Transcriptional downregulation of mir-133b by rest promotes prostate cancer metastasis to bone via activating tgf-β signaling
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT High avidity of bone metastasis is an important characteristic in prostate cancer (PCa). Downexpression of miR-133b has been reported to be implicated in the development,
progression and recurrence in PCa. However, clinical significance and biological roles of miR-133b in bone metastasis of PCa remain unclear. Here we report that miR-133b is downregulated in
PCa tissues and further decreased in bone metastatic PCa tissues. Downexpression of miR-133b positively correlates with advanced clinicopathological characteristics and shorter bone
metastasis-free survival in PCa patients. Upregulating miR-133b inhibits invasion, migration in vitro and bone metastasis in vivo in PCa cells. Mechanistically, we find that miR-133b
suppresses activity of TGF-β signaling via directly targeting TGF-β receptor I and II, which further inhibits bone metastasis of PCa cells. Our results further reveal that overexpression of
REST contributes to miR-133b downexpression via transcriptional repression in PCa tissues. Importantly, silencing miR-133b enhances invasion and migration abilities in vitro and bone
metastasis ability in vivo in REST-silenced PCa cells. The clinical correlation of miR-133b with TGFBRI, TGFBRII, REST and TGF-β signaling activity is verified in PCa tissues. Therefore, our
results uncover a novel mechanism of miR-133b downexpression that REST transcriptionally inhibits miR-133b expression in PCa cells, and meanwhile support the notion that administration of
miR-133b may serve as a rational regimen in the treatment of PCa bone metastasis. SIMILAR CONTENT BEING VIEWED BY OTHERS CANCER-DERIVED EXOSOMAL MIR-375 TARGETS DIP2C AND PROMOTES
OSTEOBLASTIC METASTASIS AND PROSTATE CANCER PROGRESSION BY REGULATING THE WNT SIGNALING PATHWAY Article 25 November 2022 MIR-199B-5P-DDR1-ERK SIGNALLING AXIS SUPPRESSES PROSTATE CANCER
METASTASIS VIA INHIBITING EPITHELIAL-MESENCHYMAL TRANSITION Article 26 November 2020 LOSS OF MIR-936 LEADS TO ACQUISITION OF ANDROGEN-INDEPENDENT METASTATIC PHENOTYPE IN PROSTATE CANCER
Article Open access 12 October 2022 INTRODUCTION Prostate cancer (PCa) is the second most frequently diagnosed cancer among men and the fifth leading cause of cancer-related deaths
worldwide1. Despite mortality rate of PCa patients has been improved in the majority of developed countries, it remains poor, even rising in some developing countries, including China2.
Tumor cell metastasis to distant sites, particularly bone, greatly contributes to high mortality of PCa patients3,4. Bone is among the most preferential metastatic site of PCa5. Once bone
metastasis happens, which brings numerous bone- associated complications, including hypercalcemia, intractable pain, fracture, or nerve compression syndrome, causing poor
survival6.Therefore, understanding the molecular mechanism underlying the metastatic proclivity of PCa cells to bone is a profound task that may facilitate the development of strategies to
prevent or treat bone metastasis of PCa. TGF-β signaling has been demonstrated to play an important role in several biological processes, including cell differentiation, embryogenesis and
bone remodeling7. From a molecular perspective, TGF-β signaling starts via ligand-induced cooperative assembly of the receptor complex, where the kinase domain of the type II receptor
phosphorylates the type I receptor and receptor-bound SMAD (R-Smad) proteins 2 and 3, which further drives assembly of R-Smads into a complex with a second class of Smads, the so-called
co-Smad (Smad4), leading to the nuclear accumulation of heteromeric Smad complexes8. In the nucleus, Smads regulate transcription of TGF-β target genes as transcriptional factors by
interacting with a broad range of DNA-binding partners9. In cancer, TGF-β signaling functions as either an oncogenic or tumor-suppressive pathway depending on the developmental stage and
type of tumor: in early stages, TGF-β signaling inhibits cell growth as a tumor suppressor, while in later stage of cancer, TGF-β promotes invasion and metastasis, in particular metastasis
to bone10. TGF-β signaling promote bone metastasis of cancers via transcriptionally regulating multiple bone metastasis-associated genes, including MMP1, IL-11, or PTHRP11,12,13.
Accumulating literatures have reported that TGF-β signaling plays a pivotal role in the development of bone metastasis in cancer, including breast cancer and melanoma14,15. Importantly, the
pro-bone metastasis role of TGF-β signaling in PCa has been demonstrated. Fournier and colleagues reported that activation of TGF-β signaling upregulated PMEPA1, an important negative
regulator of the TGF-β pathway, and that interrupting this negative feedback loop by PMEPA1 knockdown drove PCa cells to disseminate to bone marrow, ultimately increasing bone metastases in
a mouse PCa model16. Furthermore, therapy targeting TGF-β significantly reduced the development of bone metastases in PCa17,18. However, the underlying molecular mechanisms responsible for
constitutive activation of TGF-β in PCa bone metastasis have not been determined. Since its discovery, RE1-silencing transcription factor (REST) has been identified to act as a
transcriptional repressor typically expressed in non-neuronal tissues, where it suppresses the expression of neuronal genes, and plays an important role in neuronal development19,20.
Recently, mutations or overexpression of REST have been extensively reported in various types of cancers21,22,23. Strikingly, Kreisler and colleagues have demonstrated that hypermethylation
induced loss of REST expression in small-cell lung cancer, which was linked to malignant progression of small-cell lung cancer via leading to an epidermal growth factor-mediated
de-regulation of AKT-Serine473 phosphorylation24. These studies indicate that REST may act as an oncogene or tumor suppressor dependent on tumor type. In PCa, loss of REST has been reported
to be involved in neuroendocrine differentiation25,26. However, no data is available for the role of REST alterations in bone metastasis of PCa. microRNAs (miRNAs) are short, noncoding RNAs
that regulate downstream target genes expression at a post-transcriptional level via binding with specific sequences in the 3′ untranslated region (3′-UTR) of downstream target genes27.
miRNAs play important roles in many cellular and biological processes28, and aberrant expression of miRNAs have been reported to be implicated in the progression and metastasis of
cancers29,30,31,32,33,34. Furthermore, several miRNAs have been reported as critical mediators in the bone metastasis of PCa35,36,37. Our previous studies demonstrated that the loss of
wild-type p53 induced downexpression of miR-145, promoted bone metastasis of PCa via regulating several positive regulators of EMT38,39,40. These studies indicate that aberrant expression of
miRNAs is significantly associated with the bone metastasis of PCa. In this study, our results found that miR-133b was downregulated in PCa tissues and further decreased in bone metastatic
PCa tissues, and miR-133b expression levels was inversely associated with poor clinicopathological characteristics and bone metastasis-free survival in PCa patients. In addition,
upregulating miR-133b dramatically inhibited invasion and migration in vitro, and bone metastasis in vivo in PCa cells. Our results further demonstrated that miR-133b directly targeted
TGFBRI and TGFBRII, which further repressed activity of TGF-β signaling, invasion and migration abilities of PCa cells. Importantly, our findings revealed that REST transcriptionally
inhibited miR-133b expression, leading to miR-133b downexpression in PCa tissues. Furthermore, downregulating miR-133b reversed the effects of silencing REST on bone metastasis of PCa in
vitro and in vivo. Therefore, our results indicate that REST/miR-133b/TGF-β signaling axis plays an important role in bone metastasis of PCa. RESULTS MIR-133B IS DOWNREGULATED IN PCA TISSUES
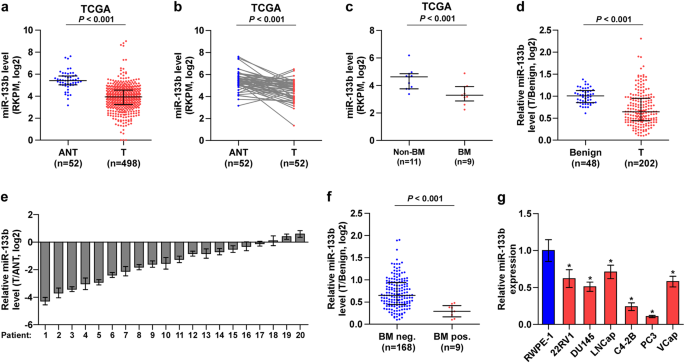
AND FURTHER DECREASED IN BONE METASTATIC PCA TISSUES To investigate the clinical significance of miR-133b in PCa, we first analyzed several publicly available miRNA sequencing data set of
PCa from The Cancer Genome Atlas (TCGA) and ArrayExpress, and found that miR-133b expression was downregulated in separate and paired PCa tissues compared with the adjacent normal tissues
(ANT) (Fig. 1a, b, and Supplementary Figure 1a and 1b), and further reduced in bone metastatic PCa tissues compared with that in non-bone metastatic PCa tissues (Fig. 1c). Consistently, low
expression of miR-133b was demonstrated in our 202 individual and 20 paired PCa tissues compared with benign prostate lesions, including benign prostate hyperplasia and prostatitis (Fig.
1d,e), particularly in bone metastatic PCa tissues (Fig. 1f). We further examined the expression levels of miR-133b in normal prostate epithelial cells RWPE-1 and other 6 PCa cells and found
that miR-133b expression were differentially decreased compared with RWPE-1, especially in bone metastatic PCa cell lines (PC-3 and C4-2B) (Fig. 1g). These results indicate that
downexpression of miR-133b may be associated with the progression and bone metastasis of PCa. DOWNEXPRESSION OF MIR-133B CORRELATES WITH ADVANCED CLINICOPATHOLOGICAL CHARACTERISTICS AND POOR
BONE METASTASIS-FREE SURVIVAL IN PCA PATIENTS We further analyzed the clinical correlation of miR-133b expression levels with clinicopathological characteristics in PCa. As shown in Fig.
2a–d, Supplementary Figure 2a–d and Supplementary Table 1, low expression of miR-133b positively correlated with Gleason grade, T classification, N classification and M classification in PCa
patients. Kaplan–Meier survival analysis indicated that PCa patients with low miR-133b expression correlated with shorter bone metastasis-free and progression-free survival (Fig. 2e and
Supplementary Figure 2f). Univariate Cox-regression analysis indicated patients with low miR-133b expression had shorter bone metastasis-free survivals (_P_ < 0.001; hazard ratio = 0.08,
95% CI = 0.03–0.22) compared to patients with high miR-133b expression (Supplementary Table 2). Multivariate Cox regression analysis revealed that low expression of miR-133b may be used as
independent factors to predict bone metastasis-free survival (Fig. 2g and Supplementary Table 2). However, low expression of miR-133b had no obvious effect on overall survival in PCa
patients (Fig. 2f, Supplementary Figure 2e and Supplementary Table 3). It was noteworthy that downexpression of miR-133b combined with lymph node metastasis or high level of serum PSA
predicted poorer bone metastasis-free and progression-free survival (Fig. 2h–j). Taken together, our results indicated that low expression of miR-133b is positively associated with poor bone
metastasis-free and progression-free survival in PCa patients. MIR-133B INHIBITS TGF-Β SIGNALING PATHWAY To investigate the underlying signaling pathways mediating the role of miR-133b in
bone metastasis of PCa, luciferase reporter plasmids of multiple signaling pathways were transfected into PCa cells respectively. As shown in Fig. 3a, TGF-β/Smad-responsive luciferase
reporter activity was consistently downregulated by upregulation of miR-133b in three bone metastatic PCa cells, and upregulated by silencing miR-133b in these three cells. Gene Set
Enrichment Analysis (GSEA) was further analyzed based on miR-133b expression from TCGA, and the result indicated that miR-133b expression levels correlated with TGF-β signaling pathway (Fig.
3b) that has been extensively demonstrated to play a pivotal role in bone metastasis of several human cancer, including PCa11,15,41. Therefore, we further examined the effects of miR-133b
on TGF-β signaling activity in PCa cells by exogenously overexpressing miR-133b and endogenously knocking down miR-133b via virus transduction in PCa cells (Supplementary Figure 3a). As
shown in Fig. 3c and Supplementary Figure 4a, upregulating miR-133b significantly repressed, while silencing miR-133b enhanced the transcriptional activity of the TGF-β/Smad-responsive
luciferase reporter in the absence or presence of ectopic TGF-β in PCa cells, and the expression levels of miR-133b in PCa cells was not affected by TGF-β treatment (Supplementary Figure
4b). Western blotting analysis revealed that upregulation of miR-133b decreased nuclear translocation of pSMAD3 in PCa cells in the absence or presence of ectopic TGF-β, whereas silencing
miR-133b increased its expression (Fig. 3d and Supplementary Figure 4c). In addition, upregulation of miR-133b reduced, while silencing miR-133b increased multiple downstream bone
metastasis-related genes of the TGF-β pathway in both the absence and presence of ectopic TGF-β (Fig. 3e). Importantly, the stimulatory effects of silencing miR-133b on invasion and
migration abilities of PCa cells were abrogated by TGF-β signaling inhibitor SD208 (Supplementary Figure 4d). Thus, our results demonstrate that miR-133b inhibits TGF-β signaling activity in
PCa cells. MIR-133B TARGETS TGFBI AND TGFBII By several available algorithms TargetScan and miRanda, we found that TGF-β receptor I (TGFBRI) and II (TGFBRII) may be potential target of
miR-133b (Supplementary Figure 5a). RT-PCR and western blotting revealed that upregulating miR-133b reduced, while silencing miR-133b increased the mRNA and protein expression levels of
TGFBRI and TGFBRII (Fig. 4a, b). Luciferase assay revealed that upregulating miR-133b decreased, while silencing miR-133b increased the reporter activity of the 3′-UTRs of TGFBRI and TGFBRII
transcripts (Fig. 4c–e). Furthermore, RNA immunoprecipitation (IP) assay demonstrated a selective association of miR-133b with TGFBRI and TGFBRII transcripts (Fig. 4f–h). Furthermore,
individual silencing TGFBRI or TGFBRII or both differentially attenuated the stimulatory effects of silencing miR-133b on invasion and migration abilities of PCa cells (Supplementary Figure
5b). Consequently, our results reveal that TGFBRI and TGFBRII are the direct targets of miR-133b in PCa cells. REST TRANSCRIPTIONALLY INHIBITS MIR-133B EXPRESSION IN PCA TISSUES To
investigate the underlying mechanism responsible for miR-133b downexpression in PCa tissues, we analyzed the deletion levels in PCa data set from TCGA from a genetic perspective and found
that only 6.25% of PCa tissues appeared deletion (~29 cases) (Supplementary Figure 6a). However, the expression level of miR-133b with deletion was no significant difference compared with
those without deletion (Supplementary Figure 6b). This finding suggested that deletion may be not responsible for miR-133b downexpression in bone metastatic PCa tissues. Furthermore, CpG
island was not found in the promoter of MIR-133B through UCSC analysis, suggesting that methylation level is not a primary mechanism regulating miR-133b expression in PCa. Numerous studies
have reported that dysregulation of miRNAs frequently occurred at a transcriptional level42. So, we further analyzed whether some transcriptional factor may be involved in miR-133b
downexpression in bone metastatic PCa tissues. The UCSC bioinformatics identified two transcriptional factors, CTGF and REST, with the potent binding ability in the promoter region of
MIR-133B (Fig. 5a). Through analyzing JASPAR, several potential REST or CTGF-binding motifs were identified inside the putative promoter region of MIR-133B (Fig. 5b). RT-PCR analysis showed
that upregulation of REST repressed, while silencing REST enhanced miR-133b expression levels, but CTGF had no any effect on miR-133b expression (Fig. 5c–e). A ChIP assay indicated that only
REST bound to the P1-binding site in the promoter region of MIR-133B in PCa cells (Fig. 5f–h). Furthermore, upregulation of REST reduced, while silencing REST elevated the promoter
luciferase activity of MIR-133B (Fig. 5i–k). However, upregulation or downregulation of REST had no effect on the luciferase activity of the MIR-133B promoter without P1-binding sites (Fig.
5i–k). These results indicated that REST transcriptionally inhibits miR-133b expression. Collectively, our results demonstrated that REST transcriptionally inhibits miR-133b expression.
CLINICAL RELATION OF MIR-133B WITH TGFBRI, TGFBRII AND REST EXPRESSION, AND TGF-Β SIGNALING ACTIVITY IN HUMAN PCA TISSUES To determine the clinical correlation of miR-133b with TGFBRI,
TGFBRII, REST expression, and TGF-β signaling activity in clinical PCa tissues, the miR-133b expression and protein levels of TGFBRI, TGFBRII, REST and nuclear SMAD2/3 expression were
examined in four bone metastatic and four non-bone metastatic PCa tissues. As shown in Fig. 6a, b, protein expression of TGFBRI, TGFBRII, REST and nuclear pSMAD3 in bone metastatic PCa
tissues (T1–4) was upregulated compared with that in non-bone metastatic PCa tissues (T5–8). Conversely, miR-133b displayed an opposite pattern. Pearson analysis revealed that miR-133b
expression inversely correlated with TGFBRI, TGFBRII and REST expression, and nuclear pSMAD3 expression (Fig. 6c–f.). Taken together, our results indicate that transcriptional repression of
miR-133b by REST in bone metastatic PCa tissues activates TGF-β signaling by upregulating TGFBRI and TGFBRII expression, finally promoting the development of PCa bone metastasis.
OVEREXPRESSION OF MIR-133B REPRESSES BONE METASTASIS OF PCA CELLS IN VIVO Our results above indicated that miR-133b was downregulated in bone metastasis PCa tissues, which positively
correlated with poor bone metastasis-free survival in PCa patients. Therefore, we further investigated the effect of miR-133b on the bone metastasis of PCa in vivo. A mouse model of bone
metastasis was used, in which the luciferase-labeled vector or miR-133b-overexpressing PC-3 cells were inoculated, respectively, into the left cardiac ventricle of male nude mice to monitor
the progression of bone metastasis by bioluminescence imaging (BLI). As shown in Fig. 7a, b, ectopic expression of miR-133b dramatically suppressed bone metastasis ability compared with the
control group by X-ray and BLI. H&E staining of the tumor sections from the indicated mice tibias showed that upregulating miR-133b decreased the tumor burden in bone (Fig. 7c).
Furthermore, mice injected with miR-133b-overexpressing cells exhibited fewer bone metastatic sites and smaller osteolytic area of metastatic tumors, as well as longer survival and bone
metastasis-free survival compared to the control group (Fig. 7d–g). Furthermore, upregulating miR-133b repressed, while silencing miR-133b increased invasion and migration abilities of PCa
cells (Supplementary Figure 7a–d). Consistently, upregulating miR-133b reduced, while silencing miR-133b enhanced several migration and invasion markers, including MMP9, MMP13 and TIMP2
(Supplementary Figure 7e and f). Consequently, our results demonstrate that upregulating miR-133b represses the bone metastasis of PCa in vivo. REST PROMOTES BONE METASTASIS VIA INHIBITING
MIR-133B IN PCA CELLS We further evaluated whether miR-133b mediated the function role of REST in bone metastasis of PCa. We first examined the effect of REST on bone metastasis of PCa in
vivo. As shown in Fig. 8a–g, we found that silencing REST repressed bone metastasis ability of PC-3 cells, including decreasing bone metastatic score and osteolytic area of tumors, and
prolonging bone metastasis-free survival compared to the control group by bioluminescence imaging (BLI), X-ray, H&E staining and survival analysis. Next, to investigate the role of
REST/miR-133b axis in bone metastasis of PCa, we further downregulating miR-133b in REST-silenced PC-3 cells, and we found that silencing miR-133b enhanced bone metastasis ability in vivo in
REST-silenced PCa cells (Fig. 8a–g). Consistently, silencing miR-133b enhanced the TGF-β signaling activity, invasion and migration abilities in REST-silencing PCa cells (Supplemental Fig.
8a–d). These findings indicated that REST activates TGF-β signaling, and promotes bone metastasis via inhibiting miR-133b in PCa cells. DISCUSSION In the present study, we reported that
miR-133b was downregulated in PCa tissues, particularly in bone metastatic PCa tissues. Importantly, low expression of miR-133b correlated with advanced clinicopathological characteristics
and poor bone metastasis-free survival in PCa patients. Our results further revealed that miR-133b inhibited invasion and bone metastasis of PCa cells via targeting TGFBRI and TGFBRII,
leading to the inactivation of TGF-β signaling. These results determine the tumor-suppressive role of miR-133b in bone metastasis of PCa. Constitutive activation of TGF-β signaling has been
reported in bone metastases of various types of cancer11,14,15, to which several regulatory mechanisms contributed, such as increase of autocrine TGF-β, mutation of core components of TGF-β
signaling43,44,45. Recent studies have demonstrated that aberrant of miRNAs is another important mechanism responsible for activation of TGF-β signaling, giving rise to tumor cell metastasis
in different types of cancer. The study by Bonci has reported that simultaneous loss of miR-15/miR-16 in PCa cells significantly activated TGF-β signaling, which further promoted invasion,
distant bone marrow colonization and development of osteolytic tumor46. In this study, we found that downxpression of miR-133b repressed the activity of TGF-β signaling via targeting TGFBRI
and TGFBRII in PCa cells, which further inhibited bone metastasis of PCa cells. Taken together, our findings unravel a novel mechanism of TGF-β signaling-mediated bone metastasis of PCa. As
one of the originally identified muscle-specific microRNAs, the roles of miR-133b in the development and remodeling of heart and skeletal muscle have been well documented47,48. Furthermore,
numerous studies have shown that miR-133b comes into playing important pathological role in human cancer49. In gastric cancer, bladder cancer, renal cell carcinoma, squamous cell carcinoma
of esophagus and tongue, miR-133b has been reported to be downregulated and low expression of miR-133b promoted the progression and metastasis via vary mechanism50,51,52,53,54. However, high
expression of miR-133b has been demonstrated in cervical carcinoma and glioblastoma,55,56. There studies indicated that the pro- and anti-tumor roles of miR-133b are environment and
tumor-type dependent. Interestingly, a study by Li et al. has shown that overexpression of miR-133b in LNCaP cells boosted cell proliferation and cell-cycle progression, but inhibited
apoptosis; in contrast, upregulation of miR-133b yielded an opposite role in PC-3 cells, indicating that miR-133b might enhance tumor-promoting properties in less aggressive LNCaP cells,
whereas this miR may act as a tumor suppressor in more aggressive PC-3 cells57. Furthermore, several lines of evidence have shown that miR-133b was downregulated in PCa tissues and acted as
a tumor-suppressive miRNA in PCa58,59,60,61. Importantly, Coarfa and colleagues reported that miR-133b was dramatically downexpressed in metastatic PCa tissues62. These studies support the
ideal that miR-133b is downregulated in PCa tissues, which was involved in the metastatic phenotype of PCa. However, the clinical significance and biological roles of miR-133b in bone
metastasis of PCa remain unclear. In this study, our results showed that miR-133b was downregulated in PCa tissues and further decreased in bone metastatic PCa tissues, which was associated
with poor clinicopathological characteristics and bone metastasis-free survival in PCa patients. In addition, upregulating miR-133b dramatically inhibited invasion and migration in vitro,
and bone metastasis in vivo in PCa cells by repressing activity of TGF-β signaling via targeting TGFBRI and TGFBRII in PCa cells. Therefore, our results indicate that miR-133b plays an
inhibitory role in bone metastasis of PCa. As discussed above, the downexpression of miR-133b has been widely reported in PCa. However, the underlying mechanism responsible for miR-133b
downexpression in cancers was not clarified. With this question, we first analyzed the deletion levels of miR-133b in the PCa data set from TCGA and found that 6.25% of 496 PCa tissues
occurred deletions, but the miR-133b expression levels in PCa tissues with deletions was no significant difference compared with those without gains. This finding suggested that deletion was
not responsible for miR-133b downexpression in PCa tissues. Numerous studies have reported that dysregulation of miRNAs frequently occurred at a transcriptional level42. In this study, our
results demonstrated that REST transcriptionally inhibited miR-133b expression, which resulted in miR-133b downexpression in PCa tissues. These results indicated that overexpression of REST
contributed to miR-133b downexpression in PCa tissues. Indeed, REST has been extensively demonstrated to be a transcriptional repressor involved in the control of neuroendocrine gene
expression63,64, and overexpression of REST has previously been reported in various types of cancer21,22,65. Importantly, a study by Epping has reported that the TSPYL2/REST complex
activated TGF-β signaling in breast cancer66. However, how REST promotes TGF-β signaling remains unknown. In this study, our results found that REST transcriptionally inhibited miR-133b
expression, and miR-133b repressed activity of TGF-β signaling via targeting TGFBRI and TGFBRII, indicating that REST activates TGF-β signaling via transcriptional repression of miR-133b.
Therefore, our results in combination with other reveal that REST/miR-133b axis promotes bone metastasis via constitutively activating TGF-β signaling in PCa. Several lines of evidence
reported that miR-133b was identified in the serum of cancer patients, suggesting that miR-133b may serve as a potential diagnostic and prognostic marker in human cancers. Zhang et al.
reported that expression levels of miR-133b were significantly decreased in the serum of osteosarcoma patients compared with the healthy controls67. Moreover, high expression level of
miR-133b expression was identified in the serum of breast cancer patients68. Interestingly, miRNA profiling of prostate secretion samples (PSS) from 23 PCa and 25 benign prostate hyperplasia
(BPH) patients revealed that miR-133b was significantly downregulated in PSS of PCa patients. Importantly, miR-133b has much power than PSA to discriminate PCa from BPH patients59,
suggesting that miR-133b expression levels in PSS could be used as diagnostics markers for PCa. In this study, our results showed that miR-133b expression inversely correlated with bone
metastasis-free survival in PCa patients, suggesting that miR-133b may be used as a potential bone metastasis diagnostic marker in PCa cancers. However, whether miR-133b expression in the
serum or prostate secretion samples of PCa patients may be used as a minimal invasive diagnostic marker for the bone metastasis of PCa needs to be further validated in a larger series of
studies. In summary, our results indicate that miR-133b inhibits bone metastasis of PCa by targeting TGFBRI and TGFBRII, leading to inactivation of TGF-β signaling pathway, suggesting that
administration of miR-133b may serve as a therapeutic strategy in the treatment of PCa bone metastasis. Better understanding of the underlying mechanism of miR-133b in the inactivation of
TGF-β signaling will facilitate the development of novel anti-metastatic therapeutic methods against PCa. MATERIALS AND METHODS CELL LINES AND CELL CULTURE The human PCa cell lines 22RV1,
PC-3, VCaP, DU145, LNCaP and normal prostate epithelial cells RWPE-1 were obtained from Shanghai Chinese Academy of Sciences cell bank (China). RWPE-1 cells were grown in defined
keratinocyte-SFM (1×) (Invitrogen). PC-3, LNCaP and 22Rv1 cells were cultured in RPMI-1640 medium (Life Technologies, Carlsbad, CA, US) supplemented with penicillin G (100 U/ml),
streptomycin (100 mg/ml) and 10% fetal bovine serum (FBS, Life Technologies). DU145 and VCaP cells were grown in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% FBS.
The C4-2B cell line was purchased from the MD Anderson Cancer Center and maintained in T-medium (Invitrogen) supplemented with 10 % FBS. All cell lines were grown under a humidified
atmosphere of 5% CO2 at 37 °C. PLASMIDS, TRANSFECTION AND GENERATION OF STABLE CELL LINES The human miR-133b gene was PCR-amplified from genomic DNA and cloned into a pMSCV-puro retroviral
vector (Clontech, Japan). The (CAGAC) 12/pGL3 TGF-β/Smad-responsive luciferase reporter plasmid and control plasmids (Clontech, Japan) were used to quantitatively assess the transcriptional
activity of TGF-β signaling components. The 3′-UTR of TGFBRI and TGFBRII were PCR-amplified from genomic DNA and cloned into pmirGLO vectors (Promega, USA), and the list of primers used in
cloning reactions is shown in Supplementary Table 4. AntagomiR-133b, small interfering RNA (siRNA) for TGFBRI and TGFBRII knockdown and corresponding control siRNAs were synthesized and
purified by RiboBio. Human REST cDNA was purchased form (Vigene Biosciences, Shandong, China). Transfection of miRNA, siRNAs, and plasmids was performed using Lipofectamine 3000 (Life
Technologies, USA) according to the manufacturer’s instructions. RNA EXTRACTION, REVERSE TRANSCRIPTION, AND REAL-TIME RT-PCR Total RNA from tissues or cells was extracted using the RNA
Isolation Kit (Qiagen, USA) according to the manufacturer’s instructions. Messenger RNA (mRNA) and miRNA were reverse transcribed from total mRNA using the RevertAid First Strand cDNA
Synthesis Kit (Thermo Fisher, USA) according to the manufacturer’s protocol. Complementary DNA (cDNA) was amplified and quantified on the CFX96 system (BIO-RAD, USA) using iQ SYBR Green
(BIO-RAD, USA). The primers are provided in Supplementary Table 5. Primers for U6 and miR-133b were synthesized and purified by RiboBio (Guangzhou, China). U6 or glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as the endogenous controls. Relative fold expressions were calculated with the comparative threshold cycle (2-ΔΔCt) method. PATIENTS AND TUMOR TISSUES A total
of 182 individual and 20 paired PCa tissues, and 48 benign prostate lesions tissues were obtained during surgery or needle biopsy at The Clinical Biobank of Collaborative Innovation Center
for Medical Molecular Diagnostics of Guangdong Province, The Affiliated Jiangmen Hospital of Sun Yat-sen University (Guangdong, China), and the Second Affiliated Hospital of Guangzhou
Medical University (Guangdong, China) between January 2008 and December 2016. Patients were diagnosed based on clinical and pathological evidence, and the specimens were immediately
snap-frozen and stored in liquid nitrogen tanks. For the use of these clinical materials for research purposes, prior patient’ consents and approval from the Institutional Research Ethics
Committee were obtained. The clinicopathological features of the patients are summarized in Supplementary Table 6-8. The median of miR-133b expression in PCa tissues was used to stratify
high and low expression of miR-133b. MIRNA IMMUNOPRECIPITATION Cells were co-transfected with HA-Ago2, followed by HA-Ago2 immunoprecipitation using anti-HA-antibody. Real-time PCR analysis
of the IP material was performed to test the association of the mRNA of TGFBRI and TGFBRII with the RISC complex. The specific processes were performed as previously described69. WESTERN
BLOT Western blot was performed according to a standard method, as previously described70. Antibodies against TGFBRI, anti–TGFBRII and p84 were purchased from Abcam, anti–pSMAD3 and
anti–SMAD3 from Cell Signaling Technology. Nuclear extracts were prepared using the Nuclear Extraction Kit (Active Motif), according to the manufacturer’s instructions. As a loading control,
membranes were stripped and reprobed with an anti-α-tubulin antibody (Sigma-Aldrich, USA). LUCIFERASE REPORTER ASSAY Cells (4 × 104) were seeded in triplicate in 24-well plates and cultured
for 24 h and performed as previously described71. Luciferase and Renilla signals were measured 36 h after transfection using a Dual Luciferase Reporter Assay Kit (Promega). CHROMATIN
IMMUNOPRECIPITATION (CHIP) Cells were cultured as described above. Cross-linking was performed with formaldehyde (Merck) at a final concentration of 1 % and terminated after 5 min by the
addition of glycine at a final concentration of 0.125 M. Cells were collected with SDS buffer, pelleted by centrifugation, and resuspended in IP buffer. Chromatin was sheered by sonication
(HTU SONI 130; Heinemann) to generate DNA fragments with an average size of 500 bp. Preclearing and incubation with anti-Flag (F1804, Sigma) antibodies or IgG control (M-7023, Sigma) for 16
h was performed. Immunoprecipitated DNA was decrosslinked, digested with proteinase K and purified. Immunoprecipitated DNA was analyzed by qPCR and the primers of MIR133B promoter was
presented in Supplementary Table 4. Enrichment was expressed as the percentage of the input for each condition. INVASION AND MIGRATION ASSAYS The invasion and migration assays were performed
as described previously72. The cell count was performed under a microscope (x100). ANIMAL STUDY All mouse experiments were approved by The Institutional Animal Care and Use Committee of Sun
Yat-sen University and the approval-No. was L102012016110D. For the bone metastasis study, BALB/c-nu mice (5–6 weeks old) were anaesthetized and inoculated into the left cardiac ventricle
with 1 × 105 PC-3 cells in 100 μl of PBS. Osteolytic lesions were identified on radiographs as radiolucent lesions in the bone. The area of the osteolytic lesions was measured using the
Metamorph image analysis system and software (Universal Imaging Corporation), and the total extent of bone destruction per animal was expressed in square millimeters. Each bone metastasis
was scored based on the following criteria: 0, no metastasis; 1, bone lesion covering < 1/4 of the bone width; 2, bone lesion involving 1/4–1/2 of the bone width; 3, bone lesion across
1/2–3/4 of the bone width; and 4, bone lesion > 3/4 of the bone width. The bone metastasis score for each mouse was the sum of the scores of all bone lesions from four limbs. STATISTICAL
ANALYSIS All values are presented as the mean ± SD. Significant differences were determined using the GraphPad 5.0 software (USA). Student’s _t_ test was used to determine statistical
differences between two groups. The _Χ_2 test was used to analyze the relationship between miR-133b expression and clinicopathological characteristics. Survival curves were plotted using the
Kaplan–Meier method and compared by log-rank test. _P_ < 0.05 was considered statistical significant. All experiments were repeated three times. REFERENCES * Torre, L. A. et al. Global
cancer statistics, 2012. _CA Cancer J. Clin._ 65, 87–108 (2015). Article PubMed Google Scholar * Chen, W. et al. Cancer statistics in China, 2015. _CA Cancer J. Clin._ 66, 115–132 (2016).
Article PubMed Google Scholar * Body, J. J., Casimiro, S. & Costa, L. Targeting bone metastases in prostate cancer: improving clinical outcome. _Nat. Rev. Urol._ 12, 340–356 (2015).
Article PubMed Google Scholar * Weinfurt, K. P. et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer.
_Ann. Oncol._ 16, 579–584 (2005). Article PubMed CAS Google Scholar * Langley, R. R. & Fidler, I. J. The seed and soil hypothesis revisited–the role of tumor-stroma interactions in
metastasis to different organs. _Int. J. Cancer_ 128, 2527–2535 (2011). Article PubMed PubMed Central CAS Google Scholar * Saad, F. et al. Pathologic fractures correlate with reduced
survival in patients with malignant bone disease. _Cancer_ 110, 1860–1867 (2007). Article PubMed Google Scholar * Mohammad, K. S. et al. Pharmacologic inhibition of the TGF-beta type I
receptor kinase has anabolic and anti-catabolic effects on bone. _PLoS ONE_ 4, e5275 (2009). Article PubMed PubMed Central CAS Google Scholar * Shi, Y. & Massague, J. Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. _Cell_ 113, 685–700 (2003). Article PubMed CAS Google Scholar * Huse, M. et al. The TGF beta receptor activation process: an
inhibitor- to substrate-binding switch. _Mol. Cell_ 8, 671–682 (2001). Article PubMed CAS Google Scholar * Pickup, M., Novitskiy, S. & Moses, H. L. The roles of TGFbeta in the tumour
microenvironment. _Nat. Rev. Cancer_ 13, 788–799 (2013). Article PubMed PubMed Central CAS Google Scholar * Kang, Y. et al. Breast cancer bone metastasis mediated by the Smad tumor
suppressor pathway. _Proc. Natl Acad. Sci. USA_ 102, 13909–13914 (2005). Article PubMed CAS Google Scholar * Sethi, N., Dai, X., Winter, C. G. & Kang, Y. Tumor-derived JAGGED1
promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. _Cancer Cell_ 19, 192–205 (2011). Article PubMed PubMed Central CAS Google Scholar *
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. _Cancer Cell_ 3, 537–549 (2003). Article PubMed CAS Google Scholar * Yin, J. J. et al. TGF-beta signaling
blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. _J. Clin. Invest._ 103, 197–206 (1999). Article PubMed PubMed Central CAS Google Scholar *
Javelaud, D. et al. Stable overexpression of Smad7 in human melanoma cells impairs bone metastasis. _Cancer Res._ 67, 2317–2324 (2007). Article PubMed CAS Google Scholar * Fournier, P.
G. et al. The TGF-beta signaling regulator PMEPA1 suppresses prostate cancer metastases to bone. _Cancer Cell_ 27, 809–821 (2015). Article PubMed PubMed Central CAS Google Scholar * Hu,
Z. et al. Systemic delivery of oncolytic adenoviruses targeting transforming growth factor-beta inhibits established bone metastasis in a prostate cancer mouse model. _Hum. Gene Ther._ 23,
871–882 (2012). Article PubMed PubMed Central CAS Google Scholar * Wan, X. et al. Effect of transforming growth factor beta (TGF-beta) receptor I kinase inhibitor on prostate cancer
bone growth. _Bone_ 50, 695–703 (2012). Article PubMed CAS Google Scholar * Mori, N., Schoenherr, C., Vandenbergh, D. J. & Anderson, D. J. A common silencer element in the SCG10 and
type II Na + channel genes binds a factor present in nonneuronal cells but not in neuronal cells. _Neuron_ 9, 45–54 (1992). Article PubMed CAS Google Scholar * Chong, J. A. et al. REST:
a mammalian silencer protein that restricts sodium channel gene expression to neurons. _Cell_ 80, 949–957 (1995). Article PubMed CAS Google Scholar * Blom, T. et al. Molecular genetic
analysis of the REST/NRSF gene in nervous system tumors. _Acta Neuropathol._ 112, 483–490 (2006). Article PubMed CAS Google Scholar * Lawinger, P. et al. The neuronal repressor REST/NRSF
is an essential regulator in medulloblastoma cells. _Nat. Med._ 6, 826–831 (2000). Article PubMed CAS Google Scholar * Fuller, G. N. et al. Many human medulloblastoma tumors overexpress
repressor element-1 silencing transcription (REST)/neuron-restrictive silencer factor, which can be functionally countered by REST-VP16. _Mol. Cancer Ther._ 4, 343–349 (2005). PubMed CAS
Google Scholar * Kreisler, A. et al. Regulation of the NRSF/REST gene by methylation and CREB affects the cellular phenotype of small-cell lung cancer. _Oncogene_ 29, 5828–5838 (2010).
Article PubMed CAS Google Scholar * Terry, S. & Beltran, H. The many faces of neuroendocrine differentiation in prostate cancer progression. _Front. Oncol._ 4, 60 (2014). Article
PubMed PubMed Central Google Scholar * Tawadros, T. et al. IB1/JIP-1 controls JNK activation and increased during prostatic LNCaP cells neuroendocrine differentiation. _Cell Signal._ 17,
929–939 (2005). Article PubMed CAS Google Scholar * Bartel, D. P. MicroRNAs: target recognition and regulatory functions. _Cell_ 136, 215–233 (2009). Article PubMed PubMed Central CAS
Google Scholar * Velu, V. K., Ramesh, R. & Srinivasan, A. R. Circulating microRNAs as biomarkers in health and disease. _J. Clin. Diagn. Res._ 6, 1791–1795 (2012). PubMed PubMed
Central Google Scholar * Ren, D. et al. Maintenance of cancer stemness by miR-196b-5p contributes to chemoresistance of colorectal cancer cells via activating STAT3 signaling pathway.
_Oncotarget_ 8, 49807–49823 (2017). PubMed PubMed Central Google Scholar * Zhang, X. et al. Upregulation of miR-572 transcriptionally suppresses SOCS1 and p21 and contributes to human
ovarian cancer progression. _Oncotarget_ 6, 15180–15193 (2015). PubMed PubMed Central Google Scholar * Hu, G. et al. MicroRNA-145 attenuates TNF-alpha-driven cartilage matrix degradation
in osteoarthritis via direct suppression of MKK4. _Cell Death Dis._ 8, e3140 (2017). Article PubMed PubMed Central CAS Google Scholar * Feliciano, A. et al. miR-99a reveals two novel
oncogenic proteins E2F2 and EMR2 and represses stemness in lung cancer. _Cell Death Dis._ 8, e3141 (2017). Article PubMed PubMed Central CAS Google Scholar * Yang, R. M. et al. miR-3656
expression enhances the chemosensitivity of pancreatic cancer to gemcitabine through modulation of the RHOF/EMT axis. _Cell Death Dis._ 8, e3129 (2017). Article PubMed PubMed Central CAS
Google Scholar * Ji, Q. et al. miR-216a inhibits osteosarcoma cell proliferation, invasion and metastasis by targeting CDK14. _Cell Death Dis._ 8, e3103 (2017). Article PubMed PubMed
Central CAS Google Scholar * Ren, D. et al. Oncogenic miR-210-3p promotes prostate cancer cell EMT and bone metastasis via NF-kappaB signaling pathway. _Mol. Cancer_ 16, 117 (2017).
Article PubMed PubMed Central Google Scholar * Colden, M. et al. MicroRNA-466 inhibits tumor growth and bone metastasis in prostate cancer by direct regulation of osteogenic
transcription factor RUNX2. _Cell Death Dis._ 8, e2572 (2017). Article PubMed PubMed Central CAS Google Scholar * Siu, M. K. et al. Transforming growth factor-beta promotes prostate
bone metastasis through induction of microRNA-96 and activation of the mTOR pathway. _Oncogene_ 34, 4767–4776 (2015). Article PubMed CAS Google Scholar * Ren, D. et al. Wild-type p53
suppresses the epithelial-mesenchymal transition and stemness in PC-3 prostate cancer cells by modulating miR145. _Int. J. Oncol._ 42, 1473–1481 (2013). Article PubMed CAS Google Scholar
* Ren, D. et al. Double-negative feedback loop between ZEB2 and miR-145 regulates epithelial-mesenchymal transition and stem cell properties in prostate cancer cells. _Cell Tissue Res._
358, 763–778 (2014). Article PubMed CAS Google Scholar * Guo, W. et al. HEF1 promotes epithelial–mesenchymal transition and bone invasion in prostate cancer under the regulation of
microRNA-145. _J. Cell. Biochem._ 114, 1606–1615 (2013). Article PubMed CAS Google Scholar * Dai, Y. et al. The TGF-beta signalling negative regulator PICK1 represses prostate cancer
metastasis to bone. _Br. J. Cancer_ 117, 685–694 (2017). Article PubMed CAS PubMed Central Google Scholar * Chang, Y. S. et al. EGF receptor promotes prostate cancer bone metastasis by
downregulating miR-1 and activating TWIST1. _Cancer Res._ 75, 3077–3086 (2015). Article PubMed PubMed Central CAS Google Scholar * Macias, M. J., Martin-Malpartida, P. & Massague,
J. Structural determinants of Smad function in TGF-beta signaling. _Trends Biochem. Sci._ 40, 296–308 (2015). Article PubMed PubMed Central CAS Google Scholar * Bhola, N. E. et al.
TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. _J. Clin. Invest._ 123, 1348–1358 (2013). Article PubMed PubMed Central CAS Google Scholar *
Scheel, C. et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. _Cell_ 145, 926–940 (2011). Article PubMed PubMed Central CAS Google
Scholar * Bonci, D. et al. A microRNA code for prostate cancer metastasis. _Oncogene_ 35, 1180–1192 (2016). Article PubMed CAS Google Scholar * Nohata, N., Hanazawa, T., Enokida, H.
& Seki, N. microRNA-1/133a and microRNA-206/133b clusters: dysregulation and functional roles in human cancers. _Oncotarget_ 3, 9–21 (2012). Article PubMed PubMed Central Google
Scholar * Mitchelson, K. R. & Qin, W. Y. Roles of the canonical myomiRs miR-1, -133 and -206 in cell development and disease. _World J. Biol. Chem._ 6, 162–208 (2015). Article PubMed
PubMed Central Google Scholar * Li, D. et al. miR-133b, a particular member of myomiRs, coming into playing its unique pathological role in human cancer. _Oncotarget_ 8, 50193–50208
(2017). PubMed PubMed Central Google Scholar * Liu, Y. et al. Identification of miRNomes in human stomach and gastric carcinoma reveals miR-133b/a-3p as therapeutic target for gastric
cancer. _Cancer Lett._ 369, 58–66 (2015). Article PubMed CAS Google Scholar * Pignot, G. et al. microRNA expression profile in a large series of bladder tumors: identification of a
3-miRNA signature associated with aggressiveness of muscle-invasive bladder cancer. _Int. J. Cancer_ 132, 2479–2491 (2013). Article PubMed CAS Google Scholar * Hidaka, H. et al. Tumor
suppressive microRNA-1285 regulates novel molecular targets: aberrant expression and functional significance in renal cell carcinoma. _Oncotarget_ 3, 44–57 (2012). Article PubMed PubMed
Central Google Scholar * Kano, M. et al. miR-145, miR-133a and miR-133b: tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. _Int. J. Cancer_ 127, 2804–2814
(2010). Article PubMed CAS Google Scholar * Wong, T. S. et al. Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. _Clin. Cancer Res._ 14, 2588–2592
(2008). Article PubMed CAS Google Scholar * Qin, W. et al. MicroRNA-133b is a key promoter of cervical carcinoma development through the activation of the ERK and AKT1 pathways.
_Oncogene_ 31, 4067–4075 (2012). Article PubMed CAS Google Scholar * Xu, G. & Li, J. Y. Differential expression of PDGFRB and EGFR in microvascular proliferation in glioblastoma.
_Tumour Biol._ 37, 10577–10586 (2016). Article PubMed CAS Google Scholar * Li, X. et al. Identification of miR-133b and RB1CC1 as independent predictors for biochemical recurrence and
potential therapeutic targets for prostate cancer. _Clin. Cancer Res._ 20, 2312–2325 (2014). Article PubMed CAS Google Scholar * Pashaei, E., Pashaei, E., Ahmady, M., Ozen, M. &
Aydin, N. Meta-analysis of miRNA expression profiles for prostate cancer recurrence following radical prostatectomy. _PLoS ONE_ 12, e0179543 (2017). Article PubMed PubMed Central CAS
Google Scholar * Guzel, E. et al. Identification of microRNAs differentially expressed in prostatic secretions of patients with prostate cancer. _Int. J. Cancer_ 136, 875–879 (2015).
Article PubMed CAS Google Scholar * Karatas, O. F. et al. miR-1 and miR-133b are differentially expressed in patients with recurrent prostate cancer. _PLoS ONE_ 9, e98675 (2014). Article
PubMed PubMed Central CAS Google Scholar * Patron, J. P. et al. MiR-133b targets antiapoptotic genes and enhances death receptor-induced apoptosis. _PLoS ONE_ 7, e35345 (2012). Article
PubMed PubMed Central CAS Google Scholar * Coarfa, C. et al. Comprehensive proteomic profiling identifies the androgen receptor axis and other signaling pathways as targets of
microRNAs suppressed in metastatic prostate cancer. _Oncogene_ 35, 2345–2356 (2016). Article PubMed CAS Google Scholar * Lim, J. S. et al. Intratumoural heterogeneity generated by Notch
signalling promotes small-cell lung cancer. _Nature_ 545, 360–364 (2017). Article PubMed PubMed Central CAS Google Scholar * Abderrahmani, A. et al. The transcriptional repressor REST
determines the cell-specific expression of the human MAPK8IP1 gene encoding IB1 (JIP-1). _Mol. Cell Biol._ 21, 7256–7267 (2001). Article PubMed PubMed Central CAS Google Scholar *
Guardavaccaro, D. et al. Control of chromosome stability by the beta-TrCP-REST-Mad2 axis. _Nature_ 452, 365–369 (2008). Article PubMed PubMed Central CAS Google Scholar * Epping, M. T.
et al. TSPYL2 is an essential component of the REST/NRSF transcriptional complex for TGFbeta signaling activation. _Cell Death Differ._ 22, 1353–1362 (2015). Article PubMed PubMed Central
CAS Google Scholar * Zhang, C., Yao, C., Li, H., Wang, G. & He, X. Serum levels of microRNA-133b and microRNA-206 expression predict prognosis in patients with osteosarcoma. _Int. J.
Clin. Exp. Pathol._ 7, 4194–4203 (2014). PubMed PubMed Central CAS Google Scholar * Chan, M. et al. Identification of circulating microRNA signatures for breast cancer detection. _Clin.
Cancer Res._ 19, 4477–4487 (2013). Article PubMed CAS Google Scholar * Li, X. et al. miR150 inhibits proliferation and tumorigenicity via retarding G1/S phase transition in
nasopharyngeal carcinoma. _Int. J. Oncol._ 1097–1108 (2017) https://doi.org/10.3892/ijo.2017.3909. * Wang, M. et al. N-cadherin promotes epithelial-mesenchymal transition and cancer stem
cell-like traits via ErbB signaling in prostate cancer cells. _Int. J. Oncol._ 48, 595–606 (2016). Article PubMed CAS Google Scholar * Zhang, X. et al. Phospholipid Phosphatase 4
promotes proliferation and tumorigenesis, and activates Ca2 + -permeable Cationic Channel in lung carcinoma cells. _Mol. Cancer_ 16, 147 (2017). Article PubMed PubMed Central Google
Scholar * Zhang, X. et al. Thymosin beta 10 is a key regulator of tumorigenesis and metastasis and a novel serum marker in breast cancer. _Breast Cancer Res:_ 19, 15 (2017). Article CAS
Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by grants from the National Natural Science Foundation of China (No. 81402227, 81503281, 81502219, 81660362) and
Guangdong Natural Science Foundation (No. 2014A030310157, 2015A020212014, 2014A030310034). AUTHOR INFORMATION Author notes * These authors contributed equally: Shuai Huang, Qingde Wa,
Jincheng Pan. AUTHORS AND AFFILIATIONS * Department of Orthopaedic Surgery, the Second Affiliated Hospital of Guangzhou Medical University, 510260, Guangzhou, China Shuai Huang & Yan
Huang * Department of Orthopaedic Surgery, the First Affiliated Hospital of Sun Yat-sen University, 510080, Guangzhou, China Shuai Huang, Xinsheng Peng, Dong Ren, Qiji Li, Yuhu Dai, Qing
Yang & Peiheng He * Department of Orthopaedic Surgery, the Affiliated Hospital of Zunyi Medical college, 563003, Zunyi, China Qingde Wa * Department of Urology Surgery, the First
Affiliated Hospital of Sun Yat-sen University, 510080, Guangzhou, China Jincheng Pan * Department of Pathology, Jiangmen Central Hospital, Affiliated Jiangmen Hospital of Sun Yat-sen
University, Jiangmen, 529030, China Xin Zhang & Wei Zhou * Department of Urology, Jiangmen Central Hospital, Affiliated Jiangmen Hospital of Sun Yat-sen University, Jiangmen, 529030,
China Dan Yuan & Jiazheng Cao * Department of Orthopaedic Surgery, Jiangmen Central Hospital, Affiliated Jiangmen Hospital of Sun Yat-sen University, Jiangmen, 529030, China Yuming Li *
Department of Pharmacy, The First Affiliated Hospital of Sun Yat-Sen University, 510080, Guangzhou, China Yubo Tang Authors * Shuai Huang View author publications You can also search for
this author inPubMed Google Scholar * Qingde Wa View author publications You can also search for this author inPubMed Google Scholar * Jincheng Pan View author publications You can also
search for this author inPubMed Google Scholar * Xinsheng Peng View author publications You can also search for this author inPubMed Google Scholar * Dong Ren View author publications You
can also search for this author inPubMed Google Scholar * Qiji Li View author publications You can also search for this author inPubMed Google Scholar * Yuhu Dai View author publications You
can also search for this author inPubMed Google Scholar * Qing Yang View author publications You can also search for this author inPubMed Google Scholar * Yan Huang View author publications
You can also search for this author inPubMed Google Scholar * Xin Zhang View author publications You can also search for this author inPubMed Google Scholar * Wei Zhou View author
publications You can also search for this author inPubMed Google Scholar * Dan Yuan View author publications You can also search for this author inPubMed Google Scholar * Jiazheng Cao View
author publications You can also search for this author inPubMed Google Scholar * Yuming Li View author publications You can also search for this author inPubMed Google Scholar * Peiheng He
View author publications You can also search for this author inPubMed Google Scholar * Yubo Tang View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHORS Correspondence to Peiheng He or Yubo Tang. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION
PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Edited by G. Calin ELECTRONIC SUPPLEMENTARY
MATERIAL SUPPLEMENTARY TABLE 1 SUPPLEMENTARY TABLE 2 SUPPLEMENTARY TABLE 3 SUPPLEMENTAL TABLE 4 SUPPLEMENTAL TABLE 5 SUPPLEMENTARY TABLE 6 SUPPLEMENTARY TABLE 7 SUPPLEMENTARY TABLE 8
SUPPLEMENTAL FIGURE LEGENDS SUPPLEMENTAL FIGURE 1 SUPPLEMENTAL FIGURE 2 SUPPLEMENTAL FIGURE 3 SUPPLEMENTAL FIGURE 4 SUPPLEMENTAL FIGURE 5 SUPPLEMENTAL FIGURE 6 SUPPLEMENTAL FIGURE 7
SUPPLEMENTAL FIGURE 8 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Huang, S., Wa, Q., Pan, J. _et al._ Transcriptional downregulation of miR-133b by REST promotes prostate cancer metastasis to bone via activating TGF-β signaling. _Cell
Death Dis_ 9, 779 (2018). https://doi.org/10.1038/s41419-018-0807-3 Download citation * Received: 06 November 2017 * Revised: 23 April 2018 * Accepted: 11 May 2018 * Published: 13 July 2018
* DOI: https://doi.org/10.1038/s41419-018-0807-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative