Increased risk of thrombosis in jak2 v617f-positive patients with primary myelofibrosis and interaction of the mutation with the ipss score
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Dear Editor, Thrombosis remains a major unmet need in polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), accounting for three to four-fold higher
probability of risk compared to controls [1]. In PMF, this risk is poorly characterized given that, unlike PV and ET, death due to disease progression and/or evolution to leukemia represents
a competing risk that significantly overwhelms that attributable to vascular complications [2,3,4,5]. We analyzed a large cohort of PMF patients enrolled in the European ERNEST registry
with the aim to identify patients at higher risk in which anti-thrombotic prophylaxis could be suggested. Primary prophylaxis with aspirin is debated in PMF and, since thrombosis is not
considered a major issue, some experts prescribe this agent only as a secondary prophylaxis after an arterial event [6]. The ERNEST project was launched in 2013 to prospectively enroll
primary and post–polycythemia vera (post-PV) and post–essential thrombocythemia (post-ET) MF patients with the epidemiological goal of assuring reliability, representativeness and
comparability of real-word data across international centers with expertise in the management of MF [7]. The project, promoted by the European _LeukemiaNet_ (ELN), was coordinated by FROM -
Research Foundation at Papa Giovanni XXIII Hospital in Bergamo (Italy) - and supported by Novartis through a research collaboration. From February 2013 to May 2014, the ERNEST registry
enrolled 1292 MF-WHO diagnosed patients from 13 centers in 5 European countries. This manuscript reports analysis of a subset of 584 PMF patients for whom updated information until the end
of 2020 were provided by 6 centers from Italy, Spain, Sweden. Prefibrotic myelofibrosis as well as post-ET/PV myelofibrosis were not included. Descriptive statistics were used to summarize
the characteristics of PMF patients at diagnosis. Each patient was classified according to baseline IPSS and followed from the date of PMF diagnosis until occurrence of thrombosis or
censoring (last contact/study-end fixed at December, 31th 2020), whichever came first. Lower and higher-risk IPSS categories were compared grouping together low and intermediate-1 risk
categories and intermediate-2 and high-risk categories, respectively. Univariate and multivariable Fine and Gray’s competing risk models were fitted to estimate the association between
characteristics of PMF patients and post-diagnosis thrombosis, considering death as a competing event, and estimated sub-distribution Hazard Ratios (sHRs) with the corresponding 95% CIs were
reported. Cumulative incidence functions (CIF) of thrombosis were estimated using competing risk methods and compared over time using Gray’s test. The effect of cytoreductive drugs (i.e.,
hydroxyurea (HU) and ruxolitinib (Ruxo)) on thrombotic risk was investigated by adjusting for immortal time bias with the Mantel-Byar method [8]. Finally, the impact of post-diagnosis
thrombosis on mortality was estimated by a multivariable Cox model including thrombosis as a time-dependent variable. After a median follow up of 3.9 years (interquartile range: 1.8–8.2), a
total of 61 out of 584 subjects (10.4%) experienced a post-diagnosis thrombosis, corresponding to 1.82% patients per year (pt/y). Of these, 30 (5.1%; rate 0.93% pt/y) were arterial and 31
(5.3%; rate 0.89% pt/y) venous events. In univariate analysis (Supplementary Table 1S), factors that significantly distinguished patients with thrombosis were younger age, lower-risk IPSS
and _JAK2_ V617F mutation; in contrast, no difference in the frequency of _MPL_ and _CALR_ mutation or triple negative status, was recorded. Hemoglobin levels were higher in the group of
patients with thrombosis while leukocyte and platelet counts were comparable. As might be expected, the proportion of patients treated with HU tended to be higher in cases who developed
post-diagnosis thrombosis. Interestingly, pre-diagnosis arterial but not venous events were predictors of future events. In multivariable competing risk model, adjusted for previous history
of thrombosis, hemoglobin levels and cytoreductive treatment, the category of lower-risk IPSS lost the statistical significance, even though a trend towards an increased risk of thrombosis
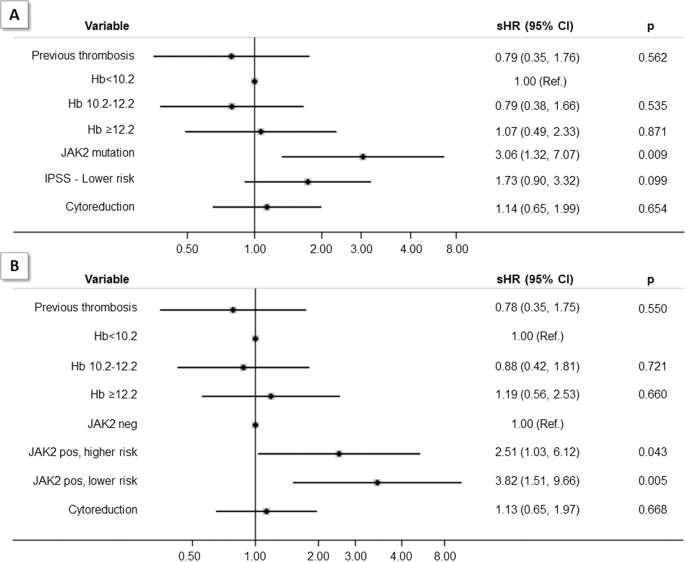
was documented (sHR = 1.73, 95% CI 0.90–3.32); in contrast, _JAK2_ mutation retained its prognostic value (sHR = 3.06, 95% CI 1.32–7.07) independently from the two IPSS groups (Fig. 1, panel
A). By adjusting for immortal time bias, we failed to show an effect of HU (_n_ = 272) and Ruxolitinib (_n_ = 69) exposure on the risk of thrombosis (sHR = 1.14, 95% CI 0.65–1.99). Few
patients in this study received aspirin (_n_ = 61) or oral anticoagulants (_n_ = 39) as primary or secondary prophylaxis thus precluding efficacy analyzes; we report major hemorrhagic events
after aspirin (_n_ = 10) and oral anticoagulants (_n_ = 4). Figure 2 illustrates the cumulative incidence function (CIF) of thrombosis stratified by IPSS (panel A) and _JAK2_ V617F mutation
(panel B), considering death as a competing event. Cumulative incidence of thrombosis after 10 years for patients with lower-risk IPSS was similar to the group of _JAK2_ V617F mutated
patients (14% and 15%). However, in patients with lower vs. higher-risk IPSS, the cumulative incidence was substantially higher (_p_ = 0.042) and the corresponding sHR was 1.73. The two
groups did not differ for generic risk factors of thrombosis such as smoking (3% vs. 2%, _p_ = ns), diabetes (20% vs. 18%, _p_ = ns) and arterial hypertension (80% vs. 65%, _p_ = ns). In
patients with _JAK2 V617F_ mutation the risk was over 3-fold higher than in un-mutated cases (sHR = 3.57, 95% CI 1.61–7.92) particularly in lower-risk IPSS patients (Fig. 2, panel C). In
this latter subgroup, in comparison with _JAK2_ unmutated patients, sHR was 4.28 (95% CI 1.86 –9.83) while for _JAK2_ V617F mutated patients in higher-risk IPSS category was 2.75 (95% CI
1.12–6.74). The combination of _JAK2 V617F_ mutation and lower-risk IPSS was independently associated with thrombosis as shown in multivariable model by adjusting for prior thrombosis,
hemoglobin levels and cytoreductive treatment (Fig. 1, panel B). We speculate that the reason for this finding may be related to most proliferative condition in _JAK2_ mutated lower-risk
IPSS patients as shown by the normal hemoglobin levels and significantly higher platelet counts (_p_ = 0.001) with respect to _JAK2_ mutated patients with higher-risk IPSS (Supplementary
Table 2S). No difference for other indicators of myeloproliferation, such as splenomegaly or _JAK2_ V617F allele burden, was found. These findings are confirmed in _JAK2_ unmutated patients
in which the likelihood of thrombosis after 10 years tended to be higher among patients included in the lower (7%) than higher-risk IPSS category (3%) (_p_ = 0.203). The effect of the
JAK2/IPSS combination on the risk of thrombosis was confirmed in an external cohort of patients with PMF (_N_ = 380) that included 115 patients with _JAK2_ V617F and lower-risk IPSS (30%),
in which the cumulative incidence of thrombosis was 15% after 10 years. Conversely, in _JAK2_ mutated and higher-risk IPSS the CIF was 9% (Dr. Barosi, Pavia, Italy). By adjusting for age,
gender, IPSS and cytoreduction, thrombosis had an independent effect on the risk of death (HR = 1.78, 95% CI 1.23–2.56). The 40 fatal thrombotic events occurred simultaneously with other
complications leading to death (i.e., MF progression (9, 22.5%), infections (3, 7.5%), bone marrow transplant toxicities (1, 2.5%), unknown causes (60%)); in 3 patients only (7.5%)
thrombosis was assigned to cause death. The strengths of this study are the large cohort of PMF patients followed for a median time of 3.9 years, the number of thrombotic events and the
external validation of the main results. The weaknesses are linked to its retrospective design with possibility of bias regarding patient selection and missing data, particularly on aspirin
primary prophylaxis. In summary, we provided evidence that PMF patients have a relevant and life-threatening risk of thrombosis and that the lower-risk IPSS categories (low/intermediate-1)
harboring the _JAK2 V617F_ mutation are at the highest risk. In clinical practice, this risk could justify studies testing primary prophylaxis with low-dose aspirin in all positive _JAK2
V617F_ patients, particularly in those with lower-risk IPSS scores, if not otherwise contraindicated. DATA AVAILABILITY Aggregated data available by request. Patient-level data will not be
shared. REFERENCES * Hultcrantz M, Björkholm M, Dickman PW. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms: a population‐based cohort study. Ann Intern
Med. 2018;168:317–25. Article PubMed PubMed Central Google Scholar * Barbui T, Carobbio A, Cervantes F, Vannucchi AM, Guglielmelli P, Antonioli E, et al. Thrombosis in primary
myelofibrosis: incidence and risk factors. Blood. 2010;115:778–82. Article CAS PubMed Google Scholar * Elliott MA, Pardanani A, Lasho TL, Schwager SM, Tefferi A. Thrombosis in
myelofibrosis: prior thrombosis is the only predictive factor and most venous events are provoked. Haematologica. 2010;95:1788–91. Article CAS PubMed PubMed Central Google Scholar * Kc
D, Falchi L, Verstovsek S. The underappreciated risk of thrombosis and bleeding in patients with myelofibrosis: a review. Ann Hematol. 2017;96:1595–604. Article PubMed Google Scholar *
Barbui T, Carobbio A, De Stefano V. Thrombosis in myeloproliferative neoplasms during cytoreductive and antithrombotic drug treatment. Res Pr Thromb Haemost. 2022;6:e12657. CAS Google
Scholar * Cervantes F. How I treat myelofibrosis. Blood. 2014;124:2635–42. Article CAS PubMed Google Scholar * Barbui T, Masciulli A, Scarano M, Tognoni G, Sisti S, Di Lelio A, et al.
Towards a better understanding of epidemiology, survival and treatment in myeloproliferative neoplasms: results of the European LeukemiaNet Registry (ERNEST study). Blood. 2014;124:1849.
Article Google Scholar * Mantel N, Byar DP. Evaluation of response-time data involving transient states: an illustration using heart-transplant data. J Am Stat Assoc. 1974;69:81–6. Article
Google Scholar Download references ACKNOWLEDGEMENTS The ERNEST registry is supported by Novartis Pharma through a research collaboration. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
FROM Research Foundation, ASST Papa Giovanni XXIII, Bergamo, Italy Tiziano Barbui, Arianna Ghirardi, Alessandra Carobbio, Arianna Masciulli & Greta Carioli * ASST Papa Giovanni XXIII,
Bergamo, Italy Alessandro Rambaldi, Maria Chiara Finazzi & Marta Bellini * University of Milan, Milan, Italy Alessandro Rambaldi & Maria Chiara Finazzi * Department of Molecular
medicine, University of Pavia, Pavia, Italy Elisa Rumi * Hematology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy Elisa Rumi, Daniele Vanni & Oscar Borsani * University of
Insubria, Varese, Italy Francesco Passamonti & Barbara Mora * ASST Sette Laghi, Varese, Italy Francesco Passamonti, Barbara Mora & Marco Brociner * CRIMM, Azienda Ospedaliera
Universitaria Careggi, Florence, Italy Paola Guglielmelli, Chiara Paoli & Alessandro Maria Vannucchi * University of Florence, Florence, Italy Paola Guglielmelli, Chiara Paoli &
Alessandro Maria Vannucchi * Hematology Department, Hospital Clínic, Barcelona, Spain Alberto Alvarez-Larran & Ana Triguero * Hematopathology Unit, Pathology Department, Hospital Clínic,
Barcelona, Spain Marta Garrote * Division of Hematology, NU Hospital Group, Uddevalla, Sweden Helna Pettersson & Björn Andréasson * Center for the Study of Myelofibrosis, IRCCS
Policlinico S. Matteo Foundation, Pavia, Italy Giovanni Barosi Authors * Tiziano Barbui View author publications You can also search for this author inPubMed Google Scholar * Arianna
Ghirardi View author publications You can also search for this author inPubMed Google Scholar * Alessandra Carobbio View author publications You can also search for this author inPubMed
Google Scholar * Arianna Masciulli View author publications You can also search for this author inPubMed Google Scholar * Greta Carioli View author publications You can also search for this
author inPubMed Google Scholar * Alessandro Rambaldi View author publications You can also search for this author inPubMed Google Scholar * Maria Chiara Finazzi View author publications You
can also search for this author inPubMed Google Scholar * Marta Bellini View author publications You can also search for this author inPubMed Google Scholar * Elisa Rumi View author
publications You can also search for this author inPubMed Google Scholar * Daniele Vanni View author publications You can also search for this author inPubMed Google Scholar * Oscar Borsani
View author publications You can also search for this author inPubMed Google Scholar * Francesco Passamonti View author publications You can also search for this author inPubMed Google
Scholar * Barbara Mora View author publications You can also search for this author inPubMed Google Scholar * Marco Brociner View author publications You can also search for this author
inPubMed Google Scholar * Paola Guglielmelli View author publications You can also search for this author inPubMed Google Scholar * Chiara Paoli View author publications You can also search
for this author inPubMed Google Scholar * Alberto Alvarez-Larran View author publications You can also search for this author inPubMed Google Scholar * Ana Triguero View author publications
You can also search for this author inPubMed Google Scholar * Marta Garrote View author publications You can also search for this author inPubMed Google Scholar * Helna Pettersson View
author publications You can also search for this author inPubMed Google Scholar * Björn Andréasson View author publications You can also search for this author inPubMed Google Scholar *
Giovanni Barosi View author publications You can also search for this author inPubMed Google Scholar * Alessandro Maria Vannucchi View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS TB conceived and designed the study, supervised the analysis and wrote the paper. AM directed the project. AC, AG and GC performed statistical
analysis. GB provided data for external validation. AR, MCF, MB, ER, DV, OB, FP, BM, MB, PG, CP, AAL, AT, MG, HP, BA, AMV collected data. All authors revised and approved the final version
of the manuscript. CORRESPONDING AUTHOR Correspondence to Tiziano Barbui. ETHICS DECLARATIONS COMPETING INTERESTS TB has received personal fees as an advisory board member from AOP Orphan
Pharmaceuticals, Italfarmaco, and Novartis, outside the submitted work. AR has received personal fees as an advisory board member from Astellas, Amgen, Celgene BMS, Gilead, Italfarmaco,
Novartis, Omeros, Roche, and Sanofi. PG has received speaker honoraria from Novartis and AbbVie and fees from Novartis and AbbVie for participation on advisory boards. AMV has received
personal fees for serving on the advisory board and speaker’s fees from AOP Orphan Pharmaceuticals, Incyte, BMS, and Novartis. The remaining authors declare no competing financial interests.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
TABLE 1S TABLE 2S RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Barbui, T., Ghirardi, A., Carobbio, A. _et al._ Increased risk of thrombosis in JAK2 V617F-positive patients with primary myelofibrosis and interaction of the mutation with
the IPSS score. _Blood Cancer J._ 12, 156 (2022). https://doi.org/10.1038/s41408-022-00743-0 Download citation * Received: 16 September 2022 * Revised: 13 October 2022 * Accepted: 14
October 2022 * Published: 16 November 2022 * DOI: https://doi.org/10.1038/s41408-022-00743-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative