Lumbar instability remodels cartilage endplate to induce intervertebral disc degeneration by recruiting osteoclasts via Hippo-CCL3 signaling
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Degenerated endplate appears with cheese-like morphology and sensory innervation, contributing to low back pain and subsequently inducing intervertebral disc degeneration in the aged
population.1 However, the origin and development mechanism of the cheese-like morphology remain unclear. Here in this study, we report lumbar instability induced cartilage endplate
remodeling is responsible for this pathological change. Transcriptome sequencing of the endplate chondrocytes under abnormal stress revealed that the Hippo signaling was key for this
process. Activation of Hippo signaling or knockout of the key gene Yap1 in the cartilage endplate severed the cheese-like morphological change and disc degeneration after lumbar spine
instability (LSI) surgery, while blocking the Hippo signaling reversed this process. Meanwhile, transcriptome sequencing data also showed osteoclast differentiation related gene set
expression was up regulated in the endplate chondrocytes under abnormal mechanical stress, which was activated after the Hippo signaling. Among the discovered osteoclast differentiation gene
set, CCL3 was found to be largely released from the chondrocytes under abnormal stress, which functioned to recruit and promote osteoclasts formation for cartilage endplate remodeling.
Over-expression of Yap1 inhibited CCL3 transcription by blocking its promoter, which then reversed the endplate from remodeling to the cheese-like morphology. Finally, LSI-induced cartilage
endplate remodeling was successfully rescued by local injection of an AAV5 wrapped Yap1 over-expression plasmid at the site. These findings suggest that the Hippo signaling induced
osteoclast gene set activation in the cartilage endplate is a potential new target for the management of instability induced low back pain and lumbar degeneration.
Lumbar degeneration is a common disorder of the spine in the aged population, it is usually a source of low back pain.2 and a cause of disability worldwide.3 The prevalence of lumbar
degeneration dramatically increases with age. Greater than 90% of people over the age of 50 present with lumbar degeneration.4,5 The risk factors that may cause this pathology include
mechanical trauma, genetic predispositions, unhealthy lifestyles and certain metabolic disorders.6,7,8 However, the mechanism that initiates this process is not clear at present.
The intervertebral disc is the largest avascular structure in the body and cells within the disc rely on diffusive transport via the vertebral cartilage endplate (CEP) to receive nutrients,
eliminate waste products and maintain disc homeostasis.9 CEP is a hyaline cartilage located on the upper and lower sides of the intervertebral disc. Biomechanically, the endplate is known as
the center of stress concentration.10,11 Despite the crucial roles of the CEP in nutrition and biomechanical stability, vertebral endplates are extremely susceptible to mechanical failure.
CEP degeneration, along with subchondral bone marrow changes were originally noticed on magnetic resonance imaging, which is known as Modic changes (MCs).12,13 At this time, the CEP
remodeling is commonly observed to occur. CEP remodeling leads to ossification with abundant pores as a cheese like morphology, which we named the “cheese-endplate”. The cheese-endplate will
lead to the difficulties in nutrient dispersion and the imbalances of disc homeostasis.14
Studies exploring the events leading to disc degeneration have shown that failure often begins at the endplates15 and the mechanisms driving CEP transition have not been elucidated. Since
CEP is the center of stress concentration and excessive load is observed to appear on the CEP,16 studies believed the etiology of the degenerative changes could be due to mechanical
micro-insults or damage secondary to macro-insults.17 In clinic, it is observed that patients with CEP ossification and degeneration are usually accompanied by significant lumbar
instability. Nowadays, the primary clinical treatment for these conditions is to reconstruct lumbar stability with girth or internal fixation. Thus, if we can determine the underlying
biological mechanism to explain CEP degeneration that caused by lumbar instability, we would be able to identify an effective coping method to replace surgical approach for lumbar stability
reconstruction, which is the major goal for this study. Moreover, we consider that “cheese-endplate” is more than just an image finding in intervertebral disc degeneration (IVDD) patients,
but rather represent an underlying pathology that should be a target for therapy.18 Therefore, we tried to further explore the biological mechanism of disc degeneration caused by instability
to demonstrate whether the lumbar endplate degeneration caused by instability will be delayed or treated through biological targeted therapy, which will provide new ideas for clinical
diagnosis and management of lumbar degeneration.
Cartilage endplate chondrocytes (CEPCs) are the cells embedded in the CEP,19,20 which are indispensable in maintaining the integrity of the structure and function of CEP.21,22,23 In this
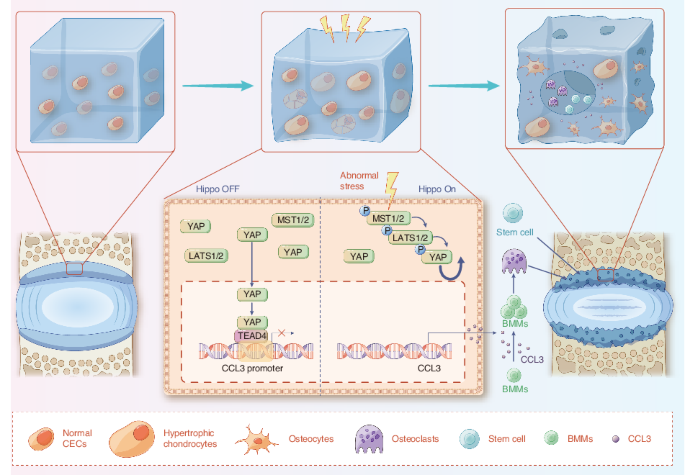
study, we found CEPCs hold the ability to sense mechanical signals through which the abnormal stress activates Hippo signaling within the CEPCs. In addition, it is well understood that
Yes-associated protein (YAP), a key factor within the Hippo signaling, plays as a mechanosensitive factor that transmits the local mechanical cues to the nucleus and tune cellular
homeostasis.24 Recent research has confirmed that the Hippo signaling integrates diverse biochemical and mechanical cell-cell and cell-extracellular matrix signals, in that way to regulate
cell proliferation and apoptosis.25 At the same time, it shows that Yap1, the coding gene of YAP, differentially regulates chondrocyte differentiation, which promotes chondrocyte
proliferation early but inhibits subsequent maturation during skeletal development.26 However, the role of Hippo signaling in lumbar spine instability (LSI) induced endplate degeneration has
not been investigated.
In this study, we investigated the role of lumbar instability induced abnormal stress in turning on the Hippo signaling that initiates cartilage endplate remodeling through activation of
osteoclast genes. Our study demonstrated that YAP is the guard for the endplate cartilage that maintains CEP homeostasis. Loss of YAP increased CCL3 release from the CEP chondrocytes, which
recruits and promotes osteoclast differentiation for cartilage endplate remodeling. Finally, we demonstrated that inhibition of Hippo signaling activation or re-supply YAP in the CEP
effectively rescued it from transforming into a cheese-like endplate and prevented the subsequent lumbar degeneration.
In clinic, cartilage endplate remodeling was found to occur in patients with lumbar spine degeneration. Radiological images showed these patients presented severe ossified endplate with
pores similar to that in the cheese (Fig. 1a). To better understand this pathology, we collected clinical samples of CEP from the patients with lumbar instability. Histological staining
showed the CEP was ossified as Collagen II expression decreased, accompanied with Collagen X and Osteocalcin expression increased (Fig. 1b). In order to investigate the mechanism of this
pathological change, lumbar spine instability (LSI) model was established on mice for the following test (Fig. 1c).1,27 Three-dimensional observations and quantified analysis by Micro-CT
(Fig. 1d) revealed significant changes in bone morphometry of the vertebral endplate at 8 weeks after surgery. Meanwhile, the Micro-CT analysis demonstrated that the porosity and trabecular
separation (Tb. Sp) of L5 vertebral CEP increased significantly in LSI-8 weeks (8w) mice relative to the sham group. Concomitant with the gross observation of the degenerated disc,
histological staining further revealed that the endplate was highly porotic. As shown by the Safranin O and fast green (SOFG) staining (Fig. 1e), it was observed the CEP region in the sham
group remained intact, while it became porotic and ossified in the LSI group. The green-stained bone matrix was completely surrounded the cavities, suggesting the interfacial soft tissue was
remodeled after LSI surgery. We used endplate score to visualize these pathological changes in the CEP of LSI mice, which was significantly higher than that in the sham group (Fig. 1f). To
further elucidate the remodeling process of CEP, the endplates of mice after LSI surgery for 8 weeks were subjected for immunofluorescent (IF) staining with Collagen II and Collagen X (Fig.
1g, h). The results showed the Collagen II expression decreased significantly, while the Collagen X started to show up in the CEP of mice from the LSI group, indicating the chondrocyte
hypertrophy and cartilage calcification processes were initiated. Meanwhile, the inflammatory factor TNF-α (Fig S1A) and apoptotic marker TUNEL (Fig S1B), along with the expression of
vascular-related marker CD31 (Fig S1C) and the nerve-related marker CGRP (Fig S1D), in the CEP pores further supported our observation of cartilage endplate remodeling in the LSI model. The
above results demonstrate LSI induced abnormal stress initiates CEP remodeling to a cheese-like ossified tissue.
Destruction of cartilage endplate in IVDD patients and LSI model. a Cheese-like endplate in patients with lumbar degeneration. b Representative H&E and immunohistochemical (IHC) staining of
endplates in patients with lumbar degeneration. c Schematic diagram of the LSI surgery. d Top, representative 3D Micro-CT images of the mouse caudal endplates of L4/5 (coronal view) at 8
weeks after LSI or sham surgery. Bottom, quantitative analysis of the total porosity and trabecular separation (Tb. Sp). e Representative SOFG staining images of the CEP in mice with LSI or
sham surgery for 8 weeks. f Endplate scores of the caudal endplates based on (e). g, h Left, representative IF staining images of the Collagen II and Collagen X (green) in the CEP. Right,
quantitative analysis of the percentage of each area in CEP. **P