Cbl-b deficiency provides protection against uvb-induced skin damage by modulating inflammatory gene signature
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Exposure of skin to ultraviolet (UV) radiation induces DNA damage, inflammation, and immune suppression that ultimately lead to skin cancer. However, some of the pathways that
regulate these events are poorly understood. We exposed mice to UVB to study its early effects in the absence of Cbl-b, a known suppressor of antitumor immune response in the skin. Cbl-b−/−
mice were protected from UV-induced cell damage as shown by the lower number of cyclobutane pyrimidine dimers and sunburn cells in exposed skin compared to wild-type mice. Microarray data
revealed that deficiency of Cbl-b resulted in differential expression of genes involved in apoptosis evasion, tumor suppression and cell survival in UV-exposed skin. After UVB, Cbl-b−/− mice
upregulated gene expression pattern associated with regulation of epidermal cell proliferation linked to Wnt signaling mediators and enzymes that relate to cell removal and tissue
remodeling like MMP12. Additionally, the skin of Cbl-b−/− mice was protected from chronic inflammatory responses and epidermal hyperplasia in a 4-weeks UVB treatment protocol. Overall, our
results suggest a novel role for Cbl-b in regulating inflammation and physiologic clearance of damaged cells in response to UVB by modulating inflammatory gene signature. SIMILAR CONTENT
BEING VIEWED BY OTHERS GALECTIN-7 REPROGRAMS SKIN CARCINOGENESIS BY FOSTERING INNATE IMMUNE EVASIVE PROGRAMS Article Open access 24 January 2023 OTULIN MAINTAINS SKIN HOMEOSTASIS BY
CONTROLLING KERATINOCYTE DEATH AND STEM CELL IDENTITY Article Open access 08 October 2021 MACROPHAGE MIGRATION INHIBITORY FACTOR (MIF) AND ITS HOMOLOG D-DOPACHROME TAUTOMERASE (D-DT) ARE
SIGNIFICANT PROMOTORS OF UVB- BUT NOT CHEMICALLY INDUCED NON-MELANOMA SKIN CANCER Article Open access 18 July 2023 INTRODUCTION Chronic exposure to ultraviolet (UV) irradiation is the
leading cause of skin cancer including melanoma, squamous and basal cell carcinoma1,2. By the impingement of UV radiation, skin develops DNA photoproducts (PPs) such as cyclobutane
pyrimidine dimers (CPDs) and 6–4PPs3. If such products are not eliminated by the repair mechanisms of the skin, mutations and subsequently cancers may arise2. The mechanisms that protect
skin from the potential consequences of UV-induced DNA lesions involve active DNA repair by nucleotide excision, base excision and mismatch repair or as last resort induction of apoptosis
and removal of cells with damages in their genome3. Additionally, genes involved in modulation of innate and adaptive immune responses including Cbl-b, may also play an important role in the
immunomodulatory and carcinogenic effects of UVB exposure3,4,5. Cbl-b is a member of the mammalian Cbl family of proteins, a group of E3 ubiquitin ligases that consist of c-Cbl, Cbl-b, and
Cbl-3. Cbl-b is a negative regulator of T-cell receptor signaling and its deficiency leads to spontaneous autoimmunity6,7. Moreover, Cbl-b is a modulator of many biological processes such as
the induction of immune tolerance and antitumor immunity8. In addition, it has been demonstrated that Cbl-b-deficient mice developed fewer UVB-induced skin malignancies by spontaneously
rejecting tumor cells in a CD8+ T-cell and NK-cell dependent manner4,6,7. Nevertheless, the role of Cbl-b in the early events that follow UVB exposure (relevant for the subsequent
carcinogenic effect of sunlight) has not been evaluated. In the present work, we irradiated Cbl-b−/− and wild-type (WT) mice with UVB to study the early responses of exposed skin in the
absence of this ubiquitin ligase. RESULTS CBL-B-/- MICE CARRY FEWER UVB-INDUCED SBCS AND DNA PPS Cbl-b participates in the rejection of UVB-induced tumor cells by enhancing cytotoxic immune
responses mediated by tumor specific CD8+ cells9. We hypothesized that upon UVB irradiation, Cbl-b is highly upregulated and participates in the immunomodulatory effects of UVB in exposed
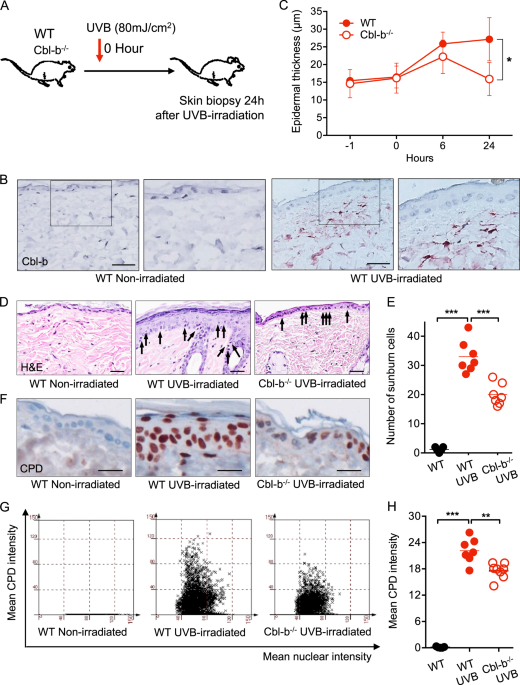
skin. To evaluate the expression of Cbl-b in response to UVB, WT mice were irradiated with 80 mJ/cm2 of UVB (Fig. 1a). Samples of dorsal skin taken 24 h after UV exposure showed that cells
mainly from the superficial dermis expressed cytoplasmic Cbl-b (Fig. 1b). Moreover, human skin irradiated with twice the minimal erythema dose of UVB also had Cbl-b+ cells infiltrating the
superficial layer of the dermis (Suppl. Fig. 1). One of the early consequences of toxic UV exposure is the formation of SBCs. A SBC is a damaged epidermal cell undergoing apoptosis
characterized by a pyknotic nucleus and condensed cytoplasm10,11. To investigate the role of Cbl-b in acute UVB-induced cytotoxicity, WT and Cbl-b−/− UVB-irradiated mice were sacrificed at
different time points to analyse epidermal thickness, SBCs, and CPDs. Cbl-b−/− mice showed less epidermal thickening during the first 24 h after UVB exposure (Fig. 1c). We observed that 6 h
after UVB irradiation, both WT and Cbl-b−/− mice had a similar number of SBCs (Suppl. Fig. 2C). However, after 24 h, the skin of Cbl-b−/− mice developed fewer SBCs compared to WT mice (i.e.,
20 vs. 33 SBCs per microscopic field) (Fig. 1d, e). To evaluate the degree of DNA damage in UVB-irradiated skin, we carried out staining of thymine dimers, a specific type of CPD.
Twenty-four hour after UVB irradiation; the skin of Cbl-b−/− mice had significantly fewer CPDs compared to the skin of WT mice (Fig. 1f–h). To rule out differences of skin pigmentation
between Cbl-b−/− and WT mice (that may influence the number of SBCs and CPD in tissue) prior UVB exposure, we quantified pigmentation by noninvasive skin reflectance spectroscopy and the
levels of melanin by Fontana-Masson staining and there was no influence of Cbl-b deficiency on skin pigmentation (Suppl. Fig. 2A, B). Together, these findings indicate that Cbl-b is an
element of response to UVB expressed in cells of the dermis of mice and humans. Cbl-b plays a role in physiologic clearance of SBCs and its deficiency may provide protection from UVB
exposure in a nonpigment-related manner. CBL-B DEFICIENCY INDUCES EXPRESSION OF EPIDERMAL GROWTH-RELATED GENES AND WNT SIGNALING IN RESPONSE TO UVB IRRADIATION To gain insight of the gene
expression profile that may be involved in protection against DNA damage induced by UVB irradiation in Cbl-b−/− mice, we isolated RNA from skin samples taken before and 24 h after UVB
irradiation of Cbl-b−/− and WT mice to carry out microarray analyses. We established a cut off threshold of _p_ value under 0.05 and a fold change level of ±1.5 to look for significant
changes in gene expression. By doing so, we found 94 genes differentially regulated in Cbl-b−/− vs. WT mice after UVB exposure (Fig. 2a). There was an overexpression of several genes
involved in negative regulation of apoptosis (Wisp2, Fmod, Nceh1, Cygb, and Nnt)12,13,14,15,16, tumor suppression (Nr4a1, Sox15, and Msap1)17,18, oxidative-stress response (Atp10d, Cxcl11,
Nnt, and Cygb)12,16,19, tissue remodeling (Mmp12)20, and cell survival (Tlr6, Nr1d1, Tpsb2, IL-22r1, Nr4a1, Prox1, and Fam198a)21,22,23 in UVB-exposed Cbl-b−/− compared to WT mice. Many
tumor-related (Tiam2, Tspan6, Hmgn5, Osr1, Pdcd4, Cntfr, and Vaultrc5)24,25 and inflammation-related (Saa3 and Defb8)26 genes were downregulated. A gene interaction network showed that
within the upregulated genes after UVB irradiation, a cluster of five genes (Mmp12, Nr4a1, Prox1, Flt4, and F3) was directly associated to regulation of epithelial cell proliferation (Fig.
2b). To understand whether the differentially expressed genes found in Cbl-b−/− mice resulted from a direct response to UVB irradiation or a background alteration of Cbl-b deficiency, we
carried out microarray analyses of the skin from nonirradiated Cbl-b−/− and WT control mice (Fig. 2c). We observed that Cbl-b deficiency resulted in nine genes with differential expression
as background, we plotted together the fold change values of expression from these genes comparing Cbl-b−/− vs. WT mice of nonirradiated and UVB-irradiated animals (Fig. 2d). Three of these
nine genes (Saa3, Defb8, and Nrd1) showed at least a twofold difference of expression after UVB irradiation between irradiated and nonirradiated animals, whereas the other six genes had
minor fold change variations. Saa3 flipped from a 2.57-fold positive ratio to a 4.45-fold negative ratio; Nr1d1 flipped from a 2.97-fold negative to a positive 1.66-fold ratio; Defb8
decreased from a 2.14-fold negative ratio to a 5.38-fold negative ratio comparing nonirradiated vs. UVB-irradiated mice. We did not observe alterations in the expression of genes that are
directly involved in DNA repair processes such as NER or BER between Cbl-b−/− vs. WT mice after UVB irradiation, however, immunohistochemical stainings revealed that Cbl-b−/− mice had higher
expression of IL-10 24 h after exposure suggesting an increased immunomodulatory effect of UVB in these mice (Suppl. Fig. 3). Our data suggest that in response to UVB, cellular pathways
involved in cell proliferation, activation, inflammation, invasion, and migration were greatly affected by the lack of Cbl-b (Suppl. Table 1). Among upregulated genes we found several
members of the Wnt signaling pathway like Sox15, Wisp2, Nr4a1, Prox1, and Mmp1227,28,29. Therefore, we stained UVB-irradiated skin for β-catenin and MMP12 to evaluate Wnt activation and
found a high number of β-catenin+ and MMP12+ cells in dermis and epidermis of Cbl-b−/− compared to WT mice (Fig. 3a, b), suggesting an activation of Wnt signaling and a possible
participation in tissue remodeling and removal of SBCs. Altogether, these results indicate that Cbl-b−/− mice have differential transcriptional profile in UVB-irradiated skin that includes
some genes from the background of Cbl-b deficiency, playing a role in regulation of cell proliferation and tumor/inflammation inhibition. Moreover, β-catenin activation suggests that Wnt
signaling may crosstalk with Cbl-b as previous findings have suggested30. CBL-B−/− MICE SHOW LOWER DEGREE OF UVB-INDUCED INFLAMMATION AND EPIDERMAL HYPERPLASIA Chronic inflammation is known
to be a promotion factor in photocarcinogenesis. We tested whether deficiency of Cbl-b protects the skin from chronic inflammation. The dorsal skin of WT and Cbl-b−/− mice was exposed to 6
doses of 80 mJ/cm2 and subsequent 6 doses of 220 mJ/cm2 during 23 days as described in Fig. 4a. Macroscopic double skin fold thickness (DSFT) and microscopic epidermal thickness were
measured over time as readout of inflammatory response. Cbl-b−/−, but not WT mice were protected from chronic inflammation evidenced by a lower DSFT from the first week throughout the entire
period of the UVB irradiation protocol (Fig. 4b). Epidermal thickening was reduced in UVB-irradiated Cbl-b−/− mice compared to WT by the end of the UVB exposure protocol (Fig. 4c).
UVB-induced immunosuppression is crucial in the promotion of skin cancer. CD4+CD25+Foxp3+ regulatory T cells (Tregs) secreting IL-10 play an important role in the effect of UVB and
participate in immune tolerance to UV-generated malignant cells10. After repetitive exposure to UVB we observed no significant changes in Tregs in the skin-draining lymph nodes of Cbl-b−/−
compared to WT mice (not shown). However, the expression of IL-10 was significantly reduced in the skin of Cbl-b−/− mice (Fig. 4d). These results suggest that Cbl-b participates in
sustaining chronic inflammation without interfering in the induction and/or recruitment of Tregs. Nonetheless, regulatory cytokines like IL-10 could be produced under Cbl-b regulation, thus,
a lack of Cbl-b expression may abolish some of the immunomodulatory effects of UVB. DISCUSSION The regulatory function of Cbl-b has been explored in lymphocyte activation, autoimmunity and
carcinogenesis. UVB-induced tumors are rejected in Cbl-b−/− mice by an efficient activation of tumor specific cytotoxic CD8+ T cells6. In this study we addressed the immediate response to
UVB of Cbl-b−/− mice to understand the early events that may lead to the improved tumor immunity previously observed in these mice6. Irradiation of human skin showed infiltration of Cbl-b+
cells (Suppl. Fig. 1)suggesting that this ubiquitin ligase may have a relevant role in the effects of UVB such as carcinogenesis associated to excessive sun exposure. Cbl-b+ cells
infiltrated the skin of WT mice after UVB exposure (Fig. 1b), possibly indirectly affecting removal of SBCs and CPDs in epidermis. In fact, the response to UVB-induced DNA damage can be
modulated by inflammatory cytokines such as IL-10 and IL-1831,32. IL-10 staining 24 h after UVB exposure (Suppl. Fig. 3) demonstrated that Cbl-b deficiency resulted in a higher expression of
this cytokine but after chronic exposure for up to 4 weeks and 12 sessions, qPCR results (Fig. 4d) showed a drastic drop in the expression of IL-10 in Cbl-b−/− mice. Controversially,
microarray results (Fig. 2a) and staining of XPC (not shown) did not indicate alterations in DNA repair machinery in Cbl-b−/− mice although previous investigations have associated
UVB-induced DNA damage with high levels of IL-1033. Interestingly, Cbl-b deficient effector T cells are resistant from Treg/IL-10 mediated suppression. Nevertheless, despite the
immunosuppressive environment of the skin, Cbl-b deficiency promoted the clearance of SBCs. After exposure to UVB, Cbl-b−/− mice showed a reduced epidermal thickening compared to WT mice,
suggesting a lower degree of overall cell injury (Fig. 1c). The quantification of CPDs and SBCs provides an accurate assessment of the damage in irradiated tissue and may predict the risk of
tumor development. Indeed, SBCs are considered a surrogate marker of UV-induced DNA damage to the skin34. By using two different protocols of UVB exposure we studied the effects of Cbl-b
deficiency on SBCs and CPDs formation and inflammation. We found that 24 h after exposure to a single dose of UVB, the skin of Cbl-b−/− mice developed fewer SBCs and DNA dimers (Fig. 1c–h).
The peak of UVB acute effects are regularly observed between 6 and 24 h, depending on UVB wavelength and dose35,36. SBC are visible as early as 30 min after irradiation and reach their
highest in numbers within 24 h37, offering with the first 24 h after exposure a well delimited time-span to study immediate effects of UVB. The relevance of these early events post UVB
irradiation are highlighted by studies in which the application of topical DNA repair enzymes reverts suppression in induction of contact hypersensitivity and protects from Langerhans cell
depletion in UV-irradiated mice38. Moreover, DNA repair-deficient XP39 and IL-12p40−/− mice exhibited an increased number of SBCs compared to WT after UVB irradiation34,40. Our results
indicate that DNA PPs and SBCs are linked to Cbl-b expression, suggesting that Cbl-b may be involved directly or indirectly in controlling DNA repair mechanisms. Alternatively, the lack of
Cbl-b may result in an enhanced removal of cells carrying DNA damage by an efficient cytotoxic activity of CD8+ and NK cells as demonstrated by previous results6,7. Moreover, we found no
indication of a gains or losses in the mechanisms that cope with detoxification of ROS and DNA damage in Cbl-b−/− mice since staining of 8-hydroxyguanosine, XPC and 6,4-PPs did not differ
between WT vs. Cbl-b−/− mice (not shown). Having said so, it is known that Cbl-b carries a UBA domain shared with the DNA repair protein Rad23, however, the functional role of the Cbl-b UBA
domain is not known41. The transcriptional profile of WT compared to Cbl-b−/− mice after UVB irradiation showed 85 differentially regulated genes in addition to 9 genes with background
differential expression in nonirradiated mice. Ubiquitin ligases like Cbl-b are known negative regulators of tyrosine kinase signaling. Their regulatory effect in cells of the immune system
has been largely described42 and more recently, Cbl-b has been implicated in the maintenance of mammary stem cell phenotype involving a negative regulation of the AKT-mTOR pathway43. Our
results suggest that Cbl-b deficiency may affect the transcriptional profile by shifting the activation of Wnt and tyrosine kinase signaling. The genes affected by UVB irradiation play a
role in regulation of cellular processes like cell cycle, cell adhesion, invasion, and migration. It is known that Wnt signaling orchestrates DNA damage response through rescue of irradiated
cells from apoptosis in a process regulated by c-Cbl targeting β-catenin44,45, a similar mechanism could also operate with Cbl-b. This observation can be supported by the presence of high
number of β-catenin positive cells in UVB-irradiated Cbl-b−/− mice (Fig. 3a). Although, β-catenin was upregulated after UVB irradiation together with other epidermal cell proliferation
regulators, skin thickness of Cbl-b−/− mice was lower compared to control WT mice after chronic exposure to UVB (Fig. 3b), suggesting that Cbl-b may limit the mechanisms that ameliorate UV
damage to exposed cells and participate in the early events of phototoxicity and subsequent carcinogenesis. Moreover, metalloproteinases are enzymes that mediate photoaging and tissue
remodeling46. Some metalloproteinases like MMP13 are negatively regulated by Cbl-b47. UVB-irradiated Cbl-b−/− mice had an increase of MMP12 expression in cells of the epidermis (Fig. 3b),
suggesting that upon UVB these mice may have a higher rate of tissue remodeling and elimination of SBC. Excessive exposure to UVB is a known promotion factor of carcinogenesis. Our data
indicate that Cbl-b deficiency protects from some of the effects of repetitive exposure to UVB as measured by lower cellular skin infiltration, swelling, and epidermal hyperplasia (Fig. 4b,
c). Despite the low dose of UVB and the short period of irradiation used in our experiments (in comparison with studies of UVB tumor models48), IL-10 (a known mediator of UVB-induced
immunosuppression2,5,8,10) was initially upregulated 24 h after a single exposure to UVB. Chronic exposure to UVB led to an increased expression of IL-10 in skin of WT mice, however,
Cbl-b−/− mice failed to upregulate this cytokine and showed lower levels of IL-10 (Fig. 4d). Animal models of photocarcinogenesis have demonstrated that regulatory T cells mediate a large
proportion of the immunosuppressive effects of UVB with a peaks of infiltration in irradiated skin 7 days after initial exposure and subsequent normalization49. Nonetheless, our study was
consistent with previous findings of unchanged Treg numbers in the skin after exposure of Cbl-b−/− mice to UVB and a possible higher cytotoxic activity of CD8+ T cells by a decreased
sensitivity to Treg-mediated immunosuppression6. Whether the origin of the observed protective Cbl-b−/− phenotype is due to the decreased DNA damage in the epidermis, an autocrine effect or
via interaction with the altered immune system in these mice remains to be determined. In summary, UVB irradiation in Cbl-b deficient mice leads to a lower number of DNA PPs and SBCs, higher
expression of Wnt signaling mediators like β-catenin and a differential gene expression signature enriched with cell proliferation regulators compared to WT control mice. This suggests that
Cbl-b is involved in the immediate response to UVB cytotoxicity. Thus, if cells can indeed better tolerate UVB and survive in the absence of Cbl-b (without harboring internal damage), then
our findings may open new avenues to target this gene in novel sun protection strategies. MATERIALS AND METHODS ANIMALS BL6 WT and Cbl-b−/− mice were obtained from the Institute of Molecular
Biotechnology (IMBA) Vienna, Austria and held in our facility at the Medical University of Graz. All animals were maintained with alternating 12 h light and dark cycles, as well as
controlled temperature and humidity in our facilities. Mice were shaved on the dorsal skin 1 day prior experimental procedures. All animal procedures were approved by the Federal Ministry of
Science and Research, Austrian Government through protocol no. BMWF-66.010/0019-II/3b/2011. All methods were performed in accordance with the relevant guidelines and regulations. UVB
IRRADIATION OF ANIMALS Mice were exposed to a single dose of 80 mJ/cm2 of UVB. A Waldmann UV236B irradiation system equipped with two fluorescent CF-L 36 W/UV6 light tubes (emission range,
280–360 nm; peak, 324 nm; Waldmann Medizintechnik, Villingen-Schwenningen, Germany) was used for UVB irradiation, at a mean irradiance of 2.20 mW/cm2 at a distance of 15 cm and the
irradiance of exposure was monitored by a calibrated Waldmann photometer. The applied UVB dose corresponded to approximately one minimal erythema dose, as determined by exposure to a series
of UVB doses at the increments by factor of 1.4, as previously described50. To study the chronic effects, mice were exposed to repetitive UVB doses on alternate days for 23 days at a dose of
80 mJ/cm2 for 11 days and then at a dose of 220 mJ/cm2 from day 12 to 23 by using the same irradiation system. Skin inflammation was monitored by measuring the DSFT of dorsal skin with an
engineer’s micrometer (Mitutoyo Corporation) and mice were killed 6 or 24 h after last UVB exposure for tissue collection. Noninvasive skin pigmentation was determined by skin reflectance
spectroscopy on shaved dorsal skin with a DermaSpectometer (Cortex Technologies, DK). HUMANS SKIN SAMPLES Paraffin-embedded materials were available for immunohistochemical stainings from a
previous clinical study. In accordance with the study protocol (IRB approval number: 15–129 ex 93/94) healthy volunteers had been irradiated on their buttocks with solar simulated UV
radiation equivalent to two times of the minimal erythema dose and skin biopsies had been taken 24 h after exposure. HISTOLOGICAL EVALUATION Epidermal hyperplasia was assessed on
H&E-stained sections of dorsal skin by measuring the histologic thickness of the epidermis. SBCs were counted in the interfollicular epidermis of H&E-stained dorsal skin sections in
at least 10 random fields (at a final magnification 20×). All measurements were performed in a blinded manner. Photographic images were acquired by using a DP71 digital camera (Olympus)
attached to an Olympus BX51 microscope. IMMUNOHISTOCHEMICAL AND IMAGE ANALYSIS Skin sections of mice were pretreated with EDTA at pH 8 and then incubated with antithymine dimer (clone KTM53;
Kamiya Biomedical Co, Seattle, WA) (1:2000), anti-MMP12 (clone EP1261Y; Abcam ab52897) anti-IL-10 (clone JES5-2A5; Abcam ab189392) or anti-β-catenin monoclonal antibody (clone E247; Abcam,
Oxford UK). Sections of human and mice skin were stained with anti Cbl-b (clone 246C5a; Abcam ab54362). The Dako K 5003 detection system (Dako, Glostrup, Denmark) was used for visualization
according to the manufacturer’s instructions. Melanin determination was performed by standard Fontana-Masson staining (Ab1506669, Abcam, Oxford, UK) according to manufacturer instructions.
To quantify CPDs, antibody-stained tissue sections were scanned on TissueFAXS system by TissueFAXS cell tissue analysis software (TissueGnostics GmbH, Vienna, Austria) and the epidermis was
electronically dissected with ImageJ software for intensity measurement by TissueQuest software (TissueGnostics GmbH, Vienna, Austria). QUANTITATIVE RT-PCR RNA was extracted from dorsal skin
tissue using QIAGEN fibrous mini kit (QIAGEN) and cDNA was made by using First strand cDNA synthesis kit (Roche). Quantitative RT-PCR for IL-10 was performed on an Applied Biosystems 7900HT
system by using RT. SYBR Green/ROX qPCR Master Mix (SABiosciences). The 2-ΔCt method was used to normalize the transcript to GAPDH. MICROARRAY ANALYSIS The Affymetrix Gene Chip Microarray
platform was used for identifying genes that are transcriptionally influenced. We processed three biological replicates of dorsal skin RNA samples collected from WT and Cbl-b−/− mice before
and 24 h after exposure to a single dose of 80 mJ/cm2 UVB on the Affymetrix Mouse Gene 1.0 ST Array (Affymetrix, Santa Clara, CA, USA) to determine the transcriptional profile. Total RNA was
extracted as described above and checked for quality on the BioAnalyzer BA2100 (Agilent, Foster City, CA). For amplification, 400 ng of the total RNA was used with the Ambion Whole
Transcript Expression Kit for Affymetrix GeneChip, Whole Transcript Expression Arrays (Life Technologies; Carlsbad, California). The first strand cDNA was created according to the
manufacturer protocol, which then synthesized the second strand cDNA. During the following in vitro transcription, complementary RNA (cRNA) was generated. After purification, a second cycle
of first strand cDNA synthesis was performed implementing dUTPs for fragmentation. RNaseH hydrolyzed the cRNA, followed by an enzymatic fragmentation and biotin-labeling (Affymetrix GeneChip
Whole Transcript Terminal Labeling and Hybridization for use with Ambion Whole Transcript; Affymetrix, Santa Clara, CA, USA). We hybridized the fragmented samples overnight rotating the
arrays at 60 rpm. After washing at the Affymetrix Genechip fluidics station 450, protocol FS450_0007 (Affymetrix GeneChip® HT hybridization, Wash, and Stain Kit; Affymetrix, Santa Clara, CA,
USA) the arrays were scanned with the Affymetrix Scanner GCS3000, AGCC (Command Console software AGCC 3.1.1) with default analysis settings for generation of CEL-files. The arrays were
evaluated based on the internal array controls using Affymetrix Genexpression Console (1.1.2). Gene interaction analysis was done using the GeneMANIA webserver51. CEL-files were imported
into Partek Genomic Suite v6.6 software (Partek Inc., St Louis, MO) for performing robust multichip average normalization including background correction, quantile normalization across all
arrays, and median polished summarization based on log transformed expression values. A fold change of ±1.5 in gene expression was considered as differentially relevant. To gain insight into
functional processes we used Ingenuity Pathway Analysis (IPA; Qiagen, Redwood City, CA). Differentially expressed genes in Cbl-b−/− vs. WT mice before and after UVB irradiation were
compared and plotted in a waterfall graph. Raw data is available on the Gene Expression Omnibus (GEO) database with the dataset number GSE79073. STATISTICAL ANALYSIS Data were expressed as
mean ± SEM. Statistical differences among experimental groups were determined by using two-tailed unpaired _t_ test or one-way ANOVA (for microarray analysis). Statistical significance was
set at _P_ < 0.05. REFERENCES * Ramos, J., Villa, J., Ruiz, A., Armstrong, R. & Matta, J. UV dose determines key characteristics of nonmelanoma skin cancer. _Cancer Epidemiol.
Biomark. Prev._ 13, 2006–2011 (2004). CAS Google Scholar * Lo, J. A. & Fisher, D. E. The melanoma revolution: from UV carcinogenesis to a new era in therapeutics. _Science_ 346,
945–949 (2014). Article PubMed PubMed Central CAS Google Scholar * Matsumura, Y. & Ananthaswamy, H. N. Toxic effects of ultraviolet radiation on the skin. _Toxicol. Appl.
Pharmacol._ 195, 298–308 (2004). Article PubMed CAS Google Scholar * Chiang, J. Y., Jang, I. K., Hodes, R. & Gu, H. Ablation of Cbl-b provides protection against transplanted and
spontaneous tumors. _J. Clin. Invest._ 117, 1029–1036 (2007). Article PubMed PubMed Central CAS Google Scholar * Loser, K. et al. Epidermal RANKL controls regulatory T-cell numbers via
activation of dendritic cells. _Nat. Med._ 12, 1372–1379 (2006). Article PubMed CAS Google Scholar * Loeser, S. et al. Spontaneous tumor rejection by cbl-b-deficient CD8+ T cells. _J.
Exp. Med._ 204, 879–891 (2007). Article PubMed PubMed Central CAS Google Scholar * Paolino, M. et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer
cells. _Nature_ 507, 508–512 (2014). Article PubMed CAS PubMed Central Google Scholar * Lutz-Nicoladoni, C., Wolf, D. & Sopper, S. Modulation of immune cell functions by the E3
ligase Cbl-b. _Front. Oncol._ 5, 58 (2015). Article PubMed PubMed Central Google Scholar * Loeser, S. & Penninger, J. M. The ubiquitin E3 ligase Cbl-b in T cells tolerance and tumor
immunity. _Cell Cycle_ 6, 2478–2485 (2007). Article PubMed CAS Google Scholar * Beissert, S. & Schwarz, T. Ultraviolet-induced immunosuppression: implications for
photocarcinogenesis. _Cancer Treat. Res._ 146, 109–121 (2009). Article PubMed CAS Google Scholar * Seite, S., Fourtanier, A., Moyal, D. & Young, A. R. Photodamage to human skin by
suberythemal exposure to solar ultraviolet radiation can be attenuated by sunscreens: a review. _Br. J. Dermatol._ 163, 903–914 (2010). Article PubMed CAS Google Scholar * Meimaridou, E.
et al. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. _Nat. Genet._ 44, 740–742 (2012). Article PubMed PubMed Central CAS
Google Scholar * Ji, J. et al. WISP-2 in human gastric cancer and its potential metastatic suppressor role in gastric cancer cells mediated by JNK and PLC-gamma pathways. _Br. J. Cancer_
113, 921–933 (2015). Article PubMed PubMed Central CAS Google Scholar * Choudhury, A. et al. Silencing of ROR1 and FMOD with siRNA results in apoptosis of CLL cells. _Br. J. Haematol._
151, 327–335 (2010). Article PubMed CAS Google Scholar * Sekiya, M. et al. Absence of Nceh1 augments 25-hydroxycholesterol-induced ER stress and apoptosis in macrophages. _J. Lipid Res._
55, 2082–2092 (2014). Article PubMed PubMed Central CAS Google Scholar * Latina, A. et al. DeltaNp63 targets cytoglobin to inhibit oxidative stress-induced apoptosis in keratinocytes
and lung cancer. _Oncogene_ 35, 1493–1503 (2015). Article PubMed CAS Google Scholar * Thu, K. L. et al. SOX15 and other SOX family members are important mediators of tumorigenesis in
multiple cancer types. _Oncoscience_ 1, 326–335 (2014). Article PubMed PubMed Central Google Scholar * Lin, B. et al. Conversion of Bcl-2 from protector to killer by interaction with
nuclear orphan receptor Nur77/TR3. _Cell_ 116, 527–540 (2004). Article PubMed CAS Google Scholar * Barker, C. E., Ali, S., O’Boyle, G. & Kirby, J. A. Transplantation and
inflammation: implications for the modification of chemokine function. _Immunology_ 143, 138–145 (2014). Article PubMed PubMed Central CAS Google Scholar * Tewari, A., Grys, K., Kollet,
J., Sarkany, R. & Young, A. R. Upregulation of MMP12 and its activity by UVA1 in human skin: potential implications for photoaging. _J. Invest. Dermatol._ 134, 2598–2609 (2014). Article
PubMed CAS Google Scholar * Kourtidis, A. et al. An RNA interference screen identifies metabolic regulators NR1D1 and PBP as novel survival factors for breast cancer cells with the
ERBB2 signature. _Cancer Res._ 70, 1783–1792 (2010). Article PubMed PubMed Central CAS Google Scholar * Suzuki, S. et al. Nur77 as a survival factor in tumor necrosis factor signaling.
_Proc. Natl Acad. Sci._ 100, 8276–8280 (2003). Article PubMed CAS PubMed Central Google Scholar * Ragusa, S. et al. PROX1 promotes metabolic adaptation and fuels outgrowth of Wnt(high)
metastatic colon cancer cells. _Cell Rep._ 8, 1957–1973 (2014). Article PubMed CAS Google Scholar * Chen, J. S., Su, I. J., Leu, Y. W., Young, K. C. & Sun, H. S. Expression of T-cell
lymphoma invasion and metastasis 2 (TIAM2) promotes proliferation and invasion of liver cancer. _Int. J. Cancer_ 130, 1302–1313 (2012). Article PubMed CAS Google Scholar * Kumar, N.,
Wethkamp, N., Waters, L. C., Carr, M. D. & Klempnauer, K. H. Tumor suppressor protein Pdcd4 interacts with Daxx and modulates the stability of Daxx and the Hipk2-dependent
phosphorylation of p53 at serine 46. _Oncogenesis_ 2, e37 (2013). Article PubMed PubMed Central CAS Google Scholar * Hansen, M. T. et al. A link between inflammation and metastasis:
serum amyloid A1 and A3 induce metastasis, and are targets of metastasis-inducing S100A4. _Oncogene_ 34, 424–435 (2015). Article PubMed CAS Google Scholar * Thu, K. L. et al. SOX15 is a
candidate tumor suppressor in pancreatic cancer with a potential role in Wnt/beta-catenin signaling. _Oncogene_ 33, 279–288 (2014). Article PubMed CAS Google Scholar * Hashimoto Y.
Effect of Wnt signaling protein (Wisp2/CCN5) on angiogenesis and invasion in prostate cancer. _J. Clin. Oncol._ 30 (2012) (suppl 5, abstract 227). * Chtarbova, S. et al. Murine Nr4a1 and
Herpud1 are upregulated by Wnt-1, but the homologous human genes are independent from beta-catenin activation. _Biochem. J._ 367, 723–728 (2002). Pt 3. Article PubMed PubMed Central CAS
Google Scholar * Ding, Y., Shen, S., Lino, A. C., Curotto de Lafaille, M. A. & Lafaille, J. J. Beta-catenin stabilization extends regulatory T-cell survival and induces anergy in
nonregulatory T cells. _Nat. Med._ 14, 162–169 (2008). Article PubMed CAS Google Scholar * Schwarz, T. & Schwarz, A. DNA repair and cytokine responses. _J. Invest. Dermatol._ 14,
63–66 (2009). Article CAS Google Scholar * Schwarz, A., Maeda, A., Stander, S., van Steeg, H. & Schwarz, T. IL-18 reduces ultraviolet radiation-induced DNA damage and thereby affects
photoimmunosuppression. _J. Immunol._ 176, 2896–2901 (2006). Article PubMed CAS Google Scholar * Nishigori, C. et al. Evidence that DNA damage triggers interleukin 10 cytokine production
in UV-irradiated murine keratinocytes. _Proc. Natl Acad. Sci._ 93, 10354–10359 (1996). Article PubMed CAS PubMed Central Google Scholar * Maeda, A. et al. Enhanced photocarcinogenesis
in interleukin-12-deficient mice. _Cancer Res._ 66, 2962–2969 (2006). Article PubMed CAS Google Scholar * Soter, N. A. Acute effects of ultraviolet radiation on the skin. _Semin.
Dermatol._ 9, 11–15 (1990). PubMed CAS Google Scholar * Woodcock, A. & Magnus, I. A. The sunburn cell in mouse skin: preliminary quantitative studies on its production. _Br. J.
Dermatol._ 95, 459–468 (1976). Article PubMed CAS Google Scholar * Bayerl, C., Taake, S., Moll, I. & Jung, E. G. Characterization of sunburn cells after exposure to ultraviolet
light. _Photodermatol. Photoimmunol. Photomed._ 11, 149–154 (1995). Article PubMed CAS Google Scholar * Wolf, P., Cox, P., Yarosh, D. B. & Kripke, M. L. Sunscreens and T4N5 liposomes
differ in their ability to protect against ultraviolet-induced sunburn cell formation, alterations of dendritic epidermal cells, and local suppression of contact hypersensitivity. _J.
Invest. Dermatol._ 104, 287–292 (1995). Article PubMed CAS Google Scholar * van Oosten, M. et al. Differential role of transcription-coupled repair in UVB-induced G2 arrest and apoptosis
in mouse epidermis. _Proc. Natl Acad. Sci._ 97, 11268–11273 (2000). Article PubMed PubMed Central Google Scholar * Schwarz, A. et al. Interleukin-12 suppresses ultraviolet
radiation-induced apoptosis by inducing DNA repair. _Nat. Cell Biol._ 4, 26–31 (2002). Article PubMed CAS Google Scholar * Davies, G. C. et al. Cbl-b interacts with ubiquitinated
proteins; differential functions of the UBA domains of c-Cbl and Cbl-b. _Oncogene_ 23, 7104–7115 (2004). Article PubMed CAS Google Scholar * Liu, Q., Zhou, H., Langdon, W. Y. &
Zhang, J. E3 ubiquitin ligase Cbl-b in innate and adaptive immunity. _Cell Cycle_ 13, 1875–1884 (2014). Article PubMed PubMed Central CAS Google Scholar * Mohapatra, B. et al. An
essential role of CBL and CBL-B ubiquitin ligases in mammary stem cell maintenance. _Development_ 144, 1072–1086 (2017). Article PubMed PubMed Central CAS Google Scholar * Chitalia, V.
et al. c-Cbl, a ubiquitin E3 ligase that targets active beta-catenin: a novel layer of Wnt signaling regulation. _J. Biol. Chem._ 288, 23505–23517 (2013). Article PubMed PubMed Central
CAS Google Scholar * Karimaian, A., Majidinia, M., Bannazadeh Baghi, H. & Yousefi, B. The crosstalk between Wnt/beta-catenin signaling pathway with DNA damage response and oxidative
stress: Implications in cancer therapy. _DNA Repair_ 51, 14–19 (2017). Article PubMed CAS Google Scholar * Pittayapruek, P., Meephansan, J., Prapapan, O., Komine, M. & Ohtsuki, M.
Role of matrix metalloproteinases in photoaging and photocarcinogenesis. _Int. J. Mol. Sci._ 17, E868 (2016). Article PubMed CAS Google Scholar * Yu, J. et al. Ubiquitin ligase Cbl-b
acts as a negative regulator in discoidin domain receptor 2 signaling via modulation of its stability. _FEBS Lett._ 588, 1509–1514 (2014). Article PubMed CAS Google Scholar * Sha, Y. et
al. Modulation of UVB-induced carcinogenesis by activation of alternative DNA repair pathways. _Sci. Rep._ 8, 705 (2018). Article PubMed PubMed Central CAS Google Scholar * Yamazaki, S.
et al. Ultraviolet B-induced maturation of CD11b-type langerin(−) dendritic cells controls the expansion of Foxp3(+) regulatory T cells in the skin. _J. Immunol._ 200, 119–129 (2018).
Article PubMed CAS Google Scholar * Singh, T. P., Mayer, G. & Wolf, P. In vivo siRNA targeting of CD28 reduces UV-induced DNA damage and inflammation. _J. Invest. Dermatol._ 134,
861–864 (2014). Article PubMed CAS Google Scholar * Warde-Farley, D. et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene
function. _Nucleic Acids Res._ 38, W214–W220 (2010). Web Server issue. Article PubMed PubMed Central CAS Google Scholar Download references ACKNOWLEDGMENTS This work was supported by
the Austrian Science Fund FWF (W1241) and the Medical University of Graz through the PhD programs MolMed and Molecular Fundamentals of Inflammation (DK-MOLIN). The authors thank Melita
Ticevic and Renu Sarao, Institute of Molecular biotechnology (IMBA), Vienna, Austria, and Gerlinde Mayer, Medical University of Graz, Austria for technical support. Special thanks go to
Honnavara N. Ananthaswamy, The University of Texas MD Anderson Cancer Center, Houston, Texas, for critical reading and editing of the manuscript. AUTHOR INFORMATION Author notes * Tej Pratap
Singh Present address: Inflammation Biology Section, Laboratory of Molecular Immunology, NIAID, NIH, Building 10, Room 11N112, 10 Center Drive, Bethesda, MD, 20892, USA * These authors
contributed equally: Tej Pratap Singh, Pablo A. Vieyra-Garcia AUTHORS AND AFFILIATIONS * Research Unit for Photodermatology, Department of Dermatology and Venereology, Medical University of
Graz, Graz, Austria Tej Pratap Singh, Pablo A. Vieyra-Garcia & Peter Wolf * Center for Medical Research, Medical University of Graz, Graz, Austria Tej Pratap Singh, Pablo A.
Vieyra-Garcia & Karin Wagner * Institute of Molecular Biotechnology (IMBA), Vienna, Austria Josef Penninger Authors * Tej Pratap Singh View author publications You can also search for
this author inPubMed Google Scholar * Pablo A. Vieyra-Garcia View author publications You can also search for this author inPubMed Google Scholar * Karin Wagner View author publications You
can also search for this author inPubMed Google Scholar * Josef Penninger View author publications You can also search for this author inPubMed Google Scholar * Peter Wolf View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.P.S., P.V., and P.W. designed the study and experiments, analysed and interpreted the data and wrote
the manuscript; T.P.S, P.V., and K.W. performed experiments; K.W., P.V., and T.P.S analysed data; J.P. contributed to designing experiments and editing the manuscript. CORRESPONDING AUTHOR
Correspondence to Peter Wolf. ETHICS DECLARATIONS CONFLICT OF INTEREST J.P. holds shares in a company that tries to develop Cbl-b blockers. The other authors declare no competing financial
interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Edited by M.
Malewicz ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY FIGURE 1 SUPPLEMENTARY FIGURE 2 SUPPLEMENTARY FIGURE 3 SUPPLEMENTARY TABLE 1: GENE CO-EXPRESSION ANALYSES RIGHTS AND PERMISSIONS OPEN
ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Singh, T.P., Vieyra-Garcia,
P.A., Wagner, K. _et al._ Cbl-b deficiency provides protection against UVB-induced skin damage by modulating inflammatory gene signature. _Cell Death Dis_ 9, 835 (2018).
https://doi.org/10.1038/s41419-018-0858-5 Download citation * Received: 24 July 2017 * Revised: 28 June 2018 * Accepted: 04 July 2018 * Published: 06 August 2018 * DOI:
https://doi.org/10.1038/s41419-018-0858-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative