The psychiatric phenotypes of 1q21 distal deletion and duplication
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Copy number variants are amongst the most highly penetrant risk factors for psychopathology and neurodevelopmental deficits, but little information about the detailed clinical
phenotype associated with particular variants is available. We present the largest study of the microdeletion and -duplication at the distal 1q21 locus, which has been associated with
schizophrenia and intellectual disability, in order to investigate the range of psychiatric phenotypes. Clinical and cognitive data from 68 deletion and 55 duplication carriers were analysed
with logistic regression analysis to compare frequencies of mental disorders between carrier groups and controls, and linear mixed models to compare quantitative phenotypes. Both children
and adults with copy number variants at 1q21 had high frequencies of psychopathology. In the children, neurodevelopmental disorders were most prominent (56% for deletion, 68% for duplication
carriers). Adults had increased prevalence of mood (35% for deletion [OR = 6.6 (95% CI: 1.4–40.1)], 55% for duplication carriers [8.3 (1.4–55.5)]) and anxiety disorders (24% [1.8 (0.4–8.4)]
and 55% [10.0 (1.9–71.2)]). The adult group, which included mainly genetically affected parents of probands, had an IQ in the normal range. These results confirm high prevalence of
neurodevelopmental disorders associated with CNVs at 1q21 but also reveal high prevalence of mood and anxiety disorders in a high-functioning adult group with these CNVs. Because carriers of
neurodevelopmental CNVs who show relevant psychopathology but no major cognitive impairment are not currently routinely receiving clinical genetic services widening of genetic testing in
psychiatry may be considered. SIMILAR CONTENT BEING VIEWED BY OTHERS 1Q21.1 DISTAL COPY NUMBER VARIANTS ARE ASSOCIATED WITH CEREBRAL AND COGNITIVE ALTERATIONS IN HUMANS Article Open access
22 March 2021 THE CONTRIBUTION OF COPY NUMBER VARIANTS TO PSYCHIATRIC SYMPTOMS AND COGNITIVE ABILITY Article 03 February 2023 EFFECTS OF EIGHT NEUROPSYCHIATRIC COPY NUMBER VARIANTS ON HUMAN
BRAIN STRUCTURE Article Open access 20 July 2021 INTRODUCTION The microdeletion and the microduplication at 1q21 (chr1: 146.57–147.39; GRCh37/hg19) are enriched in patients with
schizophrenia (OR estimate for the deletion: 5.2; for the duplication: 2.9)1 and in patients with intellectual disabilities (ID)/developmental delay (DD) and autism spectrum disorder (ASD)
(OR estimate for the deletion: 35; for the duplication: 18)2,3. The estimates for the population frequency of the microdeletion and -duplication at this locus derived from UK Biobank are
0.027% and 0.044%, respectively4 although this may underestimate the true frequency in the population because more severely affected carriers are likely to be underrepresented in a
middle-aged research cohort. The clinical phenotype of 1q21 deletion5,6 and duplication6 was originally established in clinical cohorts with intellectual disability, autism or congenital
anomalies. A systematic review of 22 studies containing data from 59 children and 48 adults with the 1q21.1 duplication7 reported high frequencies of neurodevelopmental problems including
50.8% for DD/ID, 35.6% for autism or autistic features and 8.5% for seizures. In 2015, the Simons VIP Consortium published a comparison of 19 deletion carriers and 19 duplication carriers
with familial noncarrier controls8. Amongst deletion carriers, the most prevalent neuropsychiatric features were mood and anxiety disorders (26%) and seizures (18%), whereas the predominant
neuropsychiatric disorders in duplication carriers were ASD (41%) and ADHD (29%). A major limitation of the extant literature is that most studies have had small sample sizes and few reports
have included detailed psychiatric phenotyping, and there is very little detailed clinical data from adults. In the present multi-centre study, we tripled the sample size compared to the
Bernier et al.8 study (123 compared to 38 CNV carriers, also included in the present study). The aim of the current study was to analyse psychopathology in the largest international cohort
so far assembled that comprises carriers of the reciprocal microdeletion and –duplication at 1q21 across children and adults. We specifically wanted to ascertain whether mirror
phenotypes—similar to the established dose effect on head size6—also occur in the psychological domain and whether—and what type of—psychopathology was prevalent in an adult group largely
composed of family carriers and thus not affected by the ascertainment bias of clinical samples. PATIENTS AND METHODS Participants were recruited by three research groups/consortia: the
Simons Variation in Individuals Project (VIP) consortium (_n_ = 51); the CNV Research Group, Lausanne University Hospital, Lausanne, Switzerland (_n_ = 12); and the Neuroscience and Mental
Health Research Institute, Cardiff University, UK (_n_ = 60). Unaffected family members were recruited as control participants (Simons VIP: _n_ = 51; Lausanne: _n_ = 10; Cardiff: _n_ = 9).
STUDY SAMPLE Participants assessed in Cardiff were recruited by three projects; children by the ‘Experiences of CHildren with cOpy number Variants’ (ECHO) and ‘Intellectual Disability and
Mental Health: Assessing the Genomic Impact on Neurodevelopment’ (IMAGINE-ID, http://imagine-id.org/)9 studies, and adults through the ‘Defining Endophenotypes From Integrated Neurosciences’
(DEFINE) study. Participants were given details of our study following a diagnosis of a 1q21.1 CNV at one of the UK National Health Service genetics clinics. The study was also advertised
on support websites and social media groups for carriers of 1q21.1 CNVs. Adult participants were screened for the capacity to consent using a telephone-based protocol. If they were deemed to
lack capacity, a personal consultee was contacted. All participants, or their personal consultee, provided informed written consent. For participants under the age of 16, parent/guardian
consent and participant assent were obtained. For 16–18-year-old participants consent was obtained from participant and parent/guardian. All interviews were taped and decisions on
psychiatric symptoms and diagnosis were made during consensus meetings led by a psychiatrist. The South-East Wales Research Ethics Committee approved the recruitment and assessment protocols
used in this study (14/WA/0035). For the IMAGINE-ID study, protocols were approved by the NHS London Queen Square research ethics committee (14/LO/1069). Participants in the Simons VIP
consortium were ascertained clinically and recruited online and through clinical laboratory referrals in the United States. Data were collected at three participating U.S. sites. Further
details on the methods are available in Simons VIP, 201210. Participants with known additional clinically recognized CNVs were excluded. Assessments were standardized across sites through
ongoing training and inter-rater reliability checks. The study was approved by the Institutional Review Boards at Columbia University (AAAF3927), Geisinger Health System (2011-0320),
Children’s Hospital of Boston (12-009720), Baylor College of Medicine (H-27549) and the University of Washington (39149), and all participants 18 or older or the participant’s designated
legal guardian provided written informed consent (and children under 18 were assented, when appropriate, based upon mental capacity). Participants from the CNV Research Group (Lausanne) were
taking part in a larger research project on CNVs at the 1q21.1 locus. Proband carriers were referred to the study by clinical geneticists. The study was reviewed and approved by the local
ethics committee (CER-VD; PB_2016-02137) and written informed consent was obtained from participants or legal representatives before investigation. Participants were assessed at the Lausanne
University Hospital, Switzerland. COHORT DEMOGRAPHICS Data from 123 participants, comprising 68 deletion and 55 duplication carriers, were analysed for this study and compared with data
from 70 familial controls. Participants were grouped and analysed according to carrier status (deletion, duplication and control) and age group (child < 18 years, adult ≥18 years) to
avoid biasing against psychopathologies which are more prevalent at specific life-stages. For full details of participant demographics see Suppl. Table S1. No significant age or gender
differences were observed between deletion carriers, duplication carriers and controls either in the child or adult cohort (Suppl. Tables S1 and S2). Carrier status for the 1q21.1 deletion
or duplication was confirmed through clinical chromosome microarrays and/or medical records. We only included carriers with a deletion or duplication that spanned at least 80% of the typical
distal locus (chr1:146,527,987–147,394,444 (hg19)), but not the nearby TAR locus (Suppl. Fig. S1 and S2), based on information from clinical genetics reports or in-house genotyping. Of the
123 CNV carriers, the inheritance status was known for 86 participants (70%). Seventy-three of these individuals had a ‘known inherited’ status (85%, with one parent with a confirmed 1q21.1
CNV carrier status), 13 individuals (15%) had a ‘known de-novo’ status (both parents with negative genetic tests for a 1q21.1 CNV). PSYCHOPATHOLOGY AND NEURODEVELOPMENTAL FUNCTION To assess
general psychopathology in children (diagnosis as well as symptomatology), the Cardiff group used the Child and Adolescent Psychiatric Assessment (CAPA)11. Autism symptomatology was measured
by the Social Communication Questionnaire (SCQ)12. The Strengths and Difficulties Questionnaire13 was used to establish conduct problems, emotional problems, hyperactivity problems, peer
problems and prosocial functioning as well as a total SDQ score. Motor functioning was measured by the Developmental Coordination Disorder Questionnaire (DCDQ)14. Full methodological details
have already been reported elsewhere9. Adult psychopathology was assessed using the Psychiatric Assessment Schedule for Adults with Developmental Disabilities (PAS-ADD)15. Psychotic
symptoms were assessed using the Structured Interview for Prodromal Symptoms (SIPS)16. Adult participants who displayed psychotic symptoms on the SIPS were further assessed using the Scale
for the Assessment of Positive and Negative Symptoms (SAPS) and (SANS), respectively17. Adult participants in the Cardiff cohort also completed the SCID-II, a semi-structured interview for
making DSM Axis II: Personality Disorder diagnoses. However, in this paper we are not reporting frequencies of personality disorders because this information was not available in the other
cohorts. All diagnoses were confirmed by a licensed psychiatrist based on face-to-face interviews or review of the interview protocols. If the PAS-ADD showed evidence of autistic symptoms,
the DISCO, a semi-structured interview, or the “Royal College of Psychiatrists Diagnostic Interview Guide for the Assessment of Adults with Autism Spectrum Disorder” was used to confirm the
diagnosis18,19. The Lausanne CNV Research Group used the Diagnostic Interview for Genetic Studies (DIGS)20. Each of the tools used corresponded to the DSM-IV/5 criteria for psychopathologies
being assessed in this report. The Lausanne control participants had no psychopathological assessments and were therefore not included in the relevant comparisons. Children were assessed
using the ADOS21. In the Simons VIP, children were assessed using the ADOS21 and ADI-R22 (used for suspected ASD cases) and the DISC (Diagnostic Interview Schedule for Children)23 or DISC-YC
(Young Child Edition), for children between 3 and 624, and parent behaviour rating scales. Adults were assessed using the ADOS, ADI-R (used for suspected ASD cases up to age 26) and the
self-report SCL-90-R25], followed by a clinical interview. Diagnoses of control participants were also based on the SCL-90-R (_n_ = 23) or self-report (_n_ = 28). All centres also collected
information about seizure history (separated into febrile and unprovoked seizures) although this information was not available for most of the controls. COGNITIVE FUNCTIONING In Cardiff,
general cognitive ability was assessed by trained raters using the Wechsler Abbreviated Scale of Intelligence (WASI)26. For the children, data were also available for reaction time,
sustained attention, spatial planning, and spatial working memory as measured by the Cambridge Neuropsychological Test Automated Battery (CANTAB)27, and set-shifting ability as measured by
the Wisconsin Card Sorting Test (WCST)28. Adults recruited by the Simons VIP group were also assessed using the WASI, while children were assessed using the Differential Ability Scales (DAS)
cognitive battery29 (2.5 years and older) or the Mullen Scales of Early Learning30 (all ages, dependent upon developmental level, resulting in a ratio IQ where applicable). The CNV Research
Group used Wechsler intelligence testing for all participants as previously described31. DATA ANALYSIS Data analyses were conducted using SPSS v.23 (RRID: SCR_002865) (IBM Corp.) and the R
Project for Statistical Computing (RRID:SCR_001905) (http://www.r-project.org/). CATEGORICAL VARIABLES The frequency of clinical phenotypes was analysed separately for children and adults.
We compared the frequency of any DSM-IV/5 diagnosis between carriers and controls to test whether copy number variation at the locus is associated with a higher prevalence of
psychopathology. For children, neurodevelopmental (ASD, ADHD, ID, social communication disorder and specific learning disorder), anxiety, mood, conduct, eating and substance use disorders
were pooled across subcategories. We also pooled diagnostic categories for adults (anxiety, mood disorder, conduct disorder, neurodevelopmental, psychotic and substance use disorders). Using
a random-effects logit model we tested for the effect of group status (e.g. Deletion vs Control, or Duplication vs Control) (independent variable) on the frequencies of psychiatric
diagnoses (_Dependent Variable:_ 0 = _No Diagnosis_, 1 = _Diagnosis Present_) while adjusting for age, sex (_Fixed Effects_) and FSIQ (only in children because of wider availability of
measures) (_Random Effect_). Control individuals from families with a participant with either a deletion or a duplication were combined and comparisons for both CNVs were conducted with the
same control sample. For one comparison (adult duplication effect on anxiety) we had to remove the covariate sex because all anxiety cases (controls and duplication) were female. Because our
control sample consisted of family members of the carriers, case-control pairs from the same family are likely to be more similar because of shared genetic and environmental background
variation. We could not always control for this because of collinearity (arising from the pooling of controls and imbalance of control recruitment across sites). Where it was possible, we
ran analyses including family ID as a random factor in our models. We report results without family ID as default in Tables 1 and 2, but have added the findings of models including the
effects of family ID in the footnotes of these Tables, where these could be reliably computed. CONTINUOUS VARIABLES We conducted linear mixed-effect models for FSIQ, VIQ and PIQ, with group
status (deletion vs duplication vs controls), age, and sex as fixed effects, and family as a random effect to take into account relatedness between 1q21.1 CNV carriers and controls. Post hoc
Tukey tests were conducted to evaluate which groups differed for FSIQ, VIQ and PIQ. Statistical analysis of the difference in head circumferences between the participants with 1q21 deletion
and duplication was conducted with t-test for independent groups, following conversion of raw scores to age-standardised _Z_-scores. We transformed neuropsychiatric trait scores (only
available for the Cardiff children) using the Tukey Ladder of Powers transformation to make the data fit the normal distribution as closely as possible9. We then standardised all transformed
test scores into _Z-_scores using the mean and SD of the control group as reference—i.e., the difference in the individual’s score and the mean score for the control group was divided by
the SD for the control group. We constructed these _Z-_scores so that a negative score denoted a worse outcome. We used linear mixed-effects models with test score as the outcome and carrier
status (deletion, vs duplication vs controls), age and sex as fixed effects and family as a random effect. RESULTS PSYCHOPATHOLOGY CHILDREN The frequency of psychiatric disorders in the
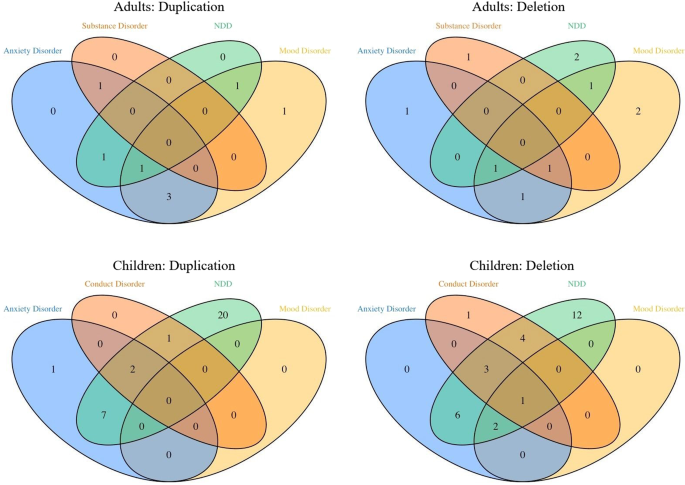
children with 1q21 CNVs is documented in Table 1 and Fig. 1. Both children with the deletion (OR = 7.11 (1.34–48.60), _p_ = 0.03) and those with the duplication (OR = 12.24 (2.62–74.84), _p_
= 0003) had higher frequencies of any psychiatric diagnosis compared to the family control sample. The prevalence of any neurodevelopmental disorder (NDD, including ASD, ADHD, ID, social
communication disorder and specific learning disorder) was 56% in the deletion and 68% in the duplication carrier group as compared to 0% in the family controls. The most prevalent NDD was
ADHD both in children with the deletion (19/50 cases assessed for NDD; 38%) and children with the duplication (21/44 cases assessed for NDD; 47.7%) (Suppl. Table S3). The prevalence of any
anxiety disorder was also higher in the deletion (25%) and duplication (23%) carrier groups as compared to the controls (11%), but these differences did not reach significance. Generalised
anxiety disorder and specific phobia were the most common diagnoses in both the deletion and the duplication groups (Suppl. Table S4). Children with 1q21.1 deletion had higher scores
relative to control siblings for _ADHD traits_ as measured by the CAPA (ADHD symptomatology, _z_ = −2.85, _p_ = 0.005) and the SDQ (Hyperactivity subscale, _z_ = −2.10, _p_ = 0.002), with
higher scores indicating higher difficulty levels/psychopathology. Children with the 1q21.1 duplication had higher scores than control siblings for _ADHD traits_ as measured by the CAPA
(ADHD symptomatology, _z_− = −3.11, _p_ = 0.005) and the SDQ (Hyperactivity subscale, _z_ = −2.38, _p_ = 0.003), and _traits of oppositional defiance_ as measured by the CAPA (ODD
symptomatology, _z_ = −1.50, _p_ = 0.006) and the SDQ (Conduct subscale, _z_ = −2.08, _p_ = 0.018). Comparing the two CNV groups, children with the deletion had higher levels of mood
symptomatology (_z_ difference = −0.56, _p_ = 0.033), and ODD symptomatology (_z_ difference = −1.01, _p_ = 0.008) than children with the duplication. Full results of the CAPA scores are
presented in the Supplementary Material 2. ADULT SAMPLE The frequencies of mental disorders in the adults with 1q21 CNVs are documented in Table 2 and Fig. 1. DELETION CARRIERS VERSUS FAMILY
CONTROLS Fifty-nine percent of the carriers had at least one psychiatric disorder compared to 28% in the control group (OR = 4.0, _p_ = 0.03). The prevalence of any mood disorder was higher
in the deletion carrier group as compared to the family controls (35% versus 9%, OR = 6.0, _p_ = 0.02). No differences were found for any of the other diagnostic categories. The ORs were
similar in the model with family ID included although the difference in mood disorder was no longer significant (from _p_ = 0.02 to 0.14). DUPLICATION CARRIERS VERSUS FAMILY CONTROLS
Seventy-three percent of the carriers (versus 28% of the family controls) met criteria for any psychiatric disorder (OR = 6.0, _p_ = 0.03). The prevalence of any mood disorder was higher in
the duplication carrier group as compared to the family controls, (55% versus 9%, OR = 8.3, _p_ = 0.02). The prevalence of any anxiety disorder was also higher in the duplication carrier
group as compared to the family controls (55% versus 16%, OR = 10.0, _p_ = 0.01). No differences were found for any of the other diagnostic categories. The ORs were similar in the model with
family ID included although the difference in any psychiatric diagnosis now only reached trend level (_p_ = 0.06). SEIZURE RATES Seven out of 36 children with deletions (19.4%) and 5/35
children with duplications (14.3%) had a history of unprovoked seizures. History of febrile seizures was reported for no child with deletion and 2/35 children with duplications (6%). Figures
in adults were too low to allow for robust inferences but did not provide evidence for increased seizure rates (Suppl. Table S5). COGNITIVE FUNCTION IQ data is presented in Table 3. Full
data on CANTAB subscores are presented in the Supplementary Material 2. CHILDREN The three groups differed for FSIQ (_F_ = 7.01, df = 2, _p_ = 0.002), PIQ (_F_ = 7.62, df = 2, _p_ <
0.001) and VIQ (_F_ = 4.37, df = 2, _p_ = 0.016). Post hoc tests (Tukey) indicated that deletion carriers had a lower FSIQ (_p_ = 0.002), PIQ (_p_ < 0.001) and VIQ (_p_ = 0.029) than
controls and similarly that duplication carriers had lower FSIQs, PIQs and VIQs than controls (FSIQ: _p_ = 0.025; PIQ: _p_ = 0.035; VIQ: _p_ = 0.033). There were no IQ differences between
deletion and duplication carriers (FSIQ: _p_ = 0.582; PIQ: _p_ = 0.274; VIQ: _p_ = 0.996). Children with 1q21.1 deletion had deficits relative to control siblings in _spatial cognition_
including spatial planning (_z_ = −1.46, _p_ = 0.011), and spatial working memory (_z_ = −2.23, _p_ = 0.009). Children with the 1q21.1 duplication also had deficits relative to control
siblings in spatial working memory (_z_ = −2.71, _p_ = 0.001). Children with the deletion had greater deficits in sustained attention than children with the duplication (_z_ difference =
−0.62, _p_ = 0.020). ADULTS The three groups differed for FSIQ (_F_ = 9.18, df = 2, _p_ < 0.001), PIQ (_F_ = 5.64, df = 2, _p_ = 0.007) and VIQ (_F_ = 5.77, df = 2, _p_ = 0.006). Post hoc
tests (Tukey) indicated that deletion carriers had a lower FSIQ than controls (_p_ = 0.002), PIQ (_p_ = 0.014) and VIQ (_p_ = 0.025) but that there were no significant differences between
duplication carriers and controls (FSIQ: _p_ = 0.168; PIQ: _p_ = 0.590; VIQ: _p_ = 0.223). Moreover, there were no IQ differences between deletion and duplication carriers (FSIQ: _p_ =
0.428; PIQ: _p_ = 0.323; VIQ: _p_ = 0.747). HEAD CIRCUMFERENCE Age-normalised head circumference data were available for 35 deletion and 34 duplication carriers (Fig. 2). Standardised head
circumference was markedly lower for deletion vs. duplication carriers (_t_ = −6.48 [95% CI = −4.48−2.37], df = 66.4, _p_-value = 1.3 × 10−8). DISCUSSION This is the largest and most
detailed study to date documenting the psychiatric and cognitive phenotype associated with copy number variation at the 1q21 chromosomal locus. We found a clear association of deletion and
duplication of the critical region of chromosome 1q21 with psychopathology both in children ascertained through clinical genetics services and an adult group that was mainly composed of
parents of probands. As in previous studies, based on smaller samples, the main diagnostic groups of neurodevelopmental disorders were ADHD and ASD. Association of both the deletion and the
duplication with ADHD- and ASD-related psychopathology was also revealed through quantitative measures (CAPA and SDQ) in the Cardiff children sample. However, the high prevalence of anxiety
disorders in both deletion and duplication carriers (previously only reported for those with the deletion)9 and the high prevalence of mood and anxiety disorders in the high-functioning
adult group are novel findings. These findings and the recent report of high prevalence of depression in participants of a population cohort (UK Biobank) with 1q21 duplication32 underline
the association of the 1q21 locus with psychopathology beyond the classical neurodevelopmental disorders. We found no evidence for preponderance of certain psychiatric syndromes in the
deletion or duplication carriers, which some of the previous, smaller studies had suggested, in line with other studies highlighting commonalities across CNV dosage33. An implication of
comparisons between carriers of deletions and duplications across multiple CNVs is that normal function seems to be supported by normal dosage of genes/proteins in the region, and deviations
in both directions can be damaging. Furthermore, we found no evidence of psychiatric mirror phenotypes such as increased vs. reduced risk, which has been discussed for another CNV locus
(22q11.2 deletion vs. duplication)34. Similarly, seizure frequencies were high in both children with deletions and duplications. This represents an understudied topic in CNV research:
however, a recent study has indicated that the frequency of seizures in young people with 22q11.2 deletion syndrome may be higher than suspected35, indicating that individuals with certain
CNVs may need to be closely monitored and particularly under certain circumstances (e.g., fever). The one mirror phenotype of head size associated with this locus6 was confirmed in our
study. We found the deletion was associated with smaller and the duplication with larger head circumference. We initially hypothesised that both 1q21.1 deletions and duplications would
result in cognitive impairment; however, the cognitive abilities in our study varied widely between the age-categories. Although each carrier group (except for the adults with a duplication)
displayed significantly lower levels of cognitive ability than controls, the mean scores in the adult carriers were in the normal range, reflecting the notion that the child and adult
carriers recruited to this study may fall in different parts of the 1q21.1 functional spectrum based upon method of ascertainment. Like other neurodevelopmental CNVs, including 22q11.2
deletion syndrome36, the effect of 1q21 deletion and duplication on psychopathology is pleiotropic. This suggests that pathogenic CNVs are associated with cognitive impairment and
psychological dysfunction9, perhaps through alterations in brain development37, rather than a specific psychiatric syndrome. Although the spectrum of phenotypes includes intellectual
disability, and both 1q21 deletion and duplication are associated with reductions in mean IQ and educational attainment4, the present study shows that psychopathology in CNV carriers at this
locus is not confined to those with a low IQ. Even the adult group, with a mean full-scale IQ in the average range, had high prevalence of psychopathology, mainly anxiety and mood
disorders. Beyond this dissociation of intellectual impairment and psychopathology, our study reveals two subgroups of carriers of CNVs at 1q21: an intellectually impaired group that is
referred to clinical genetics services in childhood because of developmental delay and/or physical abnormalities and has a high prevalence of neurodevelopmental disorders, but also of
anxiety disorders, and a group with only subtle intellectual/educational impairment (but high prevalence of anxiety/mood disorders) which is generally not recognised unless they are referred
for genetic testing because of their children. Similar patterns likely exist for other neurodevelopmental risk factors with variable penetrance, even those associated with recognised
clinical syndromes such as 22q11.2 deletion. The mechanisms underlying this variable penetrance of pathogenic neurodevelopmental CNVs are still poorly understood and likely include both
genetic (oligogenic and polygenic)38 and environmental modifiers. Large and multigenerational cohorts of CNV carriers are particularly suited to the study of modifying effects because they
allow for the comparison of probands and their (generally less severely affected) parents and extended family members. The experience of caring for a mentally ill child may contribute to a
higher prevalence of stress-related disorders in parents. However, a recent large-scale study into the (sub)clinical symptoms of parents of children and adolescents with psychopathology
found lower frequencies (depressive symptoms: 12.8% for fathers, 14.6% for mothers; anxiety: 6 and 7.2%39) than the present study, which suggests a genetic contribution to the
psychopathology of the adult carriers. In addition to the importance for the integrated clinical care for families affected by CNVs at 1q21, these findings also have implications for
translational neuroscience, highlighting the need to model the anxious phenotype in animal models of this locus and to identify the responsible genes. The 1q21 critical region contains
several genes that are expressed in the brain, including Hydrocephalus-inducing homologue 2 (_HYDIN2_) and three Notch homologue 2 N-terminal-like (_NOTCH2NL_) genes. It has recently been
proposed that _NOTCH2NL_ is crucial for the effects on cortical development40. Another proposed genetic mechanism for the effects on head/brain size is related to the Olduvai protein domain
(formerly called DUF1220 domain). Sequences coding for this protein domain (average amino acid length ~65) are predominantly found in the 1q21 locus, primarily clustered in Neuroblastoma
breakpoint family (NBPF) genes in close proximity to the _NOTCH2NL_ genes41. The human lineage-specific increase in copy number of sequences coding for these domains has been linked with the
anthropoid brain expansion42, and individual differences in copy number were associated with brain size in people with 1q21 deletion43. Because of the SNP array used, this study focussed on
large CNV’s present at this locus and further studies, including sequencing and high resolution CNV analysis of large cohorts, will be necessary for a full elucidation of these
genotype-phenotype relationships. LIMITATIONS The collaborations reported in this study were established following the assessment of participants and hence there is some discrepancy in the
assessment measures used by each research centre. Another limitation is the relatively small number of family controls, particularly in the children group. We had to use the same control
sample for both deletion and duplication carriers, which resulted in difficulties controlling effects for familiality. IMPLICATIONS The results of the present study support the need for more
extensive genetic testing in psychiatry because it is likely that a considerable number of users of mental health services carry an undiagnosed genetic variant. A recent study in patient
groups with intellectual disability and comorbid mental disorder identified recognised pathogenic CNVs in ~10%44. If genetic testing were to be offered routinely for this patient population
in mental health services, this would not only inform their clinical care but also provide much-needed information about the frequency and severity of neurobehavioral challenges associated
with CNVs. CHANGE HISTORY * _ 18 JUNE 2021 A Correction to this paper has been published: https://doi.org/10.1038/s41398-021-01296-9 _ REFERENCES * Rees, E. et al. Analysis of copy number
variations at 15 schizophrenia-associated loci. _Br. J. Psychiatry_ 204, 108–114 (2014). Article Google Scholar * Kirov, G. et al. The penetrance of copy number variations for
schizophrenia and developmental delay. _Biol. Psychiatry_ 75, 378–385 (2014). Article CAS Google Scholar * Marshall, C. R. et al. Contribution of copy number variants to schizophrenia
from a genome-wide study of 41,321 subjects. _Nat. Genet._ 49, 27–35 (2017). Article CAS Google Scholar * Kendall, K. M. et al. Cognitive performance among carriers of pathogenic copy
number variants: analysis of 152,000 UK Biobank subjects. _Biol. Psychiatry_ 82, 103–110 (2017). Article Google Scholar * Mefford, H. C. et al. Recurrent rearrangements of chromosome
1q21.1 and variable pediatric phenotypes. _N. Engl. J. Med._ 359, 1685–1699 (2008). Article CAS Google Scholar * Brunetti-Pierri, N. et al. Recurrent reciprocal 1q21.1 deletions and
duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. _Nat. Genet._ 40, 1466–1471 (2008). Article CAS Google Scholar * Dolcetti, A. et
al. 1q21.1 Microduplication expression in adults. _Genet. Med._ 15, 282–289 (2013). Article Google Scholar * Bernier, R. et al. Clinical phenotype of the recurrent 1q21.1 copy-number
variant. _Genet. Med._ 18, 341–349 (2016). Article Google Scholar * Chawner, S. J. R. A. et al. Genotype-phenotype associations in children with copy number variants associated with high
neuropsychiatric risk in the UK (IMAGINE-ID): a case-control cohort study. _Lancet Psychiatry_ 6, 493–505 (2019). Article Google Scholar * Consortium, S. V. Simons Variation in Individuals
Project (Simons VIP): a genetics-first approach to studying autism spectrum and related neurodevelopmental disorders. _Neuron_ 73, 1063–1067 (2012). Article Google Scholar * Angold, A.
& Costello, E. J. The Child and Adolescent Psychiatric Assessment (CAPA). _J. Am. Acad. Child Adolesc. Psychiatry_ 39, 39–48 (2000). Article CAS Google Scholar * Rutter, M., Bailey,
A. & Lord, C. _The Social Communication Questionnaire_ (Western Psychological Services, 2003). * Goodman, R. The Strengths and Difficulties Questionnaire: a research note. _J. Child
Psychol. Psychiatry_ 38, 581–586 (1997). Article CAS Google Scholar * Wilson, B. N. et al. Psychometric properties of the revised Developmental Coordination Disorder Questionnaire. _Phys.
Occup. Ther. Pediatr._ 29, 182–202 (2009). Article Google Scholar * Moss, S. et al. Reliability and validity of the PAS-ADD Checklist for detecting psychiatric disorders in adults with
intellectual disability. _J. Intellect. Disabil. Res._ 42, 173–183 (1998). (Pt 2). Article Google Scholar * Miller, T. J. et al. Prodromal assessment with the structured interview for
prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. _Schizophr. Bull._ 29, 703–715 (2003). Article Google
Scholar * Andreasen, N. C. Methods for assessing positive and negative symptoms. _Mod. Probl. Pharmacopsychiatry_ 24, 73–88 (1990). Article CAS Google Scholar * Wing, L., Leekam, S. R.,
Libby, S. J., Gould, J. & Larcombe, M. The diagnostic interview for social and communication disorders: background, inter-rater reliability and clinical use. _J. Child Psychol.
Psychiatry_ 43, 307–325 (2002). Article Google Scholar * Berney, T., Brugha, T. & Carpenter, P. _Royal College of Psychiatrists Interview Guide for the Diagnostic Assessment of Able
Adults with Autism Spectrum Disorder (ASD)_ (Royal College of Psychiatrists, 2017). * Nurnberger, J. I. et al. Diagnostic interview for genetic studies. Rationale, unique features, and
training. NIMH Genetics Initiative. _Arch. Gen. Psychiatry_ 51, 849–859 (1994). Article Google Scholar * Lord, C. et al. The autism diagnostic observation schedule-generic: a standard
measure of social and communication deficits associated with the spectrum of autism. _J. Autism Dev. Disord._ 30, 205–223 (2000). Article CAS Google Scholar * Lord, C., Rutter, M. &
Le Couteur, A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. _J. Autism Dev.
Disord._ 24, 659–685 (1994). Article CAS Google Scholar * Shaffer, D., Fisher, P., Lucas, C. P., Dulcan, M. K. & Schwab-Stone, M. E. NIMH Diagnostic Interview Schedule for Children
Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. _J. Am. Acad. Child Adolesc. Psychiatry_ 39, 28–38 (2000). Article CAS
Google Scholar * Fisher, P. & Lucas, C. _Diagnostic Interview Schedule for Children (DISC-IV) - Young Child_ (Columbia University, 2006). * Derogatis, L. R., Rickels, K. & Rock,
A. F. The SCL-90 and the MMPI: a step in the validation of a new self-report scale. _Br. J. Psychiatry_ 128, 280–289 (1976). Article CAS Google Scholar * Wechsler, D. _Wechsler
Abbreviated Scale of Intelligence - Second Edition (WASI-II)_ (Pearson, 2011). * Cambridge Cognition. _CANTAB__®_ _[Cognitive assessment software]_ (www.cantab.com, 2019). * Heaton, R.
_Wisconsin Card Sorting Test Manual; Revised and Expanded_ 5–57 (Psychological Assessment Resources, 1981). * Elliot, C. _Differential Ability Scales: Introductory and Technical Handbook_
(The Psychological Corporation, 1990). * Mullen, E. M. _Mullen Scales of Early Learning Manual_ ix, 85 (American Guidance Service, AGS, 1995). * Zufferey, F. et al. A 600 kb deletion
syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. _J Med Genet_ 49, 660–668 (2012). Article CAS Google Scholar * Kendall, K. M. et al. Association of rare copy
number variants with risk of depression. _JAMA Psychiatry_ 76, 818–825 (2019). * Niarchou, M. et al. Psychiatric disorders in children with 16p11.2 deletion and duplication. _Transl.
Psychiatry_ 9, 8 (2019). Article Google Scholar * Rees, E. et al. Evidence that duplications of 22q11.2 protect against schizophrenia. _Mol. Psychiatry_ 19, 37–40 (2014). Article CAS
Google Scholar * Eaton, C. B. et al. Epilepsy and seizures in young people with 22q11.2 deletion syndrome: prevalence and links with other neurodevelopmental disorders. _Epilepsia_ 60,
818–829 (2019). Article CAS Google Scholar * Niarchou, M. et al. Psychopathology and cognition in children with 22q11.2 deletion syndrome. _Br. J. Psychiatry_ 204, 46–54 (2014). Article
Google Scholar * Drakesmith, M. et al. Genetic risk for schizophrenia and developmental delay is associated with shape and microstructure of midline white-matter structures. _Transl.
Psychiatry_ 9, 102 (2019). Article Google Scholar * Tansey, K. E. et al. Common alleles contribute to schizophrenia in CNV carriers. _Mol. Psychiatry_ 21, 1153 (2016). Article CAS Google
Scholar * Wesseldijk, L. W. et al. Risk factors for parental psychopathology: a study in families with children or adolescents with psychopathology. _Eur. Child Adolesc. Psychiatry_ 27,
1575–1584 (2018). Article CAS Google Scholar * Fiddes, I. T. et al. Human-specific NOTCH2NL genes affect notch signaling and cortical neurogenesis. _Cell_ 173, 1356–1369 (2018). e1322.
Article CAS Google Scholar * Fiddes, I. T., Pollen, A. A., Davis, J. M. & Sikela, J. M. Paired involvement of human-specific Olduvai domains and NOTCH2NL genes in human brain
evolution. _Hum. Genet._ 138, 715–721 (2019). Article CAS Google Scholar * Keeney, J. G., Dumas, L. & Sikela, J. M. The case for DUF1220 domain dosage as a primary contributor to
anthropoid brain expansion. _Front Hum. Neurosci._ 8, 427 (2014). Article Google Scholar * Dumas, L. J. et al. DUF1220-domain copy number implicated in human brain-size pathology and
evolution. _Am. J. Hum. Genet._ 91, 444–454 (2012). Article CAS Google Scholar * Thygesen, J. H. et al. Neurodevelopmental risk copy number variants in adults with intellectual
disabilities and comorbid psychiatric disorders. _Br. J. Psychiatry_ 212, 287–294 (2018). Article Google Scholar Download references ACKNOWLEDGEMENTS We thank all the children and parents
who took part in the Intellectual Disability and Mental Health: Assessing the Genomic Impact on Neurodevelopment (IMAGINE-ID) study, as well as the members of the IMAGINE-ID consortium for
their contributions. We are grateful for the UK National Health Service (NHS) medical genetic clinics for their support. We thank the core laboratory team of the Division of Psychological
Medicine and Clinical Neurosciences Laboratory at Cardiff University (Cardiff, UK) for DNA sample management and genotyping. The Cardiff group is grateful for recruitment support to the
charity ‘Unique’, a support group for people with rare chromosomal disorders. The New York group is grateful to all the families who participated in the Simons Variation in Individuals
Project (Simons VIP), as well as the Simons VIP investigators. Approved researchers can obtain the Simons VIP datasets described in this study by applying at https://base.sfari.org.
SPONSORSHIP: Cardiff cohort: Wellcome Trust Strategic Award “Defining endophenotypes from integrative neuroscience” (DEFINE, 100202/Z/12/Z), Medical Research Council (Intellectual Disability
and Mental Health: Assessing Genomic Impact on Neurodevelopment (IMAGINE) study MR/N022572/1 and MR/L011166/1), by the Baily Thomas Charitable Trust (2315/1), the Waterloo Foundation
(918-1234), Wellcome Trust ISSF grant, Medical Research Council Centre grant (G0801418) and Medical Research Council Programme grant (G0800509). Recruitment was supported by the National
Centre for Mental Health, which is funded by Health and Care Research Wales (HCRW). Lausanne cohort/Jacquemont group: Swiss National Science Foundation (SNSF, grant PMPDP3_171331). Canada
Research Chairs Programme. Brain Canada Multi-Investigator Research Initiative. New York/Simons VIP cohort: Support provided by the Simons Foundation Autism Research Initiative (SFARI).
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Health, Ethics and Society, Care and Public Health Research Institute (CAPHRI), Faculty of Health, Medicine and Life Sciences,
Maastricht University, Maastricht, The Netherlands Stefanie C. Linden * Division of Psychological Medicine and Clinical Neurosciences, Medical Research Council Centre for Neuropsychiatric
Genetics and Genomics, Cardiff University, Cardiff, UK Stefanie C. Linden, Cameron J. Watson, Jacqueline Smith, Samuel J. R. A. Chawner, Thomas M. Lancaster, Ffion Evans, Nigel Williams,
Jeremy Hall, Michael J. Owen, David E. J. Linden & Marianne B. M. van den Bree * Preventive Neurology Unit, Wolfson Institute of Preventive Medicine, Queen Mary University of London,
London, UK Cameron J. Watson * School of Psychology, University of Bath, Bath, UK Thomas M. Lancaster * Behavioural and Brain Sciences Unit Institute of Child Health, University College
London, London, UK David Skuse * Cambridge Institute for Medical Research, University of Cambridge, Cambridge, UK F. Lucy Raymond * Department of Psychiatry and Neuropsychology, School for
Mental Health and Neuroscience, Faculty of Health, Medicine and Live Sciences, Maastricht University, Maastricht, The Netherlands David E. J. Linden * Simons Foundation, New York, NY, USA
LeeAnne Green-Snyder * Departments of Pediatrics and Medicine, Columbia University, New York, NY, USA Wendy K. Chung * Service des Troubles du Spectre de l’Autisme et apparentés, Centre
Hospitalier Universitaire Vaudois, University of Lausanne, Lausanne, Switzerland Anne M. Maillard * Service de Génétique Médicale, Centre Hospitalier Universitaire Vaudois, Lausanne,
Switzerland Sébastien Jacquemont Authors * Stefanie C. Linden View author publications You can also search for this author inPubMed Google Scholar * Cameron J. Watson View author
publications You can also search for this author inPubMed Google Scholar * Jacqueline Smith View author publications You can also search for this author inPubMed Google Scholar * Samuel J.
R. A. Chawner View author publications You can also search for this author inPubMed Google Scholar * Thomas M. Lancaster View author publications You can also search for this author inPubMed
Google Scholar * Ffion Evans View author publications You can also search for this author inPubMed Google Scholar * Nigel Williams View author publications You can also search for this
author inPubMed Google Scholar * David Skuse View author publications You can also search for this author inPubMed Google Scholar * F. Lucy Raymond View author publications You can also
search for this author inPubMed Google Scholar * Jeremy Hall View author publications You can also search for this author inPubMed Google Scholar * Michael J. Owen View author publications
You can also search for this author inPubMed Google Scholar * David E. J. Linden View author publications You can also search for this author inPubMed Google Scholar * LeeAnne Green-Snyder
View author publications You can also search for this author inPubMed Google Scholar * Wendy K. Chung View author publications You can also search for this author inPubMed Google Scholar *
Anne M. Maillard View author publications You can also search for this author inPubMed Google Scholar * Sébastien Jacquemont View author publications You can also search for this author
inPubMed Google Scholar * Marianne B. M. van den Bree View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Marianne B.
M. van den Bree. ETHICS DECLARATIONS CONFLICT OF INTEREST Dr. S.C. Linden, Dr. Watson, Jacqueline Smith, Dr. Chawner, Dr. Lancaster, Ffion Evans, Dr. Williams, Dr. Skuse, Dr. Raymond, Dr.
Green-Snyder, Dr. Chung, Dr. Maillard, Dr. Jacquemont and Dr. D.E.J. Linden have nothing to disclose. Dr. Hall, Dr. Owen and Dr. van den Bree report a grant from Takeda Pharmaceuticals,
outside the submitted work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLES, FIGURES AND METHODS SUPPLEMENTARY RESULTS RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and
the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Linden, S.C., Watson, C.J., Smith, J. _et al._ The psychiatric phenotypes of 1q21
distal deletion and duplication. _Transl Psychiatry_ 11, 105 (2021). https://doi.org/10.1038/s41398-021-01226-9 Download citation * Received: 23 July 2020 * Revised: 07 January 2021 *
Accepted: 14 January 2021 * Published: 04 February 2021 * DOI: https://doi.org/10.1038/s41398-021-01226-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative