Ex vivo pulsed dendritic cell vaccination against cancer
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT As the most powerful antigen-presenting cell type, dendritic cells (DCs) can induce potent antigen-specific immune responses in vivo, hence becoming optimal cell population for
vaccination purposes. DCs can be derived ex vivo in quantity and manipulated extensively to be endowed with adequate immune-stimulating capacity. After pulsing with cancer antigens in
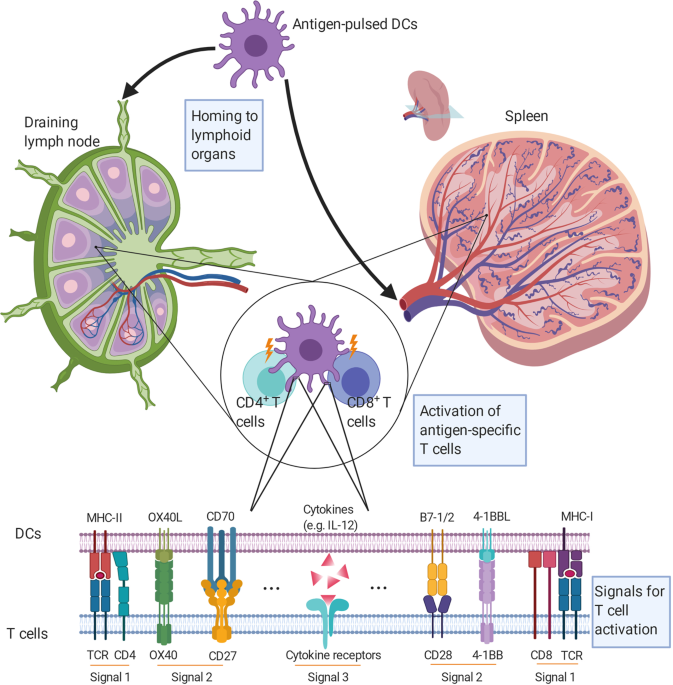
various ways, the matured DCs are administrated back into the patient. DCs home to lymphoid organs to present antigens to and activate specific lymphocytes that react to a given cancer. Ex
vivo pulsed DC vaccines have been vigorously investigated for decades, registering encouraging results in relevant immunotherapeutic clinical trials, while facing some solid challenges. With
more details in DC biology understood, new theory proposed, and novel technology introduced (featuring recently emerged mRNA vaccine technology), it is becoming increasingly likely that ex
vivo pulsed DC vaccine will fulfill its potential in cancer immunotherapy. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS A
DENDRITIC CELL VACCINE FOR BOTH VACCINATION AND NEOANTIGEN-REACTIVE T CELL PREPARATION FOR CANCER IMMUNOTHERAPY IN MICE Article Open access 29 November 2024 IMMUNOTHERAPY WITH CONVENTIONAL
TYPE-1 DENDRITIC CELLS INDUCES IMMUNE MEMORY AND LIMITS TUMOR RELAPSE Article Open access 09 April 2025 ANTITUMOUR DENDRITIC CELL VACCINATION IN A PRIMING AND BOOSTING APPROACH Article 06
August 2020 INTRODUCTION Vaccination is a type of immunotherapy that is effective in the treatment of cancer. It provides the immune system with potential antigens for recognition and
usually activates antigen-specific lymphocytes via presentation of antigens by dendritic cells (DCs), which are the most adept cells regarding antigen uptake and processing. Activated
lymphocytes, especially T cells, assume effector functions such as cytotoxicity and cytokine production to controlling cancer progression. Before the prevalence of dendritic cell vaccines,
studies on cell-based vaccines focused on inactivated tumor cells that were engineered for enhanced immunogenicity. The so-called whole-cell cancer vaccines generally require antigen uptake
by endogenous DCs, which is a process that is relatively limited in efficiency. This strategy is reasonably advantageous because the antigens are delivered directly to DCs for maximum
efficacy, and this can be done either by ex vivo pulsing or in vivo targeting of DCs. Ex vivo pulsing of DCs is a process in which DCs that are derived from autologous origins are loaded
with antigens and matured under favorable ex vivo conditions. The resulting DCs are then administered back to the patient to initiate protective immune responses. Compared with in vivo
targeting, ex vivo pulsing of DCs has a lower risk, higher efficiency, and fewer technical difficulties. U.S. FDA approval of Sipuleucel-T, a vaccine against late-stage castration-refractory
prostate cancer and notably the first therapeutic DC vaccine against cancer, ushered cancer immunotherapy into a new era. Although numerous DC-based cancer vaccines have entered clinical
trials in recent years and have registered encouraging results, many issues have yet to be addressed. In this review, we discuss recent progress in and the future of vaccination with ex vivo
pulsed DCs against cancer, with an emphasis on emerging mRNA-pulsed DC vaccines. BIOLOGY OF DCS In 1973, Steinman and Cohn discovered a cell population with branching processes and named
these cell DCs after the Greek word for tree (dendreon) [1]. Decades of in-depth study of this heterogeneous population finally verified that they are the most potent professional
antigen-presenting cells, which bridge the gap between innate and adaptive immunity and play a key role in eliciting adaptive immune responses. Steinman was awarded the Nobel Prize in 2011
for the remarkable discovery of DCs. DCs exist in two sequential stages, immature and mature DCs (mDCs) after. Most DCs in the body are immature, and they populate peripheral nonlymphoid
tissues, specialize in antigen uptake, express high levels of phagocytosis-related receptors and low levels of costimulatory molecules, such as CD80 and CD86, as well as adhesion molecules,
such as ICAM, and exhibit weak antigen-presenting capacity; hence, these cells are relatively incompetent at activating T cells [2]. Immature DCs (iDCs) migrate to peripheral lymphoid organs
after ingestion of antigen and stimulation by inflammatory factors. Maturation proceeds during migration, resulting in DCs that express high levels of major histocompatibility complex (MHC)
molecules and costimulatory molecules, such as CD40, CD70, CD80, and CD86, and secrete interleukin 12 (IL-12), IL-6, TNF-α, and IP-10. Furthermore, mDCs express increased levels of C–C
chemokine receptor type 7, a chemokine receptor that is responsible for lymph node homing. These molecules are vital for effective T-cell activation [3]. mDCs present antigens to and
activate T cells in the lymph nodes. Full activation of naïve T cells depends on the orchestration of three distinct signals. DCs present peptide-MHC complexes to T cells for recognition by
specific T-cell receptors, and the CD3 complex transduces an antigen recognition signal (Signal 1) into T cells. Signal 2 is determined by a balance between costimulatory and coinhibitory
molecules on DCs (CD80, CD86, PD-L1/2, CD40, CD70, OX40L, 4-1BBL, etc.) and T cells (CD28, CTLA-4, PD-1, CD40L, CD27, OX40, 4-1BB, etc.). Signal 3 is from the cytokine environment, which
regulates the proliferation, differentiation, and immune memory of T cells (Fig. 1). For example, IL-12 secreted by DCs promotes Th1 (T helper 1) immune responses, while IL-23, IL-6, and
IL-1β stimulate Th17 immune responses [4,5,6]. Activated T cells, such as cancer-specific cytotoxic T lymphocytes (CTLs), leave peripheral lymphoid organs for cancer foci, where they exert
their anticancer function. Moreover, DCs interact with natural killer (NK) cells and further boost anticancer effects. Recently, it was found that mDCs enhance NK cell proliferation,
activation, and cytotoxicity through the interaction of DC-expressed CX3CL1 and NK cell-expressed CX3CR1, as well as through the secretion of cytokines, such as IL-12, IL-15, and IL-18 [7].
VACCINATION WITH EX VIVO PULSED DENDRITIC CELLS—THE BASICS AND THE REGULAR STORY PREPARATION OF DCS Despite their general presence in most tissues, the absolute numbers of DCs are low. For
example, mDCs account for only ~1% of total peripheral blood mononuclear cells (PBMCs). As ex vivo derivation of DCs improves, multiple precursor cells can be used to prepare DCs, such as
nonproliferative CD14+ monocytes from peripheral blood and proliferative CD34+ precursor cells from bone marrow and the umbilical blood [8, 9]. In addition, various cell types can be
redirected to a dendritic cell fate either by direct transdifferentiation or indirect dedifferentiation followed by redifferentiation (Fig. 2). CD14+ monocytes constitute ~10% of PBMCs, and
DCs derived from peripheral blood monocytes (MoDCs) have been extensively studied and applied. In 1994, Sallusto and Romani established a method for the induction of DCs from monocytes by
granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4. GM-CSF sustains differentiation toward and subsequent development of DCs, while IL-4 suppresses the proliferation of
macrophages and granulocytes and prevents the differentiation of monocytes toward macrophages [10, 11]. Two years later, Romani and Zhou made an improved protocol public. They obtained iDCs
after induction for 6–7 days with GM-CSF and IL-4 and then developed mDCs after stimulation for 3 days with activating factors such as TNF-α. This method also registered the first successful
effort to replace bovine serum with human plasma in culture, which lays the foundation for the clinical application of ex vivo-derived DCs [12, 13]. To date, more ex vivo derivation
protocols of MoDCs have been explored, such as replacing IL-4 with IL-15 or interferon α (IFN-α) in the presence of GM-CSF to bolster the activation potency of DCs [14,15,16]. Apart from the
classical 1-week protocol, researchers invented fast protocols with which DCs could be harvested after 2–3 days of culture [17]. The maturation of DCs was commonly performed by adding the
gold standard cocktail (TNF-α, IL-1β, IL-6, and prostaglandin E2) to cultures [18]. Later, different teams developed somewhat similar modifications to the formulation (IFN-γ and LPS/MPLA)
[19, 20]. MoDCs cannot be propagated ex vivo; as a result, their application is somewhat limited. CD34+ hematopoietic stem/progenitor cells (HSPCs) can be used to prepare DCs in large
amounts ex vivo. These DCs (34DCs) are superior to MoDCs in that they elicit more potent T-cell immune responses against cancer by upregulating the expression of tumor necrosis
factor-related apoptosis-inducing ligand and enhancing cytotoxicity [21, 22]. Derivation of robust 34DCs was typically achieved in a cytokine milieu that differed from that for MoDC
derivation. The combination of fms-related tyrosine kinase 3 ligand (Flt3L), thrombopoietin (TPO), and stem cell factor (SCF) was one of the first documented formulations for such a purpose.
Alternatively, culturing in the presence of Flt3L, SCF, IL-3, and IL-6 for 3 weeks before switching to culture in the presence of Flt3L, TPO, and SCF for 1 week worked similarly well [23].
Recently, it was reported that the inclusion of Notch ligand Delta-like 1 (DLL1) in the original formulation of GM-CSF, Flt3L, and SCF significantly improved the induction of bona fide type
1 conventional DCs (cDC1s), which specialize in priming CD8+ CTLs, from CD34+ HSPCs [24]. Efforts to dissect the hematopoietic progenitor subsets that give rise to 34DCs, especially cDC1s,
revealed that, aside from the widely recognized common myeloid progenitor lineage, a large proportion of multipotent lymphoid progenitors share the same potential [25]. As the understanding
of the signals that govern cell differentiation continued to deepen, fresh strategies for deriving DCs were developed. Forced coexpression of PU.1, IRF8, and BATF3 transdifferentiated
fibroblasts into cDC1s that were competent for cell therapy [26]. Alternatively, various cell types can be efficiently dedifferentiated into induced pluripotent stem cells (iPSCs) before
being redifferentiated into DCs [27]. This process is a once-and-for-all solution that generates DCs in unprecedented quantities, although it is currently not time- or budget-friendly.
Recently, it was demonstrated that iPSCs derived from primary DCs were a superior source of DCs for immunotherapy, as the epigenetic imprints retained after dedifferentiation helped to
ensure high immunogenicity of the generated DCs [28]. PULSING OF DCS WITH CANCER ANTIGENS The pulsing of DCs with cancer antigens is the key step in preparing DC vaccines. DCs are usually
pulsed with whole-cell antigens from ultrasonicated or repeatedly frozen and thawed cancer samples, synthetic cancer antigenic peptides, DNA or RNA from cancer cells, and exosomes derived
from cancer cells [29,30,31,32] (Fig. 2). Tumor antigenic peptides are synthesized according to verified or predicted epitopes in cancer antigens and are used in many clinical trials to
pulse DCs. However, there are considerable weaknesses in this approach. For example, epitopes are restricted by the HLA (human leukocyte antigen) type of the patients, most cancer antigenic
epitopes are not yet elucidated, and peptides may only serve to elicit either CD4+ or CD8+ T-cell responses [33]. Furthermore, the half-life of the HLA-antigenic peptide complex is
relatively short, thus limiting the duration of presentation [34]. Exosomes from cancer cells carry abundant cancer antigens, and exosome-pulsed DCs are more efficacious than tumor lysates,
presumably because DNA in exosomes activates DCs through the cGAS/STING pathway and promotes DC maturation and presentation, thereby eliciting more potent immune responses against cancer
[35, 36]. However, it remains difficult to obtain large quantities of highly purified exosomes. Apart from the aforementioned method of loading, pulsing DCs ex vivo with mRNAs encoding
cancer antigens has emerged conspicuously in recent years, which will be discussed later in this review. CONVENTIONAL CLINICAL APPLICATIONS OF DC VACCINES DC vaccines have a similar clinical
objective response rate (ORR) to other conventional therapies against cancer. For example, the ORR of melanoma patients receiving DC immunotherapy is 8.5%, which is similar to the rate of
those receiving the first-line drug dacarbazine. Likewise, the ORRs of patients with prostate cancer, malignant glioma, and renal cell carcinoma receiving DC immunotherapy are 7.1%, 15.6%,
and 11.5%, respectively, which are similar to the rates of those receiving conventional chemotherapy [37]. In a phase III clinical trial of a DC vaccine for the treatment of glioblastoma,
all enrolled patients were randomly grouped after surgery and chemotherapy. The treatment group received the autologous DC vaccine (DCVax-L) and temozolomide, while the control group
received temozolomide and a placebo. DCVax-L was prepared from autologous DCs that were activated by lysates of surgically excised tumor tissues. The results indicated that the treatment
group achieved a median overall survival (mOS) of 23.1 months compared with a mOS of only 15–17 months for those who received surgery alone. In addition, 67 patients (30%) survived longer
than 30 months, 44 (24.2%) survived longer than 36 months, the regimen was well tolerated, with only 2.1% (7/331) of the patients exhibiting Grade III–IV adverse events [38]. Vaccination of
melanoma patients with DCs pulsed with mutated peptides of neoantigens enhanced T-cell immune responses directed against not only dominant neoantigens but also subdominant ones, thus
expanding the breadth of reaction and strengthening the potency of the vaccine [39]. Preconditioning with recall antigens such as Tetanus toxoid unilaterally before vaccination with DCs
pulsed with cancer antigens stimulated bilateral DC migration to lymph nodes that drained the vaccination sites, thereby enhancing the efficacy of DC vaccination. The effects depended on
both the CD4+ T-cell recall response to antigens used for preconditioning and the host CCL3 chemokine [40]. In a trial of 34DC immunotherapy of melanoma, 18 enlisted _HLA A*0201__+_ patients
were administered 34DCs pulsed with antigenic peptides of melanoma-associated tyrosinase, gp100, MART-1, and MAGE-3 antigens, along with those of control antigens. Sixteen patients
responded to at least one melanoma antigen, and ten responded to more than two melanoma antigens. Of the ten potential good responders, only one had progressive disease, and seven
experienced regression of metastases [41]. The results of these and many other studies [42,43,44] suggest that vaccination with ex vivo pulsed DCs is efficacious against cancer with minor,
if any, side effects. The anticancer efficacy of DC vaccines can be reinforced once procedures, such as loading DCs with tumor antigens, culture of DCs ex vivo and the route of
administration, are further optimized (Figs. 2, 3). NEW EMERGENCE OF MRNA-PULSED DC VACCINES AGAINST CANCER MRNA FOR DC PULSING mRNA is widely recognized as an ideal tool for the preparation
of DC vaccines [45, 46] because of its unique traits. mRNA does not integrate into the genome, avoiding any potential insertional mutagenesis. mRNA can be readily produced in large amounts
in vitro in a process that is both technologically mature and cost-efficient. mRNA can be engineered to increase immunogenicity and reduce inhibition of its translation. mRNA is degraded by
physiological mechanisms, facilitating the control of effects in a timed fashion. mRNA is not subject to splicing as pre-mRNA is, eliminating any uncertainty in protein products due to
alternative splicing. After the introduction of mRNA into DCs, specific T-cell responses targeting multiple epitopes can be elicited, mitigating the risk of immune evasion through antigen
variation [47]. Since mRNA-pulsed DC vaccines have stepped into the research spotlight, a paradigm shift away from DC vaccines pulsed in a conventional fashion is taking place. mRNA used for
DC pulsing includes cancer-derived and in vitro transcribed mRNA. Cancer-derived mRNA conveys the full repertoire of epitopes of a given cancer, expanding the range of antigens to which the
immune system responds, thus preventing evasion resulting from antigen downregulation or loss. However, preparing tumor-derived mRNA requires either a large number of tumor cells or
amplification of isolated mRNA. In either case, the majority of all mRNAs encode unaltered self-antigens, a small portion of which (tissue-specific, mutated, and aberrantly expressed ones)
are deemed as potentially appropriate targets for vaccination. The mRNAs that encode altered self-antigens and foreign antigens may become underrepresented or even lost during processing.
Moreover, the process is both time-consuming and laborious and is thus unfavorable for clinical application. mRNA encoding cancer antigens can be transcribed in vitro from templates of open
reading frame (ORF)-containing plasmids or other DNA fragments. Rational design of cap, 5′ and 3′ untranslated regions (UTRs) and poly (A) tail structure of mRNAs, and even the sorting
signals attached to the antigen, as well as the nucleotide sequence of the ORF itself and the introduction of modified nucleotides, strengthens mRNA stability, enhances translation, improves
antigen processing and presentation, avoids vigorous recognition by innate immune sensors, and culminates in augmented antigen-specific immune responses [48,49,50,51]. It has become
increasingly clear that apart from CD8+ T cells, CD4+ T cells, especially Th1 cells, are important participants in anticancer immunity [52]. mRNA-encoded proteins are synthesized in the
cytosol of DCs and are readily processed to antigenic peptides that then associate with MHC class I molecules and are presented to CD8+ T cells. However, activation of CD4+ T cells depends
on the MHC class II presentation pathway, which is not readily accessible to nonsecretory proteins in most cases. The usual solution to this problem is targeting these antigens to lysosomes
by means of fusion to lysosomal sorting signals so that both CD4+ and CD8+ arms of T-cell responses can be generated against cancer antigens [53, 54]. The strategies discussed above are far
from comprehensive, as the effects of DC vaccines are determined by multiple factors, such as the balance between costimulatory and coinhibitory molecules, as well as the balance between
activating and suppressive cytokines. In light of this, there was development of the TriMix formulation, which is a mixture of constitutively activated Toll-like receptor (TLR) 4, CD40L, and
CD70 mRNAs [55, 56]. The results of various preclinical and clinical studies on TriMix indicated that it boosted the immunostimulatory function of DCs. More importantly, education by
TriMix-DCs reprogrammed regulatory T cells (Tregs) to function like Th1 cells. An unprecedented ORR of 27% was recorded in Stage III/IV melanoma patients who were treated with TriMix-DCs
(NCT01066390) [57]. INTRODUCTION OF MRNA INTO DCS The pioneering effort of pulsing DCs with mRNAs encoding cancer antigens was developed by Boczkowski et al. at Duke University in the late
1990s [58]. In that study, mRNA was engulfed by DCs by macropinocytosis, which was problematic in that exogenous mRNA triggers signal transduction by the TLR-7 pattern recognition receptor
(PRR) pathway, and DCs activated by this signaling promptly curtail the ingestion of mRNA [59,60,61]. In addition, only a small fraction of mRNAs survived endosomal delivery, gained access
to the cytosol, and were translated into proteins. The methods of introducing mRNA into DCs have evolved significantly since then. Electroporation is probably the most widely adopted method
for the introduction of mRNA into DCs. Unlike DNA, mRNA does not have to enter the nucleus to exert its function. Therefore, a relatively weak electric pulse is sufficient for the delivery
of mRNA into the cytosol, greatly alleviating damage to the cells [62, 63]. In addition, since mRNA introduced by electroporation avoids the endosomal route where the mRNA-responsive PRRs
reside, unnecessary and potentially harmful recognition of mRNA by the innate immune system is avoided. Sonoporation exploits ultrasound to trigger the implosion of mRNA-loaded microbubbles
on target cells, thus forcing entry of mRNA into cells [64]. Nanofection delivers mRNA into cells in the form of a nanomaterial-mRNA complex, which typically travels via the endocytic
pathway and ultimately releases its cargo into the cytosol [65]. CLINICAL TRIALS OF MRNA-PULSED DC VACCINES AGAINST CANCER Numerous studies have confirmed that autologous DC vaccines
prepared with mRNAs encoding cancer antigens are both safe and efficacious. The last two decades witnessed more than 40 clinical trials adopting such a strategy (Table 1). In these trials,
mRNAs produced by in vitro transcription, as well as those derived from autologous cancers or cancer stem cells, were used to load DCs with cancer antigens. Aside from the origins of the
mRNAs, these trials also differed in activation tactics, varying from applying proinflammatory cytokines to co-delivering TriMix mRNAs. These DC vaccines were tested in the treatment of
ovarian cancer, mammary cancer, late-stage melanoma, leukemia, malignant glioma, mesothelioma, pancreatic cancer, esophageal cancer, myeloma, lung cancer, etc. For most trials, DC vaccines
were administered intradermally or intravenously, with some exceptions for intranodal and intratumoral administration. In a trial involving 15 late-stage melanoma patients (NCT01066390) who
received treatment of TriMix-DCs with mRNA encoding MAGE-A3, MAGE-C2, tyrosinase, and gp100 antigens (TriMix-DC-MEL), two achieved complete response, and another two achieved partial
response. In six of twelve patients, antigen-specific skin-infiltrating lymphocytes were detected, and in four of five patients, antigen-specific CD8+ T cells were detected in the blood
[57]. Also noteworthy is that recent trials tended to combine chemotherapy or antibody-targeted therapy with DC vaccines to strengthen efficacy (NCT00626483, NCT02649829, NCT02366728, and
NCT02649582). In a phase I trial of autologous Langerhans-type DCs pulsed with xenogeneic TRP-2 mRNA, stage IIB to IV melanoma patients who had their tumors resected were vaccinated 5 times
at 2-week intervals. Six out of nine participants stayed disease-free for a median of 51.1 months. The patients developed clinical outcome-related immune responses, including activation and
increased clonality of T cells and secretion of proinflammatory cytokines, and showed minimal signs of toxicity [66]. In addition, enhanced antitumor activities were also observed in
combinatorial treatment with mRNA-pulsed DCs and immune checkpoint inhibitors. In 2011, a phase II trial for the treatment of unresectable stage III/IV melanoma with TriMix-DCs and the
anti-CTLA-4 monoclonal antibody ipilimumab (NCT01302496) was performed, in which the 6-month disease control rate was 51%, the overall response rate was 38% (eight complete responses and
seven partial responses), and there were still seven complete responses and one partial tumor response after a median follow-up time of 36 months [67]. OUTLOOK DCs play pivotal roles in
initiating adaptive immune responses, and exploiting DCs for anticancer therapy is a promising strategy. However, it still faces challenges that call for imperative improvements. Functional
impairments were observed in endogenous DCs in peripheral blood and the tumor-draining lymph nodes of cancer patients [68], as well as DCs derived ex vivo from monocytes of cancer patients
[69]. These dysfunctions were only partially reverted by tumor resection [68] or transforming growth factor β (TGF-β) blockade [69], suggesting that further countermeasures must be taken to
erase adverse imprints left by tumors on DCs and DC precursors. Culture of DCs ex vivo offers a unique window of time in which extensive direct interventions can be implemented in DCs to
correct their functional defects. It was estimated that as few as 85 DCs are sufficient for stimulating a T-cell immune response [70]. The recruitment of naive T cells is highly efficient
[71]. It might be slightly counterintuitive that DC vaccination involving the administration of a large amount of pulsed DCs has resulted in relatively limited success. DC subsets,
derivation protocols, activation status, antigen loading, route of administration, vaccination schedules, mitigation of immunosuppression by cancer, etc. are all factors that determine the
outcome of DC vaccination (Fig. 3). DCs are heterogeneous cell populations that share similar characteristics while varying in origins and detailed functions. For most preclinical studies of
DC vaccination, the subjects are bone marrow-derived DCs (BMDCs), and for most clinical trials, MoDCs are frequently used. These subsets, while from different species, are both believed to
be counterparts of inflammatory DCs that exist in vivo rather than resident DCs [72, 73]. Inflammatory DCs act as replacements or complements of migratory cDC1s during inflammation [74] and
can present antigens by themselves or transfer antigens to lymphoid tissue-resident DCs for efficient presentation. The latter process is important, as not all migratory DCs are well
equipped for T-cell priming by themselves [75, 76]. Therefore, it might be worth attempting to derive DCs that resemble lymphoid-tissue resident DCs for vaccination purposes [77] and further
discern which DC subset is most appealing to use in vaccinations. In fact, the notion of the MoDC vaccine was challenged from inside by a recent study asserting that vaccines based on
undifferentiated monocytes were superior to DC vaccines in terms of anticancer efficacy in multiple murine tumor models. The anticancer efficacy depended on peptide transfer from monocytes
to CD8+ splenic DCs (murine equivalents of human CD141+ DCs in terms of cross-presentation activity [78]) through connexin 43 gap junctions for presentation. However, connexin 43 gap
junctions were permeable to peptides of no more than 11 amino acid residues; therefore, trafficking of only MHC class I-restricted rather than MHC class II-restricted peptides through such
junctions was possible [79]. The extent of and the mechanisms by which CD4+ T-cell responses are elicited by monocyte-based cellular vaccines have to be fully elucidated before this new
vaccine formulation can impact cancer immunotherapy. Current designs place elements from α and/or β globin genes in the 5′ and/or 3′ UTR of mRNAs to eventually increase protein productivity
[60, 80, 81]; however, by exploring the repertoire of cellular and viral elements and with the help of computer-aided design, more powerful factors might be discovered for this purpose. In
addition, many genes exert their influence on cancer immunity, and so it is expected that modulation of their expression and function by introduction of mRNAs other than those components of
TriMix might trigger even more effective anticancer immune responses. One cannot expect cancers to be easily controlled or even eradicated solely by a single approach. Combinatorial regimens
have been and will always be a topic of heated investigation. As illustrated in Fig. 3, induction of stronger immune responses against cancer antigens and amelioration of the tumor
microenvironment to facilitate infiltration and functioning of immune cells (activated T included) act in synergy to eventually attain pronounced regression of tumor nodules and elimination
of micrometastases. Recent studies of combinatorial therapy marked the latest efforts toward that ultimate goal [82,83,84,85,86]. The CRISPR base editing system guides deaminases to specific
genomic locations and changes DNA sequences, thus abrogating the expression of specific genes without causing double-stranded breaks [87]. With this powerful genetic tool, DCs can be
liberated from suppression by various negative regulators, such as the SOCS family, TGF-β receptor, and IL-10 receptor. This can be done ex vivo by introduction of relevant ribonucleoprotein
or the combination of relevant mRNA and sgRNA (single guide RNA) into DCs. Hopefully, increasingly potent DC vaccines can be generated through this modification process. Ex vivo pulsed DC
vaccines are an important platform into which new ideas and technologies have been continuously introduced. Hopes are high that, by bringing in these impetuses, DC vaccines will be properly
armed to become a regular option for cancer immunotherapy. REFERENCES * Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology,
quantitation, tissue distribution. J Exp Med. 1973;137:1142–62. CAS PubMed PubMed Central Google Scholar * Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology.
2018;154:3–20. CAS PubMed PubMed Central Google Scholar * Anguille S, Smits EL, Bryant C, Van Acker HH, Goossens H, Lion E, et al. Dendritic cells as pharmacological tools for cancer
immunotherapy. Pharmacol Rev. 2015;67:731–53. CAS PubMed Google Scholar * Terhune J, Berk E, Czerniecki BJ. Dendritic cell-induced Th1 and Th17 cell differentiation for cancer therapy.
Vaccines. 2013;1:527–49. CAS PubMed PubMed Central Google Scholar * Leal Rojas IM, Mok WH, Pearson FE, Minoda Y, Kenna TJ, Barnard RT, et al. Human blood CD1c+ dendritic cells promote
Th1 and Th17 effector function in memory CD4+ T cells. Front Immunol. 2017;8:971. PubMed PubMed Central Google Scholar * Chow KV, Lew AM, Sutherland RM, Zhan Y. Monocyte-derived dendritic
cells promote Th polarization, whereas conventional dendritic cells promote Th proliferation. J Immunol. 2016;196:624–36. CAS PubMed Google Scholar * Thomas R, Yang X. NK-DC crosstalk in
immunity to microbial infection. J Immunol Res. 2016;2016:7. Google Scholar * Unal A, Birekul A, Unal C, Karakus E, Köker Y. Dendritic cell production from allogeneic donor CD34+ stem
cells and mononuclear cells; cancer vaccine. Blood. 2016;128:5723. Google Scholar * Plantinga M, de Haar CG, Dünnebach E. van den Beemt DAMH, Bloemenkamp KWM, Mokry M, et al.
Cord-blood-stem-cell-derived conventional dendritic cells specifically originate from CD115-expressing precursors. Cancers. 2019;11:pii: E181. Google Scholar * Romani N, Gruner S, Brang D,
Kämpgen E, Lenz A, Trockenbacher B, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. CAS PubMed Google Scholar * Sallusto F, Lanzavecchia A.
Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colonystimulating factor plus interleukin 4 and downregulated by tumor
necrosis factor alpha. J Exp Med. 1994;179:1109–18. CAS PubMed Google Scholar * Romani N, Reider D, Heuer M, Ebner S, Kämpgen E, Eibl B, et al. Generation of mature dendritic cells from
human blood An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–51. CAS PubMed Google Scholar * Zhou LJ, Tedder TF. CD14+ blood monocytes can
differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci U S A. 1996;93:2588–92. CAS PubMed PubMed Central Google Scholar * Van Acker HH, Anguille S, De Reu H,
Berneman ZN, Smits EL, Van Tendeloo VF, et al. Interleukin-15-cultured dendritic cells enhance anti-tumor gamma delta T cell functions through IL-15 secretion. Front Immunol. 2018;9:658.
PubMed PubMed Central Google Scholar * Versteven M. Abstract B137: preclinical evaluation of a Wilms’ tumor protein 1-targeted interleukin-15 dendritic cell vaccine: T-cell activity and
batch production. Cancer Immunol Res. 2019;7:B137. Google Scholar * Mohty M, Mohty M, Vialle-Castellano A, Nunes JA, Isnardon D, Olive D, et al. IFN-alpha skews monocyte differentiation
into Toll-like receptor 7-expressing dendritic cells with potent functional activities. J Immunol. 2003;171:3385–93. CAS PubMed Google Scholar * Brabants E, Heyns K, De Smet S, Devreker
P, Ingels J, De Cabooter N, et al. An accelerated, clinical-grade protocol to generate high yields of type 1-polarizing messenger RNA–loaded dendritic cells for cancer vaccination.
Cytotherapy. 2018;20:1164–81. CAS PubMed Google Scholar * Jonuleit H, Kühn U, Müller G, Steinbrink K, Paragnik L, Schmitt E, et al. Pro-inflammatory cytokines and prostaglandins induce
maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–42. CAS PubMed Google Scholar * Vopenkova K, Mollova K, Buresova
I, Michalek J. Complex evaluation of human monocyte-derived dendritic cells for cancer immunotherapy. J Cell Mol Med. 2012;16:2827–37. CAS PubMed PubMed Central Google Scholar * Massa
C, Thomas C, Wang E, Marincola F, Seliger B. Different maturation cocktails provide dendritic cells with different chemoattractive properties. J Transl Med. 2015;13:175. PubMed PubMed
Central Google Scholar * Shinde P, Melinkeri S, Santra MK, Kale V, Limaye L. Autologous hematopoietic stem cells are a preferred source to generate dendritic cells for immunotherapy in
multiple myeloma patients. Front Immunol. 2019;10:1079. CAS PubMed PubMed Central Google Scholar * Bernhard H, Disis ML, Heimfeld S, Hand S, Gralow JR, Cheever MA, et al. Generation of
immunostimulatory dendritic cells from human CD34+ hematopoietic progenitor cells of the bone marrow and peripheral blood. Cancer Res. 1995;55:1099–104. CAS PubMed Google Scholar *
Bontkes HJ, De Gruijl TD, Schuurhuis GJ, Scheper RJ, Meijer CJ, Hooijberg E, et al. Expansion of dendritic cell precursors from human CD34+ progenitor cells isolated from healthy donor
blood; growth factor combination determines proliferation rate and functional outcome. J Leukoc Biol. 2002;72:321–9. CAS PubMed Google Scholar * Kirkling ME, Cytlak U, Lau CM, Lewis KL,
Resteu A, Khodadadi-Jamayran A, et al. Notch signaling facilitates in vitro generation of cross-presenting classical dendritic cells. Cell Rep. 2018;23:3658–72. e3656. CAS PubMed PubMed
Central Google Scholar * Helft J, Anjos-Afonso F, van der Veen AG, Chakravarty P, Bonnet D, Reis e Sousa C, et al. Dendritic cell lineage potential in human early hematopoietic
progenitors. Cell Rep. 2017;20:529–37. CAS PubMed PubMed Central Google Scholar * Rosa FF, Pires CF, Kurochkin I, Ferreira AG, Gomes AM, Palma LG, et al. Direct reprogramming of
fibroblasts into antigen-presenting dendritic cells. Sci Immunol. 2018;3:pii: eaau4292. Google Scholar * Senju S, Haruta M, Matsumura K, Matsunaga Y, Fukushima S, Ikeda T, et al. Generation
of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther. 2011;18:874–83. CAS PubMed Google Scholar * Horton C, Davies TJ, Lahiri P,
Sachamitr P, Fairchild PJ. Induced pluripotent stem cells reprogrammed from primary dendritic cells provide an abundant source of immunostimulatory dendritic cells for use in immunotherapy.
Stem Cells. 2020;38:67–79. CAS PubMed Google Scholar * Leone DA, Rees AJ, Kain R. Dendritic cells and routing cargo into exosomes. Immunol Cell Biol. 2018;96:683–93. CAS Google Scholar
* Saxena M, Bhardwaj N. Re-emergence of dendritic cell vaccines for cancer treatment. Trends Cancer. 2018;4:119–37. CAS PubMed PubMed Central Google Scholar * Bol KF, Schreibelt G,
Rabold K, Wculek SK, Schwarze JK, Dzionek A, et al. The clinical application of cancer immunotherapy based on naturally circulating dendritic cells. J Immunother Cancer. 2019;7:109. PubMed
PubMed Central Google Scholar * Gross S, Erdmann M, Haendle I, Voland S, Berger T, Schultz E, et al. Twelve-year survival and immune correlates in dendritic cell-vaccinated melanoma
patients. JCI Insight. 2017;2:e91438. PubMed Central Google Scholar * Mastelic-Gavillet B, Balint K, Boudousquie C, Gannon PO, Kandalaft LE. Personalized dendritic cell vaccines-recent
breakthroughs and encouraging clinical results. Front Immunol. 2019;10:766. CAS PubMed PubMed Central Google Scholar * Kukutsch NA, Roßner S, Austyn JM, Schuler G, Lutz MB. Formation and
kinetics of MHC class I-ovalbumin peptide complexes on immature and mature murine dendritic cells. J Investig Dermatol. 2000;115:449–53. CAS PubMed Google Scholar * Zhang H, Tang K,
Zhang Y, Ma R, Ma J, Li Y, et al. Cell-free tumor microparticle vaccines stimulate dendritic cells via cGAS/STING signaling. Cancer Immunol Res. 2015;3:196–205. CAS PubMed Google Scholar
* Gu X, Erb U, Büchler MW, Zöller M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int J Cancer. 2015;136:E74–84. CAS PubMed Google
Scholar * Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15:e257–67. CAS PubMed Google Scholar * Liau
LM, Ashkan K, Tran DD, Campian JL, Trusheim JE, Cobbs CS, et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed
glioblastoma. J Transl Med. 2018;16:142. Erratum in: J Transl Med. 2018;16:179. CAS PubMed PubMed Central Google Scholar * Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal
J, Petti AA, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–8. CAS PubMed PubMed
Central Google Scholar * Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma
patients. Nature. 2015;519:366–9. CAS PubMed PubMed Central Google Scholar * Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, et al. Immune and clinical
responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–8. CAS PubMed Google Scholar * Schreibelt G, Bol KF, Westdorp
H, Wimmers F, Aarntzen EH. Duiveman-de Boer T, et al. Effective clinical responses in metastatic melanoma patients after vaccination with primary myeloid dendritic cells. Clin Cancer Res.
2016;22:2155–66. CAS PubMed Google Scholar * Hsu JL, Bryant CE, Papadimitrious MS, Kong B, Gasiorowski RE, Orellana D, et al. A blood dendritic cell vaccine for acute myeloid leukemia
expands antitumor T cell responses at remission. Oncoimmunology. 2018;7:e1419114. PubMed PubMed Central Google Scholar * Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D, et
al. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7–24. CAS PubMed Google Scholar * Rauch S, Lutz J, Kowalczyk A, Schlake T, Heidenreich R. RNActive(R)
technology: generation and testing of stable and immunogenic mRNA vaccines. Methods Mol Biol. 2017;1499:89–107. CAS PubMed Google Scholar * Iavarone C, O’Hagan DT, Yu D, Delahaye NF,
Ulmer JB. Mechanism of action of mRNA-based vaccines. Expert Rev Vaccines. 2017;16:871–81. CAS PubMed Google Scholar * Van Lint S, Heirman C, Thielemans K, Breckpot K. mRNA: from a
chemical blueprint for protein production to an off-the-shelf therapeutic. Hum Vaccine Immunother. 2013;9:265–74. Google Scholar * Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ. Developing
mRNA-vaccine technologies. RNA Biol. 2012;9:1319–30. CAS PubMed PubMed Central Google Scholar * Asrani KH, Farelli JD, Stahley MR, Miller RL, Cheng CJ, Subramanian RR, et al.
Optimization of mRNA untranslated regions for improved expression of therapeutic mRNA. RNA Biol. 2018;15:756–62. PubMed PubMed Central Google Scholar * Zlotorynski E. The short tail that
wags the mRNA. Nat Rev Mol Cell Biol. 2017;19:2–3. PubMed Google Scholar * Holtkamp S, Kreiter S, Selmi A, Simon P, Koslowski M, Huber C, et al. Modification of antigen-encoding RNA
increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108:4009–17. CAS PubMed Google Scholar * Galaine J, Borg C, Godet Y, Adotévi
O. Interest of tumor-specific CD4 T Helper 1 cells for therapeutic anticancer vaccine. Vaccines. 2015;3:490–502. CAS PubMed PubMed Central Google Scholar * Bonehill A, Heirman C,
Tuyaerts S, Michiels A, Breckpot K, Brasseur F, et al. Messenger RNA-electroporated dendritic cells presenting MAGE-A3 simultaneously in HLA class I and class II molecules. J Immunol.
2004;172:6649–57. CAS PubMed Google Scholar * Aarntzen EH, Schreibelt G, Bol K, Lesterhuis WJ, Croockewit AJ, de Wilt JH, et al. Vaccination with mRNA-electroporated dendritic cells
induces robust tumor antigen-specific CD4+ and CD8+ T cells responses in stage III and IV melanoma patients. Clin Cancer Res. 2012;18:5460–70. CAS PubMed Google Scholar * Bonehill A,
Tuyaerts S, Van Nuffel AM, Heirman C, Bos TJ, Fostier K, et al. Enhancing the T-cell stimulatory capacity of human dendritic cells by coelectroporation with CD40L, CD70 and constitutively
active TLR4 encoding mRNA. Mol Ther. 2008;16:1170–80. CAS PubMed Google Scholar * Neyns B. A phase I clinical trial on the combined intravenous (IV) and intradermal (ID) administration of
autologous TriMix-DC cellular therapy in patients with pretreated melanoma (TriMixIDIV). J Clin Oncol. 2011;29:2519. Google Scholar * Wilgenhof S, Van Nuffel AM, Benteyn D, Corthals J,
Aerts C, Heirman C, et al. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol. 2013;24:2686–93.
CAS PubMed Google Scholar * Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med.
1996;184:465–72. CAS PubMed Google Scholar * Diken M, Kreiter S, Selmi A, Britten CM, Huber C, Türeci Ö, et al. Selective uptake of naked vaccine RNA by dendritic cells is driven by
macropinocytosis and abrogated upon DC maturation. Gene Ther. 2011;18:702–8. CAS PubMed Google Scholar * Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, et al. Systemic RNA
delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. PubMed Google Scholar * Diebold SS, Kaisho T, Hemmi H, Akira S. Reis e Sousa C.
Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–31. CAS PubMed Google Scholar * Gerer KF, Hoyer S, Dorrie J, Schaft N.
Electroporation of mRNA as universal technology platform to transfect a variety of primary cells with antigens and functional proteins. Methods Mol Biol. 2017;1499:165–78. CAS PubMed
Google Scholar * Van Nuffel AM, Corthals J, Neyns B, Heirman C, Thielemans K, Bonehill A, et al. Immunotherapy of cancer with dendritic cells loaded with tumor antigens and activated
through mRNA electroporation. Methods Mol Biol. 2010;629:405–52. PubMed Google Scholar * Dewitte H, Van Lint S, Heirman C, Thielemans K, De Smedt SC, Breckpot K, et al. The potential of
antigen and TriMix sonoporation using mRNA-loaded microbubbles for ultrasound-triggered cancer immunotherapy. J Control Release. 2014;194:28–36. CAS PubMed Google Scholar * McCullough KC,
Bassi I, Milona P, Suter R, Thomann-Harwood L, Englezou P, et al. Self-replicating replicon-RNA delivery to dendritic cells by chitosannanoparticles for translation in vitro and in vivo.
Mol Ther Nucleic acids. 2014;3:e173. CAS PubMed PubMed Central Google Scholar * Chung DJ, Carvajal RD, Postow MA, Sharma S, Pronschinske KB, Shyer JA, et al. Langerhans-type dendritic
cells electroporated with TRP-2 mRNA stimulate cellular immunity against melanoma: results of a phase I vaccine trial. Oncoimmunology. 2017;7:e1372081. PubMed PubMed Central Google Scholar
* Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P, et al. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus
Ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol. 2016;34:1330–8. PubMed Google Scholar * Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, et al. Clinical
significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–66. CAS PubMed Google Scholar * Ramos RN, Chin LS, Dos Santos AP, Bergami-Santos PC,
Laginha F, Barbuto JA, et al. Monocyte-derived dendritic cells from breast cancer patients are biased to induce CD4+CD25+Foxp3+ regulatory T cells. J Leukoc Biol. 2012;92:673–82. CAS PubMed
Google Scholar * Celli S, Day M, Müller AJ, Molina-Paris C, Lythe G, Bousso P. How many dendritic cells are required to initiate a T-cell response? Blood. 2012;120:3945–8. CAS PubMed
Google Scholar * van Heijst JW, Gerlach C, Swart E, Sie D, Nunes-Alves C, Kerkhoven RM, et al. Recruitment of antigen-specific CD8+ T cells in response to infection is markedly efficient.
Science. 2009;325:1265–9. PubMed Google Scholar * Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A, et al. Human inflammatory dendritic cells induce Th17 cell
differentiation. Immunity. 2013;38:336–48. CAS PubMed Google Scholar * Xu Y, Zhan Y, Lew AM, Naik SH, Kershaw MH. Differential development of murine dendritic cells by GM-CSF versus Flt3
ligand has implications for inflammation and trafficking. J Immunol. 2007;179:7577–84. CAS PubMed Google Scholar * Hespel C, Moser M. Role of inflammatory dendritic cells in innate and
adaptive immunity. Eur J Immunol. 2012;42:2535–43. CAS PubMed Google Scholar * Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, et al. Migratory dendritic cells transfer
antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–62. CAS PubMed Google Scholar * Gurevich I, Feferman T, Milo I, Tal O, Golani
O, Drexler I, et al. Active dissemination of cellular antigens by DCs facilitates CD8+ T-cell priming in lymph nodes. Eur J Immunol. 2017;47:1802–18. CAS PubMed Google Scholar * Garg AD,
Coulie PG, Van den Eynde BJ, Agostinis P. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol. 2017;38:577–93. CAS PubMed
Google Scholar * Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33:464–78. CAS PubMed PubMed Central Google
Scholar * Huang MN, Nicholson LT, Batich KA, Swartz AM, Kopin D, Wellford S, et al. Antigen-loaded monocyte administration induces potent therapeutic antitumor T cell responses. J Clin
Invest. 2020;130:774–88. CAS PubMed PubMed Central Google Scholar * Reinhard K, Rengstl B, Oehm P, Michel K, Billmeier A, Hayduk N, et al. An RNA vaccine drives expansion and efficacy of
claudin-CAR-T cells against solid tumors. Science. 2020;367:446–53. CAS PubMed Google Scholar * Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, et al. Modified mRNA
vaccines protect against Zika virus infection. Cell. 2017;169:176. CAS PubMed Google Scholar * Rodríguez-Ruiz ME, Perez-Gracia JL, Rodríguez I, Alfaro C, Oñate C, Pérez G, et al. Combined
immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann Oncol. 2018;29:1312–9. PubMed Google Scholar * Nowicki TS,
Berent-Maoz B, Cheung-Lau G, Huang RR, Wang X, Tsoi J, et al. A pilot trial of the combination of transgenic NY-ESO-1-reactive adoptive cellular therapy with dendritic cell vaccination with
or without Ipilimumab. Clin Cancer Res. 2019;25:2096–108. CAS PubMed Google Scholar * Vo MC, Jung SH, Chu TH, Lee HJ, Lakshmi TJ, Park HS, et al. Lenalidomide and programmed death-1
blockade synergistically enhances the effects of dendritic cell vaccination in a model of murine myeloma. Front Immunol. 2018;9:1370. PubMed PubMed Central Google Scholar * Hirooka Y,
Kawashima H, Ohno E, Ishikawa T, Kamigaki T, Goto S, et al. Comprehensive immunotherapy combined with intratumoral injection of zoledronate-pulsed dendritic cells, intravenous adoptive
activated T lymphocyte and gemcitabine in unresectable locally advanced pancreatic carcinoma: a phase I/II trial. Oncotarget. 2018;9:2838–47. PubMed Google Scholar * Soliman H, Khambati F,
Han HS, Ismail-Khan R, Bui MM, Sullivan DM, et al. A phase-1/2 study of adenovirus-p53 transduced dendritic cell vaccine in combination with indoximod in metastatic solid tumors and
invasive breast cancer. Oncotarget. 2018;9:10110–7. PubMed PubMed Central Google Scholar * Molla KA, Yang Y. CRISPR/Cas-mediated base editing: technical considerations and practical
applications. Trends Biotechnol. 2019;37:1121–42. CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Natural Science Foundation of
China (81872821) and the National Key S&T Special Projects (2018ZX09201018-024). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * State Key Laboratory of Biotherapy and Cancer Center, West
China Hospital, Sichuan University, and Collaborative Innovation Center for Biotherapy, Chengdu, 610041, China Yang-zhuo Gu, Xing Zhao & Xiang-rong Song * Stem Cell and Tissue
Engineering Research Center, Guizhou Medical University/Key Laboratory of Adult Stem Cell Transformation Research, Department of Immunology, Chinese Academy of Medical Sciences, Guiyang,
550004, China Xing Zhao Authors * Yang-zhuo Gu View author publications You can also search for this author inPubMed Google Scholar * Xing Zhao View author publications You can also search
for this author inPubMed Google Scholar * Xiang-rong Song View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to
Xiang-rong Song. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gu,
Yz., Zhao, X. & Song, Xr. Ex vivo pulsed dendritic cell vaccination against cancer. _Acta Pharmacol Sin_ 41, 959–969 (2020). https://doi.org/10.1038/s41401-020-0415-5 Download citation *
Received: 15 December 2019 * Accepted: 30 March 2020 * Published: 04 May 2020 * Issue Date: July 2020 * DOI: https://doi.org/10.1038/s41401-020-0415-5 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * cancer immunotherapy * dendritic cells * cancer antigens * DC vaccination * mRNA-pulsed DC vaccines * T-cell activation * tumor
microenvironment