Acute oxytocin effects in inferring others’ beliefs and social emotions in people at clinical high risk for psychosis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Social deficits are key hallmarks of the Clinical High Risk for Psychosis (CHR-P) state and of established psychotic disorders, and contribute to impaired social functioning,
indicating a potential target for interventions. However, current treatments do not significantly ameliorate social impairments in CHR-P individuals. Given its critical role in social
behaviour and cognition, the oxytocinergic (OT) system is a promising target for novel interventions in CHR-P subjects. In a double-blind, placebo-controlled, crossover design, 30 CHR-P
males were studied using functional magnetic resonance imaging (fMRI) on two occasions, once after 40IU self-administered intranasal OT and once after placebo. A modified version of the
Sally-Anne task was used to assess brain activation during inferring others’ beliefs and social emotions. The Reading the Mind in the Eyes Test was acquired prior to the first scan to test
whether OT effects were moderated by baseline social-emotional abilities. OT did not modulate behavioural performances but reduced activation in the bilateral inferior frontal gyrus compared
with placebo while inferring others’ social emotions. Furthermore, the relationship between brain activation and task performance after OT administration was moderated by baseline
social-emotional abilities. While task accuracy during inferring others’ social emotion increased with decreasing activation in the left inferior frontal gyrus in CHR-P individuals with low
social-emotional abilities, there was no such relationship in CHR-P individuals with high social-emotional abilities. Our findings may suggest that acute OT administration enhances neural
efficiency in the inferior frontal gyrus during inferring others’ social emotions in those CHR-P subjects with low baseline social-emotional abilities. SIMILAR CONTENT BEING VIEWED BY OTHERS
EFFECTS OF OXYTOCIN ADMINISTRATION ON NON-SOCIAL EXECUTIVE FUNCTIONS IN HUMANS: A PREREGISTERED SYSTEMATIC REVIEW AND META-ANALYSIS Article 18 January 2025 THE SOCIAL COGNITIVE AND NEURAL
MECHANISMS THAT UNDERLIE SOCIAL FUNCTIONING IN INDIVIDUALS WITH SCHIZOPHRENIA – A REVIEW Article Open access 21 October 2023 NEURAL CORRELATES OF PERSONAL SPACE REGULATION IN PSYCHOSIS: ROLE
OF THE INFERIOR PARIETAL CORTEX Article Open access 03 February 2025 INTRODUCTION Deficits in social functioning are core features of psychosis and predictive for the onset, development,
course and outcome of this illness1. Social functioning impairments are a distinctive characteristic of individuals at Clinical High Risk for Psychosis (CHR-P) to the point that they
represent the most impaired neurocognitive domain (Hedge’s _g_ = 0.55) in this patient population2,3,4. However, currently available approaches for CHR-P individuals fail to ameliorate their
social impairments, indicating the need for novel and more effective interventions that map onto core pathological processes underlying the CHR-P features5,6. Mind-reading, i.e., the
ability to infer others’ mental states, is integral for social interactions7 and impaired in early psychosis patients8 and CHR-P subjects9,10. Mental state attribution or perspective taking
is a complex multidimensional construct and involves the ability to infer the thoughts or beliefs of others (i.e. cognitive empathy), but also the ability to infer the emotions or feelings
of others (i.e. emotional empathy)11. Past evidence showed that cognitive and emotional aspects of empathy are partly dissociable systems12. While neurocognitive dysfunction significantly
accounts for impairments during theory of mind (ToM) tasks in CHR-P individuals10, they still remain evident after adjusting for deficits in neurocognition8, indicating the crucial
contribution of impaired emotional empathy to ToM deficits in CHR-P. Meta-analytical evidence identified the left anterior midcingulate cortex/dorsal anterior cingulate cortex, bilateral
insula/inferior frontal gyrus and bilateral supplementary motor area as being consistently activated in empathy13. While the bilateral anterior insula/inferior frontal gyrus is specifically
activated during emotional empathy, activation in the left midcingulate cortex and left anterior insula is more related to the cognitive aspect of empathy13. Previous functional magnetic
resonance imaging (fMRI) studies in CHR-P individuals reported increased activation in the prefrontal cortex including the inferior frontal gyrus, posterior cingulate cortex and the
temporoparietal cortex during inferring others’ mental status relative to healthy controls, suggesting a compensatory overactivation of brain regions critical for empathic responses during
mental state attribution14,15. Another study observed reduced bilateral inferior frontal gyrus activation during inferring others’ emotions but not beliefs in CHR-P subjects relative to
healthy subjects16. Given its critical role in human social behaviour and cognition17, the oxytocinergic (OT) system is a promising target for the treatment of social impairments in CHR-P
subjects. OT has been proposed to be a key neural substrate that interacts with central dopamine systems18 and may counteract hyperdopaminergia seen in psychosis19. Previous studies have
reported altered blood OT levels in schizophrenia patients20,21, which predicted social cue recognition21. CHR-P individuals show significantly decreased OT receptor gene methylation, which
was related to increased anhedonia-asociality22. OT administration improves mind-reading23 and trust24 in healthy volunteers. Notably, OT affects emotional rather than cognitive empathy in
healthy people25 and its effect on mind-reading depends on baseline social-emotional abilities26. A single dose of OT also improves emotion recognition27,28,29, higher-order social cognition
(such as appreciation of indirect hints and recognition of social faux pas30), as well as working memory31 in schizophrenia patients. However, evidence for OT effects on cognitive and
emotional empathy in early psychosis is sparse. A first study showed that 24 IU for 6 weeks did not improve cognitive empathy in individuals with early schizophrenia-spectrum psychotic
illness32. This study investigated, for the first time, whether acute OT administration changed the ability to successfully infer on others people’s beliefs (cognitive empathy) and social
emotions (emotional empathy) in CHR-P males, and whether this change goes along with altered neural activation. Based on previous evidence in healthy people25,33 and CHR-P individuals16, we
expected that OT would enhance successful recognition of others’ facial emotions but not beliefs and that this improvement in successful social emotion inferences would be accompanied by
increased neural activation in the emotional empathy network, in particular in the inferior frontal gyrus. As previously shown26,34, we further predicted that OT effects would be moderated
by baseline social-emotional abilities, with CHR-P individuals low in social-emotional abilities showing greater improvements after OT administration. MATERIALS AND METHODS PARTICIPANTS The
study received National Research Ethics Service approval (14/LO/1692) and all subjects gave written informed consent. The participants and OT administration have previously been described in
our Arterial Spin Labelling (ASL)35 and magnetic resonance spectroscopy (1H-MRS)36 studies. Sample size was estimated using G*Power 3 to detect neurochemical effects based on a previous
1H-MRS study37, which indicated that a sample size of 30 was sufficient to detect a medium within-subject effect size (_d__z_ = 0.53), when alpha=0.05 and power=80%. Thirty male,
help-seeking CHR-P individuals aged 18–35 were recruited from two specialist early detection services—the OASIS38 and Tower Hamlets Early Detection Service (THEDS), which belongs to the
Pan-London Network for Psychosis-prevention (PNP)39. CHR-P status was assessed using the Comprehensive Assessment of At-Risk Mental States (CAARMS) 12/2006 criteria40. In brief, subjects met
one or more of the following subgroup criteria: (a) attenuated psychotic symptoms, (b) brief limited intermittent psychotic symptoms (BLIPS, psychotic episode lasting <1 week, remitting
without treatment41,42,43), or (c) either schizotypal personality disorder or first-degree relative with psychosis40, all coupled with functional decline. Individuals were excluded if there
was a history of previous psychotic disorder (with the exception of BLIPS, some of whom may meet Acute and Transient Psychotic Disorder criteria42) or manic episode, exposure to
antipsychotics, neurological disorder or current substance-use disorder, estimated IQ < 70, acute intoxication on the day of scanning, and any contraindications to MRI or intranasal OT or
placebo. History of Axis I disorder(s) was not an exclusion criterion due to the transdiagnostic nature of the CAARMS-based definition of the CHR-P state44 and the high prevalence of such
diagnoses within these populations45. We also collected information on medication history, alcohol (Alcohol Use Disorders Identification Test), tobacco and cannabis use, functioning (Global
Functioning Role and Social scales46) and transition status. One subject reported cannabis use prior to one scan and was therefore excluded from analysis. Furthermore, to test for potential
moderation effects of baseline social-emotional abilities on OT effects26,47, the Reading the Mind in the Eyes Test (RMET48) was acquired prior to the first scan. RMET measures the ability
to interpret subtle social-emotional cues from the eye region48. It compromised 36 photographs depicting the eye region of Caucasian posers. Stimuli were presented on a black background in
the center of a computer screen. In the four corners around the picture, one target label and three distractors were simultaneously shown. Participants were asked to select the correct
mental state as fast as possible. The picture remained on the screen until participants clicked on a label with the mouse. A practice trial was conducted, and a glossary was provided in
paper form. Performance was determined by calculating the percentage of correct answers. Demographic and clinical characteristics of the sample are presented in Table 1. STUDY DESIGN Using a
randomised, double-blind, crossover design, 40 IU intranasal OT and placebo were administered in a counterbalanced fashion with a 1-week wash out period. During each challenge, subjects
underwent an MRI scan which started at 11:30 h to minimise potential effects of diurnal variation in OT or vasopressin49. Intranasal OT administration followed recommended guidelines and a
protocol adopted by a previous study conducted at our institute49. As done in our previous studies35,36, participants self-administered one puff (4 IU) of intranasal OT or matched placebo
every 30 s, alternating between nostrils, until 40 IU had been administered. See refs. 35,36 for more details. FUNCTIONAL MRI PARADIGM A modified version of the Sally-Anne task was used as
previously applied in individuals with autism spectrum disorder after OT administration50. Participants underwent two runs consecutively, each of which lasted approximately 7.5 min. Each run
consisted of 10 different stories (Supplementary Fig. 1), while every story was presented three times in a row, one for each of the three conditions: ‘control', ‘belief inference
(cognitive empathy)' and ‘social emotion inference (emotional empathy)'. The identical set of 10 stories was presented during the first and second run. At the end of each story
participants were required to answer ‘yes–no’. Questions were presented in a pseudorandom fashion, so that ‘yes’ questions in the first run were modified to be ‘no’ questions in the second
run, and vice versa. Before scanning, all participants were shown the cartoon stories in a mock scanner and required to answer questions as in the ‘control’ condition. In this manner,
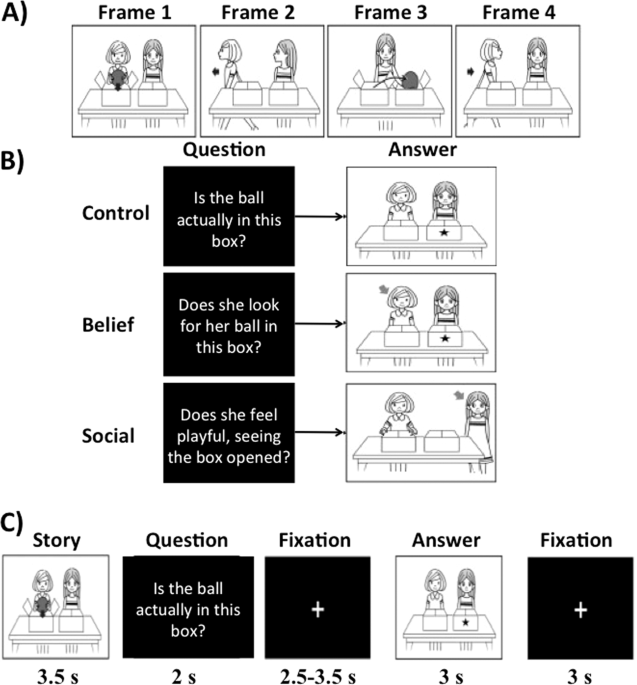
participants were trained to grasp the stories without experiencing the questions of the ‘Belief’ and ‘Social emotion’ conditions. Let us take one story as an example. As summarized in Fig.
1a, four frames show a story with two persons with neutral faces. During the story, one person (Sally) places a ball in a left box (Frame 1) and leaves the room (Frame 2). Meanwhile, another
person (Anne) moves the ball to a right box (Frame 3) and then Sally returns (Frame 4). At the end of the story, participants are asked the following three types of questions in a
pseudorandom order (Fig. 1b): ‘Is the ball actually in this box? (indicating a left box with a star)’ for the control condition, ‘Does she look for her ball in this box? (indicating Sally
with an arrow and a left box by a star)’ for the belief inference condition, and ‘Does she feel playful, seeing the box opened?’ for the social emotion inference condition. To answer the
first question (control condition), participants are required to understand the actual location of the ball. For the second question (belief inference), they are required to understand that
Sally’s actions will be based on what she believes to be true, rather than the actual location. In the third question (social emotion inference), participants were required to answer the
question about Anne’s emotional status after she completed the practical joke. To successfully infer on Anne’s emotional status, it is required not only to understand the story (control
condition) but also that Anne believes that Sally falsely believes the ball is in the left box. In other words, successful social emotion inference (emotional empathy) depended on successful
belief inference (cognitive empathy) but not vice versa. Task conditions and trial timing are summarized in Fig. 1c. Each trial consisted of 3.5 s story presentation with four frames (see
Fig. 1a); 2 s question; 2.5–3.5 s fixation cross; 3 s of a frame with comic vignette that illustrates an arrow and a star to help to answer the question; and 3 s fixation cross. Participants
were required to answer this question during the last frame with comic and fixation cross by pressing a button to answer yes or no. BEHAVIOURAL ANALYSIS We measured accuracy and reaction
time to correct responses during the task. Paired t-test was used to test OT effects for each task condition. IMAGE ACQUISITION AND PROCESSING All scans were conducted on a General Electric
Discovery MR750 3 Tesla system (General Electric, Chicago, USA) using a 32-channel head coil. During the task, we acquired T2*-weighted echo-planar images with the following parameters: 41
axial slices of 3 mm thickness, 0.3 mm interslice gap, field of view 24 × 24 cm2 and flip angle 75°. The repetition time was 2 s and the echo time 30 ms. EPIs were analysed using an
event-related design with SPM12 (www.fil.ion.ucl.ac.uk/spm). Pre-processing was performed for each subject and time point separately. In brief, slice-timing correction was first performed on
each volume using the middle slice as the reference to adjust for time differences due to multislice image acquisition. The images were then realigned to the first image in the series. The
anatomical T1 image was co-registered with the first functional image and then spatially normalized into the MNI space, generating normalization parameters, which were applied to all
functional images. Functional images were smoothed with a Gaussian kernel of 8 mm full half-width maximum (FWHM). All images were checked for movement artefacts and subjects with more than
10% corrupted volumes (all scans with more than 3 mm deviation from the previous scan in any dimension) were excluded (one subject). Voxel-wise maximum likelihood parameter estimates were
calculated during the first-level analysis using the general linear model. Our design matrix included an autoregressive AR(1) model of serial correlations and a high-pass filter with a
cut-off of 128 s. The onsets of each event were convolved with the SPM synthetic haemodynamic response function. Each event-related regressor had an onset at the time of the answer frame and
a duration that correspond to each response time (see Fig. 1c). First-level models were constructed for both treatments separately, including only the onset times for correct responses in
the control, belief and social emotion condition during both runs. In line with previous studies using the same task16,50, this approach allowed us to study whether OT administration changed
neural activation while successfully inferring others’ belief and social emotion relative to placebo. One subject had no correct responses during one of the four runs and was therefore
excluded. The final sample thus consisted of 27 subjects. Two contrast images were generated per participant: ‘belief > control’ (cognitive empathy) and ‘social emotion > control’
(emotional empathy). One-sample t tests were conducted to test for effects of condition (cognitive and emotional empathy) after placebo administration and paired t-tests were conducted to
test for treatment effects in the two interrelated contrasts of interest separately as previously done50. Significance was assessed at a cluster-level threshold of _p_ < 0.05 family-wise
error (FWE) corrected across the whole brain with an uncorrected cluster-forming threshold of _p_ < 0.00151,52 with an extent threshold of 20 voxels. IMPACT OF BASELINE SOCIAL-EMOTIONAL
ABILITIES The relationship between baseline social-emotional ability (RMET performance) and significant brain effect (left inferior frontal gyrus) after OT administration was tested using
Spearman correlation at a significance level of _p_ < 0.05. Parameter estimates were extracted from the whole left inferior frontal gyrus cluster (300 voxels) showing a significant effect
between OT and placebo during inferring others’ social emotions (‘social emotion > control’ contrast). Nonparameteric correlation was performed due to the fact that parameter estimates
of left inferior frontal gyrus activation were not normally distributed as indicated by a significant Kolmogorov–Smirnov test (_p_ = 0.033). We further used a linear regression to test
whether the relationship between brain activation in left inferior frontal gyrus and task accuracy after OT administration was moderated by RMET performance. Task accuracy was entered as
dependent variable and the interaction RMET x left inferior frontal gyrus activation as independent variable. To perform a parametric moderator analysis, parameter estimates in the left
inferior frontal gyrus after OT administration were first log transformed. RESULTS BEHAVIOURAL TASK PERFORMANCE OT did not modulate accuracy for the control (_T_ = 0.332, _p_ = 0.743),
belief (_T_ = 0.618, _p_ = 0.542) or social (_T_ = 0.775, _p_ = 0.445) condition (Fig. 2a). OT also did not influence reaction times for correct responses during the control (_T_ = 0.024,
_p_ = 0.981), belief (_T_ = 0.801, _p_ = 0.430) or social (_T_ = 1.11, _p_ = 0.277) condition (Fig. 2b). Correlations between reaction time and accuracy within and between the different task
conditions are presented in Supplementary Table 1. BRAIN ACTIVATION EFFECT OF CONDITIONS Inferring others’ beliefs (belief > control) evoked significant activation in left precuneus,
angular gyrus, inferior frontal and parietal gyrus and bilateral middle frontal gyrus (Supplementary Table 2, Supplementary Fig. 2A). Inferring others’ social emotions (social emotion >
control) evoked significant activation in left supplementary motor area, superior frontal gyrus, inferior temporal gyrus, angular gyrus, precuneus, right cerebellum, and bilateral inferior
and middle frontal gyrus, middle temporal gyrus and caudate (Supplementary Table 3, Supplementary Fig. 2B). EFFECT OF OT OT did not modulate brain activation during inferring others’ beliefs
relative to placebo (Supplementary Table 4). However, during inferring others’ social emotions, OT significantly reduced activation in the left and right inferior frontal gyrus (Table 2,
Fig. 3a). No significantly increased activation was found after OT relative to the placebo adiminstration during inferring others’ social emotions (Supplementary Table 5). Notably, the
differences in left (_T_ = 0.156, _p_ = 0.879) and right (_T_ = 0.873, _p_ = 0.419) inferior frontal gyrus activation after OT relative to placebo did not differ between those individuals
who received antidepressants (_n_ = 8) or not. MODERATER EFFECT OF BASELINE SOCIAL-EMOTIONAL ABILITIES ON OT EFFECTS We further tested whether left inferior frontal gyrus activation after OT
administration during inferring others’ social emotions (social emotion > control) was related to baseline RMET scores. There was a significant positive correlation between baseline RMET
scores and left inferior frontal gyrus activation after OT administration (_r_ = 0.388, _p_ = 0.046) (Fig. 3b). We further found that RMET score also moderated the relationship between left
frontal gyrus activation during inferring others’ social emotion and task accuracy after OT administration, as indicatined by a significant interaction RMET × left inferior frontal gyrus
(β=−3.53, _p_ = 0.020). The moderator effect was also evident after median (median = 26) splitting the sample into high (_n_ = 13) and low (_n_ = 14) RMET performance (β=−20.05, _p_ =
0.023). While task accuracy increased with decreasing activation in the left inferior frontal gyrus in CHR-P individual with low RMET scores (_r_ = −0.578, _p_ = 0.030), there was no
significant relationship between task accuracy and left inferior frontal gyrus activation in CHR-P individual with high RMET scores (_r_ = 0.088, _p_ = 0.775) (Fig. 3c). DISCUSSION To the
best of our knowledge, this is the first fMRI study to assess how acute OT administration modulated brain activation during inferring others’ beliefs and social emotions in CHR-P
individuals. The first main finding is that OT did not affect brain responses during belief inference (cognitive empathy) but reduced activation in the bilateral inferior frontal gyrus
during social emotion inference (emotional empathy). The second main finding is that neural activation in the left inferior frontal gyrus during social emotion inference after OT
administration was modulated by baseline social-emotional abilities, which also moderated the relationship between brain activation and task performance. These findings can inform subsequent
experimental therapeutics in this patient population. The first finding of this study is that while OT did not modulate neural responses during belief inference it did reduce neural
activation in the bilateral inferior frontal gyrus during social emotion inference. OT has been shown to enhance emotional but not cognitive empathy in healthy participants25,33, an effect
that could be replicated across culture and sex53. In early psychosis patients, OT treatment did not enhance cognitive empathy but improved the ability to recognize emotions in schizophrenia
patients27. We add to this line of evidence by showing that OT significantly decreased activation in the bilateral inferior frontal gyrus during emotional but not cognitive empathy in CHR-P
subjects. The inferior frontal gyrus has been increasingly implicated in emotion recognition tasks such as identification of emotional intonation54, judgment of facial expressions55 and is
an essential part of the neural mirror system, which is involved in a variety of important social behaviours, from imitation to emotional empathy56. Good imitation facilitates recognizing
emotions in other people and thus emotional empathy57. Previous seminal work demonstrated that patients with inferior frontal gyrus lesions displayed extremely impaired emotional but not
cognitive empathy12. More importantly, a previous study that used the same fMRI task in CHR-P found reduced activation in the bilateral inferior frontal gyrus when inferring others emotions
but not beliefs relative to healthy controls16. The inferior frontal gyrus is a core target of the neurofunctional effects of OT58. OT may promote assimilation of novel emotional experiences
into internal models59, which is supported by parts of the left inferior frontal gyrus60. Emotion perception is impaired in patients with schizophrenia1, CHR-P subjects61, offspring of
parents with schizophrenia62 and is related to poor functional outcomes1,61. As a potential compensatory mechanism for this deficit, stronger inferior frontal gyrus activation during
emotional perspective taking has been observed in CHR-P subjects compared with healthy controls14,15. Given that task performance during emotional empathy did not differ between OT and
placebo in the current study, decreased inferior frontal gyrus activation after OT administration might indicate enhanced neural efficiency. Such an interpretation corresponds with a recent
study showing that OT enhances neural efficiency of social perception as expressed by reduced event-related potential latencies63. Taken together, we might speculate that after receiving
placebo, CHR-P subjects compensated their deficits in emotional perspective-taking by increasing activation in the inferior frontal gyrus compared with healthy individuals, as previously
shown14,15, whereas OT may enhance neural efficiency in the inferior frontal gyrus by normalizing activation to the level of healthy persons. The second main finding was of a positive
relationship between baseline social-emotional abilities and left inferior frontal gyrus activation during social emotion inference (emotional empathy) after OT administration. Moreover,
baseline social-emotional abilities as expressed by RMET performance moderated the relationship between OT-induced task performance and inferior frontal gyrus activation during emotional
empathy. In particular, whereas inferior frontal gyrus activation decreased with increasing task performance in CHR-P subjects with low baseline social-emotional abilities, no such
relationship was found in CHR-P subjects with high baseline social-emotional abilities. These findings suggest that OT-induced improvement in neural efficiency as expressed by decreased
activation in the inferior frontal gyrus only took place in those CHR-P subjects with low baseline social-emotional abilities. In other words, CHR-P subjects with low social-emotional
abilities needed less activation in the inferior frontal gyrus to achieve comparable task performance as after placebo administration. This finding resonates with previous reports
demonstrating that OT selectively improved mind-reading as assessed with the RMET in those individuals with low emotional empathy26, high in alexithymia or high in maternal love
withdrawal34,64,65. Although RMET performance has mostly been associated with cognitive empathy26, a recent study demonstrated that the RMET measures emotion recognition rather than theory
of mind (i.e. cognitive empathy) ability66. Taken together, there is emerging evidence that OT influences emotional rather than cognitive processing and that its benefit depends on emotional
empathy capacity. There are several possible clinical implications. The dearth of preventive treatments that can effectively impact clinical outcomes—in particular social functioning
deficits— can be partially explained by lack of clear aetiopathological mechanisms underlying the therapeutic effects of preventive agents. This study advances knowledge in this area by
providing the first disease target-engagement evidence in CHR-P individuals. These neurophysiological results can inform the development of subsequent randomised controlled trials
investigating OT’s effects on CHR-P symptoms and on clinical outcomes. Identifying novel therapeutic target for outcomes other than psychosis onset is a mainstream of future research in this
area, considering that most CHR-P individuals who will not transition to psychosis will still display social functioning deficits at follow-up67. The present study has limitations. No
healthy control group was included in the current study. However, we have used a fMRI task which was already tested in CHR-P vs. healthy controls16, demonstrating significant alterations in
key brain areas that are also implicated in this study. Due to sex differences in brain responses to OT during social interaction68, only male subjects were included. Another limitation is
that this study did not collect extensive data on negative symptoms or neurocognition. Dose-response assessments are further required to test whether lower dosing might be sufficient for
optimal target engagement, i.e. improving neural efficiency in the inferior frontal gyrus. While this study demonstrated that acute OT administration modulates key brain regions during
inferring others’ social emotions, OT effects on functional outcomes in CHR-P subjects need to be tested in future longer-term clinical trials. Furthermore, in terms of the
neurophysiological mechanisms by which OT has its effects, we previously reported that OT modulates cerebral (hippocampal) perfusion in CHR-P patients35, but did not appear to modulate
regional concentrations of neurochemical metabolites36. The mechanisms by which OT increases neural efficiency in CHR-P individuals therefore warrants further research. A final limitation is
that because of the small sample size we have been unable to stratify the heterogeneous CHR-P group across its three main subgroups69. In conclusion, the present study provides the first
evidence that acute OT administration modulates brain regions while inferring others’ social emotions in CHR-P subjects. Abnormal emotion perception persists over the course of the illness70
and is predictive for poor functional outcomes in CHR-P subjects61. This study may thus provide neural targets to develop novel treatments for socio-emotional deficits in CHR-P subjects
with the aim to improve functional outcomes. REFERENCES * Couture, S. M., Penn, D. L. & Roberts, D. L. The functional significance of social cognition in schizophrenia: a review.
_Schizophr. Bull._ 32(Suppl. 1), S44–63 (2006). PubMed PubMed Central Google Scholar * Fusar-Poli, P. et al. Social dysfunction predicts two years clinical outcome in people at ultra high
risk for psychosis. _J. Psychiatr. Res._ 44, 294–301 (2010). CAS PubMed Google Scholar * Fusar-Poli, P. et al. Cognitive functioning in prodromal psychosis: a meta-analysiscognitive
functioning in prodromal psychosis. _Arch. Gen. Psychiatry_ 69, 562–571 (2012). PubMed Google Scholar * Lincoln, S. H., Norkett, E. M., Frost, K. H., Gonzalez-Heydrich, J. & D’Angelo,
E. J. A developmental perspective on social-cognition difficulties in youth at clinical high risk for psychosis. _Harv. Rev. Psychiatry_ 25, 4–14 (2017). PubMed Google Scholar * Davies, C.
et al. Efficacy and acceptability of interventions for attenuated positive psychotic symptoms in individuals at clinical high risk of psychosis: a network meta-analysis. _Front Psychiatry_
9, 187 (2018). PubMed PubMed Central Google Scholar * Davies, C. et al. Lack of evidence to favor specific preventive interventions in psychosis: a network meta-analysis. _World
Psychiatry_ 17, 196–209 (2018). PubMed PubMed Central Google Scholar * Amodio, D. M. & Frith, C. D. Meeting of minds: the medial frontal cortex and social cognition. _Nat. Rev.
Neurosci._ 7, 268–277 (2006). CAS PubMed Google Scholar * Langdon, R., Connors, M. H., Still, M., Ward, P. B. & Catts, S. Theory of mind and neurocognition in early psychosis: a
quasi-experimental study. _BMC Psychiatry_ 14, 316 (2014). PubMed PubMed Central Google Scholar * Ohmuro, N. et al. Deficits of cognitive theory of mind and its relationship with
functioning in individuals with an at-risk mental state and first-episode psychosis. _Psychiatry Res._ 243, 318–325 (2016). PubMed Google Scholar * Hur, J. W. et al. General intellectual
functioning as a buffer against theory-of-mind deficits in individuals at ultra-high risk for psychosis. _Schizophr. Res._ 149, 83–87 (2013). PubMed Google Scholar * Healey, M. L. &
Grossman, M. Cognitive and affective perspective-taking: evidence for shared and dissociable anatomical substrates. _Front. Neurol._ 9, 491 (2018). PubMed PubMed Central Google Scholar *
Shamay-Tsoory, S. G., Aharon-Peretz, J. & Perry, D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial
prefrontal lesions. _Brain_ 132(Pt 3), 617–627 (2009). PubMed Google Scholar * Fan, Y., Duncan, N. W., de Greck, M. & Northoff, G. Is there a core neural network in empathy? An fMRI
based quantitative meta-analysis. _Neurosci. Biobehav. Rev._ 35, 903–911 (2011). PubMed Google Scholar * Brüne, M. et al. An fMRI study of “theory of mind” in at-risk states of psychosis:
comparison with manifest schizophrenia and healthy controls. _Neuroimage_ 55, 329–337 (2011). PubMed Google Scholar * Derntl, B. et al. Empathy in individuals clinically at risk for
psychosis: brain and behaviour. _Br. J. Psychiatry_ 207, 407–413 (2015). PubMed Google Scholar * Takano, Y. et al. Neural basis for inferring false beliefs and social emotions in others
among individuals with schizophrenia and those at ultra-high risk for psychosis. _Psychiatry Res. Neuroimaging_ 259, 34–41 (2017). PubMed Google Scholar * Meyer-Lindenberg, A., Domes, G.,
Kirsch, P. & Heinrichs, M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. _Nat. Rev. Neurosci._ 12, 524–538 (2011). CAS PubMed Google
Scholar * Baskerville, T. A. & Douglas, A. J. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. _CNS Neurosci. Ther._ 16,
e92–e123 (2010). CAS PubMed PubMed Central Google Scholar * Feifel, D., Shilling, P. D. & MacDonald, K. A review of oxytocin’s effects on the positive, negative, and cognitive
domains of schizophrenia. _Biol. Psychiatry_ 79, 222–233 (2016). CAS PubMed Google Scholar * Jobst, A. et al. Oxytocin and vasopressin levels are decreased in the plasma of male
schizophrenia patients. _Acta Neuropsychiatr._ 26, 347–355 (2014). PubMed Google Scholar * Strauss, G. P. et al. Plasma oxytocin levels predict social cue recognition in individuals with
schizophrenia. _Schizophr. Res._ 162, 47–51 (2015). PubMed PubMed Central Google Scholar * Bang, M. et al. Reduced DNA methylation of the oxytocin receptor gene is associated with
anhedonia-asociality in women with recent-onset schizophrenia and ultra-high risk for psychosis. _Schizophr. Bull._ 45, 1279–1290 (2019). PubMed PubMed Central Google Scholar * Domes, G.,
Heinrichs, M., Michel, A., Berger, C. & Herpertz, S. C. Oxytocin improves “mind-reading” in humans. _Biol. Psychiatry_ 61, 731–733 (2007). CAS PubMed Google Scholar * Kosfeld, M.,
Heinrichs, M., Zak, P. J., Fischbacher, U. & Fehr, E. Oxytocin increases trust in humans. _Nature_ 435, 673–676 (2005). CAS PubMed Google Scholar * Hurlemann, R. et al. Oxytocin
enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. _J. Neurosci._ 30, 4999–5007 (2010). CAS PubMed PubMed Central Google Scholar * Radke, S. &
de Bruijn, E. R. Does oxytocin affect mind-reading? A replication study. _Psychoneuroendocrinology_ 60, 75–81 (2015). CAS PubMed Google Scholar * Averbeck, B. B., Bobin, T., Evans, S.
& Shergill, S. S. Emotion recognition and oxytocin in patients with schizophrenia. _Psychol. Med._ 42, 259–266 (2012). CAS PubMed Google Scholar * Fischer-Shofty, M. et al. Improving
social perception in schizophrenia: the role of oxytocin. _Schizophr. Res._ 146, 357–362 (2013). CAS PubMed Google Scholar * Woolley, J. D. et al. Oxytocin administration enhances
controlled social cognition in patients with schizophrenia. _Psychoneuroendocrinology_ 47, 116–125 (2014). CAS PubMed PubMed Central Google Scholar * Guastella, A. J. et al. A single
dose of oxytocin nasal spray improves higher-order social cognition in schizophrenia. _Schizophr. Res._ 168, 628–633 (2015). PubMed Google Scholar * Michalopoulou, P. G. et al. The effects
of a single dose of oxytocin on working memory in schizophrenia. _Schizophr. Res._ 162, 62–63 (2015). PubMed PubMed Central Google Scholar * Cacciotti-Saija, C. et al. A double-blind
randomized controlled trial of oxytocin nasal spray and social cognition training for young people with early psychosis. _Schizophr. Bull._ 41, 483–493 (2015). PubMed Google Scholar *
Shamay-Tsoory, S. G. The neural bases for empathy. _Neuroscientist_ 17, 18–24 (2011). PubMed Google Scholar * Luminet, O., Grynberg, D., Ruzette, N. & Mikolajczak, M.
Personality-dependent effects of oxytocin: greater social benefits for high alexithymia scorers. _Biol. Psychol._ 87, 401–406 (2011). PubMed Google Scholar * Davies, C. et al. Oxytocin
modulates hippocampal perfusion in people at clinical high risk for psychosis. _Neuropsychopharmacology_ 44, 1300–1309 (2019). CAS PubMed PubMed Central Google Scholar * Davies, C. et
al. Neurochemical effects of oxytocin in people at clinical high risk for psychosis. _Eur. Neuropsychopharmacol._ 29, 601–615 (2019). CAS PubMed Google Scholar * Stone, J. M. et al.
Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. _Biol. Psychiatry_ 66, 533–539 (2009). CAS PubMed Google Scholar * Fusar-Poli,
P., Byrne, M., Badger, S., Valmaggia, L. R. & McGuire, P. K. Outreach and support in south London (OASIS), 2001-2011: ten years of early diagnosis and treatment for young individuals at
high clinical risk for psychosis. _Eur. Psychiatry_ 28, 315–326 (2013). CAS PubMed Google Scholar * Fusar-Poli, P. et al. Pan-London network for psychosis-prevention (PNP). _Front.
Psychiatry_ 10, 707 (2019). PubMed PubMed Central Google Scholar * Yung, A. R. et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. _Aust. N. Z.
J. Psychiatry_ 39, 964–971 (2005). PubMed Google Scholar * Fusar-Poli, P. et al. Unmet needs for treatment in 102 individuals with brief and limited intermittent psychotic symptoms
(BLIPS): implications for current clinical recommendations. _Epidemiol. Psychiatr. Sci._ 29, e67, 1–9 (2019). * Fusar-Poli, P. et al. Diagnostic and prognostic significance of brief limited
intermittent psychotic symptoms (BLIPS) in individuals at ultra high risk. _Schizophr. Bull._ 43, 48–56 (2017). PubMed Google Scholar * Fusar-Poli, P. et al. Prognosis of brief psychotic
episodes: a meta-analysis. _JAMA Psychiatry_ 73, 211–220 (2016). PubMed Google Scholar * Fusar-Poli, P. et al. towards a standard psychometric diagnostic interview for subjects at ultra
high risk of psychosis: CAARMS versus SIPS. _Psychiatry J._ 2016, 7146341 (2016). * Fusar-Poli, P., Nelson, B., Valmaggia, L., Yung, A. R. & McGuire, P. K. Comorbid depressive and
anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. _Schizophr. Bull._ 40, 120–131 (2014). PubMed Google Scholar *
Cornblatt, B. A. et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. _Schizophr. Bull._ 33, 688–702 (2007). PubMed
PubMed Central Google Scholar * Bartz, J. A., Zaki, J., Bolger, N. & Ochsner, K. N. Social effects of oxytocin in humans: context and person matter. _Trends Cogn. Sci._ 15, 301–309
(2011). CAS PubMed Google Scholar * Baron-Cohen, S., Wheelwright, S., Hill, J., Raste, Y. & Plumb, I. The “Reading the Mind in the Eyes” Test revised version: a study with normal
adults, and adults with Asperger syndrome or high-functioning autism. _J. Child Psychol. Psychiatry_ 42, 241–251 (2001). CAS PubMed Google Scholar * Paloyelis, Y. et al. A spatiotemporal
profile of in vivo cerebral blood flow changes following intranasal oxytocin in humans. _Biol. Psychiatry_ 79, 693–705 (2016). CAS PubMed Google Scholar * Aoki, Y. et al. Oxytocin
improves behavioural and neural deficits in inferring others’ social emotions in autism. _Brain_ 137(Pt 11), 3073–3086 (2014). PubMed Google Scholar * Petersson, K. M., Nichols, T. E.,
Poline, J. B. & Holmes, A. P. Statistical limitations in functional neuroimaging. II. Signal detection and statistical inference. _Philos. Trans. R. Soc. Lond. B: Biol. Sci._ 354,
1261–1281 (1999). CAS Google Scholar * Woo, C. W., Krishnan, A. & Wager, T. D. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. _Neuroimage_ 91,
412–419 (2014). PubMed Google Scholar * Geng, Y. et al. Oxytocin enhancement of emotional empathy: generalization across cultures and effects on amygdala activity. _Front. Neurosci._ 12,
512 (2018). PubMed PubMed Central Google Scholar * Wildgruber, D. et al. Identification of emotional intonation evaluated by fMRI. _Neuroimage_ 24, 1233–1241 (2005). CAS PubMed Google
Scholar * Kesler-West, M. L. et al. Neural substrates of facial emotion processing using fMRI. _Brain Res. Cogn. Brain Res._ 11, 213–226 (2001). CAS PubMed Google Scholar * Iacoboni, M.
& Dapretto, M. The mirror neuron system and the consequences of its dysfunction. _Nat. Rev. Neurosci._ 7, 942–951 (2006). CAS PubMed Google Scholar * Iacoboni, M. Imitation, empathy,
and mirror neurons. _Annu. Rev. Psychol._ 60, 653–670 (2009). PubMed Google Scholar * Wigton, R. et al. Neurophysiological effects of acute oxytocin administration: systematic review and
meta-analysis of placebo-controlled imaging studies. _J. Psychiatry Neurosci._ 40, E1–E22 (2015). PubMed PubMed Central Google Scholar * Tops, M. et al. The role of oxytocin in
familiarization-habituation responses to social novelty. _Front. Psychol._ 4, 761 (2013). PubMed PubMed Central Google Scholar * Tops, M., Koole, S. L., IJzerman, H. &
Buisman-Pijlman, F. T. Why social attachment and oxytocin protect against addiction and stress: Insights from the dynamics between ventral and dorsal corticostriatal systems. _Pharm.
Biochem. Behav._ 119, 39–48 (2014). CAS Google Scholar * Modinos, G. et al. Association of adverse outcomes with emotion processing and its neural substrate in individuals at clinical high
risk for psychosis. _JAMA Psychiatry_ 77, 190–200 (2019). * Horton, L. E., Bridgwater, M. A. & Haas, G. L. Emotion recognition and social skills in child and adolescent offspring of
parents with schizophrenia. _Cogn. Neuropsychiatry_ 22, 175–185 (2017). PubMed Google Scholar * Tillman, R. et al. Oxytocin enhances the neural efficiency of social perception. _Front.
Hum. Neurosci._ 13, 71 (2019). PubMed PubMed Central Google Scholar * Riem, M. M., Bakermans-Kranenburg, M. J., Voorthuis, A. & van IJzendoorn, M. H. Oxytocin effects on mind-reading
are moderated by experiences of maternal love withdrawal: an fMRI study. _Prog. Neuropsychopharmacol. Biol. Psychiatry_ 51, 105–112 (2014). CAS PubMed Google Scholar * Feeser, M. et al.
Oxytocin improves mentalizing—pronounced effects for individuals with attenuated ability to empathize. _Psychoneuroendocrinology_ 53, 223–232 (2015). CAS PubMed Google Scholar * Oakley,
B. F. M., Brewer, R., Bird, G. & Catmur, C. Theory of mind is not theory of emotion: a cautionary note on the Reading the Mind in the Eyes Test. _J. Abnorm. Psychol._ 125, 818–823
(2016). PubMed PubMed Central Google Scholar * Rutigliano, G. et al. Persistence or recurrence of non-psychotic comorbid mental disorders associated with 6-year poor functional outcomes
in patients at ultra high risk for psychosis. _J. Affect Disord._ 203, 101–110 (2016). PubMed Google Scholar * Rilling, J. K. et al. Sex differences in the neural and behavioral response
to intranasal oxytocin and vasopressin during human social interaction. _Psychoneuroendocrinology_ 39, 237–248 (2014). CAS PubMed Google Scholar * Fusar-Poli, P. et al. Heterogeneity of
psychosis risk within individuals at clinical high risk: a meta-analytical stratification. _JAMA Psychiatry_ 73, 113–120 (2016). PubMed Google Scholar * Comparelli, A. et al. Emotion
recognition impairment is present early and is stable throughout the course of schizophrenia. _Schizophr. Res._ 143, 65–69 (2013). PubMed Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust and King’s
College London (P.F.P., P.M.); by a Brain & Behaviour Research Foundation NARSAD Award (grant number 22593 to P.F.P.); and by the Department of Psychosis Studies, Institute of
Psychiatry, Psychology & Neuroscience, King’s College London. D.O. is supported by the UK Medical Research Council (MR/N013700/1) and is a King’s College London member of the MRC
Doctoral Training Partnership in Biomedical Sciences. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Psychiatry (UPK), University of Basel, Basel, Switzerland André Schmidt
& Stefan Borgwardt * Early Psychosis: Interventions and Clinical-detection (EPIC) lab, Department of Psychosis Studies, Institute of Psychiatry, Psychology & Neuroscience, King’s
College London, London, UK Cathy Davies, Andrea De Micheli, Valentina Ramella-Cravaro, Umberto Provenzani, Grazia Rutigliano, Marco Cappucciati, Dominic Oliver & Paolo Fusar-Poli *
Department of Neuroimaging, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, UK Yannis Paloyelis, Fernando Zelaya & Steve Williams * Department of
Psychosis Studies, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, UK Nicholas Meyer, Paul Allen, Sukhi Shergill, Paul Morrison, Philip McGuire &
Paolo Fusar-Poli * National Institute for Health Research (NIHR) Biomedical Research Centre (BRC), South London and Maudsley NHS Foundation Trust, London, UK Andrea De Micheli & Philip
McGuire * Department of Brain and Behavioural Sciences, University of Pavia, Pavia, Italy Umberto Provenzani & Paolo Fusar-Poli * Department of Psychiatry, Hamamatsu University School of
Medicine, Shizuoka, Japan Yuta Aoki & Hidenori Yamasue * Medical Institute of Developmental Disabilities Research, Showa University, Tokyo, Japan Yuta Aoki * Department of Psychosocial
Medicine, National Center for Child Health and Development, Tokyo, Japan Yuta Aoki * Tower Hamlets Early Detection Service (THEDS), East London NHS Foundation Trust, London, UK Silvia
Murguia * Department of Psychology, University of Roehampton, London, UK Paul Allen * Institute of Pharmaceutical Science, King’s College London, London, UK David Taylor * Department of
Psychiatry and Psychotherapy, University of Lübeck, Lübeck, Germany Stefan Borgwardt * Outreach and Support in South London (OASIS) Service, South London and Maudsley NHS Foundation Trust,
London, UK Philip McGuire & Paolo Fusar-Poli Authors * André Schmidt View author publications You can also search for this author inPubMed Google Scholar * Cathy Davies View author
publications You can also search for this author inPubMed Google Scholar * Yannis Paloyelis View author publications You can also search for this author inPubMed Google Scholar * Nicholas
Meyer View author publications You can also search for this author inPubMed Google Scholar * Andrea De Micheli View author publications You can also search for this author inPubMed Google
Scholar * Valentina Ramella-Cravaro View author publications You can also search for this author inPubMed Google Scholar * Umberto Provenzani View author publications You can also search for
this author inPubMed Google Scholar * Yuta Aoki View author publications You can also search for this author inPubMed Google Scholar * Grazia Rutigliano View author publications You can
also search for this author inPubMed Google Scholar * Marco Cappucciati View author publications You can also search for this author inPubMed Google Scholar * Dominic Oliver View author
publications You can also search for this author inPubMed Google Scholar * Silvia Murguia View author publications You can also search for this author inPubMed Google Scholar * Fernando
Zelaya View author publications You can also search for this author inPubMed Google Scholar * Paul Allen View author publications You can also search for this author inPubMed Google Scholar
* Sukhi Shergill View author publications You can also search for this author inPubMed Google Scholar * Paul Morrison View author publications You can also search for this author inPubMed
Google Scholar * Steve Williams View author publications You can also search for this author inPubMed Google Scholar * David Taylor View author publications You can also search for this
author inPubMed Google Scholar * Stefan Borgwardt View author publications You can also search for this author inPubMed Google Scholar * Hidenori Yamasue View author publications You can
also search for this author inPubMed Google Scholar * Philip McGuire View author publications You can also search for this author inPubMed Google Scholar * Paolo Fusar-Poli View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to André Schmidt. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare
that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License,
which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link
to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Schmidt, A., Davies, C., Paloyelis, Y. _et al._ Acute oxytocin effects in inferring others’ beliefs and social emotions in people at
clinical high risk for psychosis. _Transl Psychiatry_ 10, 203 (2020). https://doi.org/10.1038/s41398-020-00885-4 Download citation * Received: 23 January 2020 * Revised: 19 May 2020 *
Accepted: 26 May 2020 * Published: 22 June 2020 * DOI: https://doi.org/10.1038/s41398-020-00885-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative