains. Nature. 2010;468:1067–73. Article CAS PubMed PubMed Central Google Scholar * Miller AL, Fehling SC, Garcia PL, Gamblin TL, Council LN, van Waardenburg R,
et al. The BET inhibitor JQ1 attenuates double-strand break repair and sensitizes models of pancreatic ductal adenocarcinoma to PARP inhibitors. EBioMedicine. 2019;44:419–30. Article
PubMed PubMed Central Google Scholar * Mazur PK, Herner A, Mello SS, Wirth M, Hausmann S, Sanchez-Rivera FJ, et al. Combined inhibition of BET family proteins and histone deacetylases as
a potential epigenetics-based therapy for pancreatic ductal adenocarcinoma. Nat Med. 2015;21:1163–71. Article CAS PubMed PubMed Central Google Scholar * Andricovich J, Perkail S, Kai Y,
Casasanta N, Peng W, Tzatsos A. Loss of KDM6A activates super-enhancers to induce gender-specific squamous-like pancreatic cancer and confers sensitivity to BET inhibitors. Cancer Cell.
2018;33:512–26.e8. Article CAS PubMed PubMed Central Google Scholar * Xu C, Wang X, Zhou Y, Chen FX, Wang H, Li K, et al. Synergy between arsenic trioxide and JQ1 on autophagy in
pancreatic cancer. Oncogene. 2019;38:7249–65. Article CAS PubMed Google Scholar * Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion.
Nature. 2008;451:1069–75. Article CAS PubMed PubMed Central Google Scholar * Choi HI, An GY, Yoo E, Baek M, Binas B, Chai JC, et al. The bromodomain inhibitor JQ1 up-regulates the long
non-coding RNA MALAT1 in cultured human hepatic carcinoma cells. Sci Rep. 2022;12:7779. Article CAS PubMed PubMed Central Google Scholar * Li F, Yang C, Zhang HB, Ma J, Jia J, Tang X,
et al. BET inhibitor JQ1 suppresses cell proliferation via inducing autophagy and activating LKB1/AMPK in bladder cancer cells. Cancer Med. 2019;8:4792–805. Article CAS PubMed PubMed
Central Google Scholar * Kaizuka T, Morishita H, Hama Y, Tsukamoto S, Matsui T, Toyota Y, et al. An autophagic flux probe that releases an internal control. Mol Cell. 2016;64:835–49.
Article CAS PubMed Google Scholar * Beesabathuni NS, Park S, Shah PS. Quantitative and temporal measurement of dynamic autophagy rates. Autophagy. 2023;19:1164–83. Article CAS PubMed
Google Scholar * Sui S, Zhang J, Xu S, Wang Q, Wang P, Pang D. Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells.
Cell Death Dis. 2019;10:331. Article PubMed PubMed Central Google Scholar * Sarnik J, Poplawski T, Tokarz P. BET proteins as attractive targets for cancer therapeutics. Int J Mol Sci.
2021;22:11102. Article CAS PubMed PubMed Central Google Scholar * Barrows JK, Lin B, Quaas CE, Fullbright G, Wallace EN, Long DT. BRD4 promotes resection and homology-directed repair of
DNA double-strand breaks. Nat Commun. 2022;13:3016. Article CAS PubMed PubMed Central Google Scholar * Yang L, Zhang Y, Shan W, Hu Z, Yuan J, Pi J, et al. Repression of BET activity
sensitizes homologous recombination-proficient cancers to PARP inhibition. Sci Transl Med. 2017;9:1–13. Article Google Scholar * Bouwman P, Jonkers J. Molecular pathways: how can
BRCA-mutated tumors become resistant to PARP inhibitors? Clin Cancer Res. 2014;20:540–7. Article CAS PubMed Google Scholar * Karakashev S, Zhu H, Yokoyama Y, Zhao B, Fatkhutdinov N,
Kossenkov AV, et al. BET bromodomain inhibition synergizes with PARP inhibitor in epithelial ovarian cancer. Cell Rep. 2017;21:3398–405. Article CAS PubMed PubMed Central Google Scholar
* Vessoni AT, Filippi-Chiela EC, Menck CF, Lenz G. Autophagy and genomic integrity. Cell Death Differ. 2013;20:1444–54. Article CAS PubMed PubMed Central Google Scholar * Mizushima N.
Autophagy: process and function. Genes Dev. 2007;21:2861–73. Article CAS PubMed Google Scholar * Yang A, Herter-Sprie G, Zhang H, Lin EY, Biancur D, Wang X, et al. Autophagy sustains
pancreatic cancer growth through both cell-autonomous and nonautonomous mechanisms. Cancer Discov. 2018;8:276–87. Article CAS PubMed PubMed Central Google Scholar * Bryant KL,
Stalnecker CA, Zeitouni D, Klomp JE, Peng S, Tikunov AP, et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat Med. 2019;25:628–40. Article
CAS PubMed PubMed Central Google Scholar * Kinsey CG, Camolotto SA, Boespflug AM, Guillen KP, Foth M, Truong A, et al. Protective autophagy elicited by RAF–>MEK–>ERK inhibition
suggests a treatment strategy for RAS-driven cancers. Nat Med. 2019;25:620–7. Article CAS PubMed PubMed Central Google Scholar * Raufi AG, Liguori NR, Carlsen L, Parker C, Hernandez
Borrero L, Zhang S, et al. Therapeutic targeting of autophagy in pancreatic ductal adenocarcinoma. Front Pharm. 2021;12:751568. Article CAS Google Scholar * Arun B, Akar U,
Gutierrez-Barrera AM, Hortobagyi GN, Ozpolat B. The PARP inhibitor AZD2281 (Olaparib) induces autophagy/mitophagy in BRCA1 and BRCA2 mutant breast cancer cells. Int J Oncol. 2015;47:262–8.
Article CAS PubMed PubMed Central Google Scholar * Tang MK, Kwong A, Tam KF, Cheung AN, Ngan HY, Xia W, et al. BRCA1 deficiency induces protective autophagy to mitigate stress and
provides a mechanism for BRCA1 haploinsufficiency in tumorigenesis. Cancer Lett. 2014;346:139–47. Article CAS PubMed Google Scholar * Wan B, Dai L, Wang L, Zhang Y, Huang H, Qian G, et
al. Knockdown of BRCA2 enhances cisplatin and cisplatin-induced autophagy in ovarian cancer cells. Endocr Relat Cancer. 2018;25:69–82. Article CAS PubMed Google Scholar * Khandia R,
Dadar M, Munjal A, Dhama K, Karthik K, Tiwari R, et al. A comprehensive review of autophagy and its various roles in infectious, non-infectious, and lifestyle diseases: current knowledge and
prospects for disease prevention, novel drug design, and therapy. Cells. 2019;8:674. Article CAS PubMed PubMed Central Google Scholar * Santana-Codina N, Mancias JD, Kimmelman AC. The
role of autophagy in cancer. Annu Rev Cancer Biol. 2017;1:19–39. Article PubMed PubMed Central Google Scholar * Yang A, Kimmelman AC. Inhibition of autophagy attenuates pancreatic cancer
growth independent of TP53/TRP53 status. Autophagy. 2014;10:1683–4. Article CAS PubMed PubMed Central Google Scholar * Wijshake T, Zou Z, Chen B, Zhong L, Xiao G, Xie Y, et al.
Tumor-suppressor function of Beclin 1 in breast cancer cells requires E-cadherin. Proc Natl Acad Sci USA. 2021;118:e2020478118. Article CAS PubMed PubMed Central Google Scholar Download
references ACKNOWLEDGEMENTS This study was supported by a grant from the National Cancer Institute of the National Institutes of Health 5K22CA226037 (to C-IH) and 5R37CA249007. C-IH was
supported by the Synthego Genome Engineer Innovation Award. KYJ was supported by UC Davis Maximizing Access to Research Careers (MARC) Program (NIGMS-MARC-U-STAR grant T34GM136469), UC Davis
College of Biological Sciences Dean’s Circle Summer Undergraduate Research Program, UC Davis Provost’s Undergraduate Fellowship, and Barry M. Goldwater Scholarship. SA was supported by the
Anandamahidol Foundation (Thailand). We would like to thank Vincenzo Corbo, Rafaella Casolino, Sarah Wang, and Jihao Xu for their critical comments on the manuscript. We would like to thank
the Hunter lab for providing immunofluorescence microscopy imaging, Sarah Wang for developing an ImageJ plugin that was used for quantifying LC3 puncta measurement. We gratefully acknowledge
the support for histology and immunohistochemistry from the Center for Genomic Pathology at University of California Davis. Figures were generated with BioRender.com. AUTHOR INFORMATION
Author notes * These authors contributed equally: EunJung Lee, Suyakarn Archasappawat, Keely Ji. AUTHORS AND AFFILIATIONS * Department of Microbiology and Molecular Genetics, University of
California, Davis, Davis, CA, 95616, USA EunJung Lee, Suyakarn Archasappawat, Keely Ji, Martin Jean Kim, Qi Tian, Priya S. Shah & Chang-Il Hwang * Graduate Group in Integrative
Pathobiology, University of California, Davis, Davis, CA, 95616, USA Suyakarn Archasappawat * The Herbert Wertheim UF Scripps Institute, High-Throughput Screening Center, Department of
Molecular Medicine, Scripps Florida, Jupiter, FL, 33458, USA Jocelyn Pena, Virneliz Fernandez-Vega, Louis Scampavia & Timothy P. Spicer * Department of Chemical Engineering, College of
Engineering, University of California, Davis, Davis, CA, 95616, USA Ritika Gangaraju, Nitin Sai Beesabathuni & Priya S. Shah * University of California Davis Comprehensive Cancer Center,
Sacramento, CA, 95817, USA Chang-Il Hwang Authors * EunJung Lee View author publications You can also search for this author inPubMed Google Scholar * Suyakarn Archasappawat View author
publications You can also search for this author inPubMed Google Scholar * Keely Ji View author publications You can also search for this author inPubMed Google Scholar * Jocelyn Pena View
author publications You can also search for this author inPubMed Google Scholar * Virneliz Fernandez-Vega View author publications You can also search for this author inPubMed Google Scholar
* Ritika Gangaraju View author publications You can also search for this author inPubMed Google Scholar * Nitin Sai Beesabathuni View author publications You can also search for this author
inPubMed Google Scholar * Martin Jean Kim View author publications You can also search for this author inPubMed Google Scholar * Qi Tian View author publications You can also search for
this author inPubMed Google Scholar * Priya S. Shah View author publications You can also search for this author inPubMed Google Scholar * Louis Scampavia View author publications You can
also search for this author inPubMed Google Scholar * Timothy P. Spicer View author publications You can also search for this author inPubMed Google Scholar * Chang-Il Hwang View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization, EL, C-IH; Writing – Original Draft, EL, SA, KJ, C-IH; Writing – Review &
Editing, EL, SA, KJ, C-IH; Investigation, EL, SA, KJ, JP, VF, RG, NSB, MJK, QT; Resources, PSS, LS, TPS; Funding Acquisition, C-IH; Supervision, C-IH. CORRESPONDING AUTHOR Correspondence to
Chang-Il Hwang. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. Edited by Gian Maria Fimia SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION SUPPLEMENTAL FIGURES ORIGINAL DATA
FILES SUPPLEMENTARY FILE 1 SUPPLEMENTARY FILE 2 SUPPLEMENTARY FILE 3 SUPPLEMENTARY FILE 4 REPRODUCIBILITY CHECKLIST RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lee, E., Archasappawat, S., Ji, K. _et al._ A new vulnerability to
BET inhibition due to enhanced autophagy in BRCA2 deficient pancreatic cancer. _Cell Death Dis_ 14, 620 (2023). https://doi.org/10.1038/s41419-023-06145-9 Download citation * Received: 30
May 2023 * Revised: 06 September 2023 * Accepted: 12 September 2023 * Published: 21 September 2023 * DOI: https://doi.org/10.1038/s41419-023-06145-9 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative