The real-life costs of emotion regulation in anorexia nervosa: a combined ecological momentary assessment and fmri study
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Regulation of emotions is necessary for successful attainment of short-term and long-term goals. However, over-regulation may also have its costs. In anorexia nervosa (AN), forgoing
food intake despite emaciation and endocrine signals that promote eating is an example of “too much” self-control. Here we investigated whether voluntary emotion regulation in AN patients
comes with associated disorder-relevant costs. Thirty-five patients with acute AN and thirty-five age-matched healthy controls (HCs) performed an established emotion regulation paradigm
during functional magnetic resonance imaging after an overnight fast. The task required reducing emotions induced by positively valenced pictures via distancing. We calculated a neural
regulation score from responses recorded in a reward-related brain region of interest (ventral striatum; VS) by subtracting activation measured on “positive distance” trials from that
elicited under the “positive watch” (baseline) condition. Complementing the imaging data, we used ecological momentary assessment (EMA) to probe disorder-related rumination and affect six
times/day for 2 weeks following the scanning session. The neural regulation score indicating reduced VS activation during emotion regulation was used as a predictor in hierarchical linear
models with EMA measures as outcomes. No group differences in neural activity were found for the main contrasts of the task. However, regulation of VS activity was associated with increased
body-related rumination and increased negative affect in AN, but not in HC. In line with this finding, correlational analysis with longitudinal BMI measurements revealed a link between
greater VS regulation and poorer treatment outcome after 60 and 90 days. Together, these results identify a neural correlate of altered emotion regulation in AN, which seems to be
detrimental to psychological well-being and may interfere with recovery. SIMILAR CONTENT BEING VIEWED BY OTHERS INTACT NEURAL AND BEHAVIORAL CORRELATES OF EMOTION PROCESSING AND REGULATION
IN WEIGHT-RECOVERED ANOREXIA NERVOSA: A COMBINED FMRI AND EMA STUDY Article Open access 24 January 2022 THE COSTS OF OVER-CONTROL IN ANOREXIA NERVOSA: EVIDENCE FROM FMRI AND ECOLOGICAL
MOMENTARY ASSESSMENT Article Open access 21 May 2021 ABNORMAL SENSE OF AGENCY IN EATING DISORDERS Article Open access 30 August 2023 INTRODUCTION Effective behavioral and emotional
self-regulation is critical for the success of everyday functioning and health1,2,3,4. Research on self-regulation has mainly focused on failures of volitional control, such as in problem
gambling, substance abuse, or obesity5,6. In contrast, clinical observations of patients with anorexia nervosa (AN) are suggestive of relatively elevated self-control. One of the most
puzzling questions is how patients are able to abstain from food intake despite extreme low body weight and endocrine signals that promote eating7,8. In addition, primary rewards (food and
sex) are often avoided by AN patients9,10,11,12. One hypothesis is that AN patients downregulate their responses to rewarding stimuli, which would be in line with reward-centered models of
AN13,14,15 and a growing body of literature implicating alterations in activity and connectivity of reward-related mesolimbic brain structures such as the ventral striatum (VS) in
AN16,17,18. Complementing these observations, some fMRI studies have found alterations in frontoparietal networks involved in cognitive control and executive functions19,20,21,22,23.
Researchers have proposed that not only a lack of, but also elevated self-control might come with associated costs24,25. This might also be the case for emotion regulation. Walter et al.26
demonstrated that initial successful downregulation of amygdala activity (measured using fMRI) via a reappraisal strategy (detachment; i.e., regulating emotion by adopting the perspective of
a distanced and uninvolved observer)27,28 was followed by a paradoxical increase of amygdala activity during a rest period immediately after stimulus presentation. This finding underscores
the notion that cognitive emotion regulation strategies might also have their costs. Regarding the regulation of positive emotions, distancing has been shown to be effective in
downregulating reward-related feelings29,30,31, as well as in neural activation in the VS30,32. However, to date little is known about the costs of downregulating positive emotions. Studies
in healthy volunteers have emphasized the association between self-control, eating, and weight33,34. Indeed, clinical observations and data collected using ecological momentary assessment
(EMA) suggest that, despite showing avoidance behaviors and regulating feelings of hunger, AN patients constantly ruminate about food and their bodies35,36,37. Also, research on restrained
eaters or externally imposed food restriction indicates that chronic restriction of eating leads to emotional problems, increased distractibility, and obsessions with food38,39,40. Other
studies also found an increase in eating disorder (ED) symptoms after using thought suppression as a regulation strategy41,42,43, suggesting possible consequences in ED samples. To shed
light on the possible costs of excessive volitional control of reward-related processes in AN, a sample of acute patients and age-matched healthy controls (HCs) were asked to regulate
emotions elicited by viewing positive pictures (e.g., puppies, happy family scenes, or fun sport) during fMRI. The focus of the current study was to assess the possible associations between
regulation success (as gauged by the level of reduction in activity of the VS) and both short-term (affective states and ruminations) and long-term (weight gain) outcome measures. This is an
important open question because measuring current, momentary effect in patients in natural settings may provide a more accurate index of true affective experience than questionnaires
administered in the laboratory44. We employed a modified version of an established emotion regulation paradigm26,27,45,46,47, which focuses on distancing as an emotion regulation strategy.
We hypothesized that increased regulation of emotions elicited by rewarding stimuli (positive pictures) in patients would be associated with an increase in intrusive disease-relevant
thoughts (food/body) as well as with negative effect and tension measured in the natural environment. Further, we expected higher regulation success to be associated with less favorable
long-term outcome as measured in follow-up body mass index (BMI) in the patient group. MATERIALS AND METHODS PARTICIPANTS The sample in the current study consisted of a total of 70 female
volunteers: 35 patients with acute AN according to DSM-V (12.1–29.2 years old) and 35 HC (12.1–29.5 years old). AN patients were admitted to ED programs of a university child and adolescent
psychiatry and psychosomatic medicine department and were assessed and scanned within 96 h after the beginning of a behaviorally oriented nutritional rehabilitation program. To be included
in the HC group, participants had to be of normal weight and eumenorrhoeic. Normal weight was defined as BMI equal or above the 10th age percentile (if 18 years or younger)/BMI equal or
above 18.5 kg/m2 (if older than 18 years), or below the 94th age percentile (if 18 years or younger)/BMI below 28 kg/m2 (if older than 18 years). HCs were recruited through advertisement
among middle school, high school, and university students. Exclusion criteria and possible confounding variables for both groups were obtained using a semi-structured research interview
(SIAB-EX), our own semi-structured interview, and medical records if applicable (for further details of exclusion and inclusion criteria of both groups, as well as partial overlap with our
previous study Seidel et al.36 please see Supplementary Material 1.1). Study data were collected and managed using secure, web-based electronic data capture tools REDCap (Research Electronic
Data Capture)48. This study was approved by the local Institutional Review Board, and all participants (and if underage their guardians) gave written informed consent. CLINICAL MEASURES To
complement the information obtained with the clinical interviews, we assessed ED-specific psychopathology using the Eating Disorder Inventory (EDI-2)49 and depressive symptoms using the Beck
Depression Inventory50. For habitual use of the emotion regulation strategies' reappraisal and suppression, we used the Emotion Regulation Questionnaire (ERQ)51. BMI, gender, and
age-corrected BMI-standard deviation score (BMI-SDS)52 development of patients was measured at the day of scanning and after 30, 60, and 90 days after admission to the inpatient
rehabilitation program. EMOTION REGULATION TASK During the task (see Supplementary Fig. S1), participants were asked to either passively view the set of negative, positive, and neutral
pictures or to actively downregulate any emotions arising in response to the negative and positive pictures. Negative, positive, and neutral stimuli for the emotion regulation task were
selected from the International Affective Picture System53 and the emotional pictures set (Emopics)54. All stimuli were presented onto a back-projection screen located at the rear end of the
scanner and were viewed through a mirror attached to the head coil. During the view condition, participants were instructed to simply view the picture without modulating any associated
feelings, while not to look away or distract themselves in any way. During the regulation condition they were told to try to downregulate any elicited feeling via the reappraisal strategy
“distancing”. More specifically, they were instructed to detach themselves from the upcoming emotion: “Look at the following picture directly, but try to take the position of a non-involved
observer/thinking about the present picture in a neutral way/imagine that between you and the picture is a wall of glass/imagine the picture is getting smaller and smaller”. The instruction
for each condition was given by presenting a cue word laid over the stimulus for 1.5 s, stating either “view” or “distance”. We did not include a “distance neutral” condition in the
experiment because of an assumed lack of validity of this condition; we expected no initial emotional reaction that allowed being downregulated. After each picture presentation, participants
were asked to rate how aroused they were at the current moment on a visual analog scale, ranging from “very aroused” to “not aroused at all”. To ensure understanding of the instructions and
familiarity with the procedure, all participants underwent a training session outside the MR scanner, which took about 10 min and consisted of 17 trials, training each condition. After
completion, they were asked whether they had any difficulties applying the instructions and to explain what they had done during the regulation instruction. If this report was incompatible
with prior instructions or participants reported difficulties with the task, instructions were read again and participants were asked to do another training session. All stimuli used in
training were different from those shown in the main experiment. The complete task consisted of 100 trials (20 per condition), which were presented in pseudorandomized order with each
condition constrained to not occur more than twice in a row, while the assignment of stimuli to either the “view” or “distance” condition was randomized for each participant. The subsequent
fMRI measurement lasted for ~23 min. In line with the hypotheses, the current study focused on the regulation of the positive stimuli. EMA For more specific information regarding the EMA
sampling procedure, please refer to Seidel et al.36 or to the Supplementary Material 1.3. In short, EMA sampling via smartphones started the day after the fMRI scan for a period of 14 days.
Alarms occurred at six semi-random times during a 14-h period that was adapted for each individual to suit different daily routines. Each prompt examined rumination about AN-related content
(food/weight), which was assessed via two items adapted from the SIAB-EX interview (question 60 and question 61), i.e., “How much have you been thinking about food/calories/cooking?” and
“How much have you been thinking about your weight/shape?”. Responses were given on a visual analog scale, ranging from “not at all” to “a lot”. An adapted version of the Multidimensional
Mood Questionnaire55 recommended to use in EMA research56 assessed tension, affect, and energetic arousal with two bipolar items each. Participants were asked to rate on a visual analog
scale how they had felt since the last alarm. Higher scores indicated less negative affect, less tension, and higher energetic arousal. FUNCTIONAL IMAGE ACQUISITION AND PROCESSING Images
were acquired between 8 and 9 a.m. following an overnight fast using standard sequences with a 3 T whole-body MRI scanner (TRIO, Siemens) equipped with a standard head coil (see
Supplementary Material 1.4). Functional and structural images were processed with SPM8 (www.fil.ion.ucl.ac.uk/spm) within the Nipype framework (http://nipy.sourceforge.net/nipype/37)
following standard procedures, an artifact detection tool, and DARTEL (see Supplementary Material 1.4). On the single participant level a general linear model was fit to model the brain
activation in response to each of the five conditions (neutral, positive/negative watch, positive/negative distance). We modeled the picture as boxcar function with a duration of 6 s and the
subsequent rating as stick-function (zero duration). Additional regressors included six motion parameters and one regressor for each motion or intensity outlier volume (for details see
Supplementary Material 1.4) as nuisance regressors of no interest. All events were modeled using a canonical hemodynamic response function. At the second level, we conducted independent
_t_-tests to assess group differences between the individual contrasts. On the basis of the role of the VS in reward processing in the context of emotion regulation30 and our research
question, whether there are AN-relevant consequences of regulating reward-associated emotions, the bilateral VS as defined by the AAL atlas and implemented in the WFU PickAtlas toolbox for
SPM57,58 was used as a region of interest (ROI). To control for false-positives regarding main effects (including both groups) and group differences in the VS, family-wise error (FWE, _p_
< 0.05) correction was performed using small volume correction in SPM8. Whole-brain analysis of main effects and group differences of interest (positive watch > neutral; positive watch
> positive distance; positive watch < positive distance) were also corrected at a threshold of _p_ < 0.05, FEW using random field theory59. We further averaged extracted indices of
activation (β estimates) using the MarsBaR toolbox60 from the ROI VS. STATISTICAL ANALYSIS CLINICAL AND PSYCHOMETRIC DATA We conducted a 3 × 2 repeated measures ANOVA to test for potential
group differences in the effect of the three conditions of interest (neutral, positive watch, and positive distance) on the arousal ratings. To investigate effects between reported
regulation and imaging data, we calculated an arousal regulation score by subtracting the rated arousal following “positive distance” trials from those following “positive watch”. The higher
this arousal regulation score, the more the arousal was reduced during the regulation condition as compared to the watch condition. To assess outcome measures, we calculated BMI-SDS change
scores by subtracting BMI-SDS after 30, 60, and 90 days from BMI-SDS at the day of admission. Histograms, box plots, normal probability plots, and Levene statistics were employed to verify
the underlying statistical assumptions—no major deviations were detected. FMRI To investigate effects of neural regulation on the EMA data and outcome measures, we calculated a neural
regulation score by subtracting the betas extracted from the VS ROI in the “positive distance” condition from those during “positive watch”. The higher this neural regulation score, the more
neural activity was reduced during the regulation condition as compared to the watch condition. To assess whether this neural regulation score was associated with the arousal regulation
score or clinical characteristics, Pearson’s correlation was calculated if data were normally distributed, otherwise Spearman’s rho was used. To investigate the relationship between VS
neural regulation and weight gain after 30, 60, and 90 days, we conducted three linear regressions with the respective change in BMI-SDS as outcome variable and added the VS neural
regulation score, baseline BMI-SDS, and baseline EDI-2-total as predictors. To reach normal distribution of the neural regulation score for inclusion in the hierarchical models, one outlier
was removed from the data set. HIERARCHICAL LINEAR MODELS As the research design of the EMA data yields nested data, we conducted hierarchical linear modeling (HLM 7)61 to examine the extent
to which the neural regulation score was able to predict disease-relevant ruminations, affect, and tension in the 14 days following the scan. For all outcome variables (rumination about
food, rumination about weight and shape, negative affect, and tension) separate multilevel models were estimated. These models take into account that the present data set is organized within
three different levels and that single observations (Level 1) are nested within days (Level 2), which are nested within participants (Level 3). The same statistical approach was used for
all models. First, a null model including intercepts and error terms, but without predictors on any level, was calculated to analyze within-person variability. In model 1a, 1b, 1c, and 1d
(for our four different outcomes) we allowed for random intercepts and included time (indicating time of day as a continuous variable from 1 to 6) on level 1 and day of study (1–14) on level
2. To account for autoregression, we estimated random slopes for the time variables at level 1 (time points per day) and level 2 (days)62. On level 3, the person level, we included
diagnostic group (−1 and 1), mean centered extracted beta values of the VS ROI, as well as an interaction term of group and the centered VS neural activity. To facilitate the interpretation
of the interaction patterns, we performed simple slope tests, as recommended by Preacher, Curran, and Bauer63. To explore whether group differences revealed in associations between VS
regulation and EMA data represented a generic response in brain regions involved in emotion regulation and reward processing, we carried out control analyses of relationships between EMA
variables and change in BOLD response in the amygdala64,65; a brain region tightly connected to VS and previously implicated in AN66,67 by adding a neural regulation score of the amygdala as
predictor to the HLM models instead of the VS regulation score. RESULTS DEMOGRAPHIC AND CLINICAL VARIABLES, SELF-REPORT DATA AN patients did not differ from matched HC in age or IQ, but BMI
was significantly lower and ED symptoms as well as depression scores were considerably elevated (Table 1). Further, as indicated by their scores on the subscales of the ERQ, patients were
less likely to habitually use reappraisal than HC when regulating emotions, while no differences were observed for the suppression subscale (Table 1). Regarding the arousal ratings during
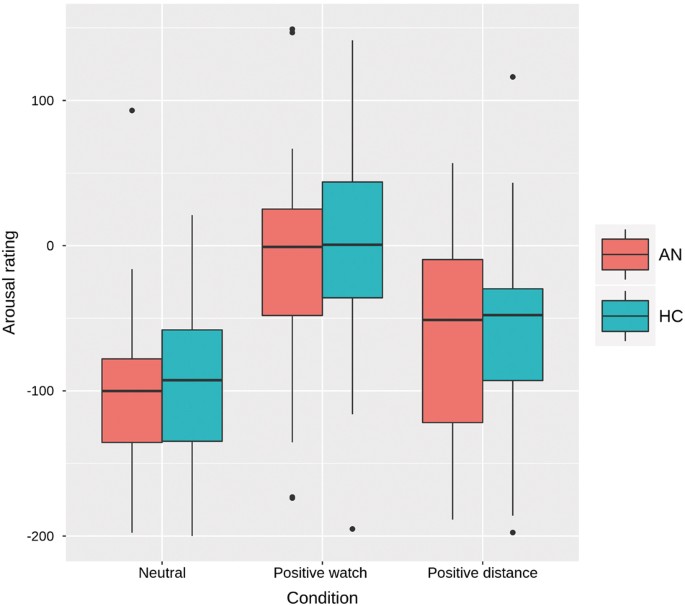
the fMRI experiment, an expected main effect of condition was found (F(2,140) = 61.29, _p_ < 0.001). Post hoc tests confirmed that arousal in the positive watch condition was higher than
in the neutral watch condition (_p_ < 0.001) and that it decreased during the emotion regulation condition compared to watch (_p_ < 0.001), but no interaction with group was evident
(see Fig. 1). The regulation score (positive watch–positive distance) as gauged by the arousal ratings did not show any correlations with age, BMI-SDS, EDI-2-total, or depression scores
(Supplementary Table S1). IMAGING DATA Exploratory whole-brain analyses of the main effects of emotion and task, which were not in the focus of the current study, are reported in the
Supplementary Fig. S2 and Supplementary Table S2). No group differences were evident on whole-brain level or in the VS ROI, neither during emotion induction (positive watch>neutral), nor
during emotion regulation (positive watch>positive distance, positive watch<positive distance). Supporting the general validity of the paradigm, a positive correlation was evident
between the extracted neural regulation score (VS, positive watch–positive distance) and the arousal regulation score (arousal ratings, positive watch–positive distance; rho = 0.27, _p_ =
0.026; see also Supplementary Fig. S3). Examining the association between self-reported emotion regulation strategies (ERQ) and VS neural regulation score, we found a positive association
between the neural regulation score and self-reported habitual use of suppression in patients only (AN: _r_ = 0.49. _p_ = 0.003; HC: _r_ = −0.14, n.s.). No correlation between reappraisal
and neural regulation score surfaced (AN: _r_ = 0.25, n.s.; HC: _r_ = −0.13, n.s.). More importantly, VS neural regulation score predicted treatment outcome after 60 (_r_ = −0.36, _p_ =
0.031) and 90 days (_r_ = −0.47, _p_ = 0.006; Fig. 2 and Supplementary Table S3) in patients beyond the effects of baseline BMI-SDS and baseline EDI-2-total, indicating that the more
patients reduced VS activity during the “positive distance” condition as compared to “positive watch”, the less weight they had gained after 60 and 90 days. HIERARCHICAL LINEAR MODELS We
tested the effect of the neural regulation score on subsequent rumination about weight, rumination about food, affect, and tension with four different models, each was based on a total of
4594 (AN = 2434, HC = 2160) data points. The null model of all outcomes (intercept as the only predictor) showed significant variance at both higher levels, hence justifying the application
of further multilevel analysis (Supplementary Table S4). In model 1, we controlled for time of day, day of study, and diagnostic group, for effects of neural regulation in the VS as well as
the interaction term neural regulation score × group on the respective outcome measure (for additional models also controlling for age, compliance rates, duration of illness, and AN subtype,
please refer to Supplementary Tables S5 and S6). As expected, the diagnostic group showed a significant effect on the intercepts of rumination about weight, rumination about food, affect,
and tension. Rumination as well as negative affect and tension were higher for AN compared to HC. Results further indicated that there was a significant main effect of neural regulation on
rumination about weight, and most importantly, a significant neural regulation score × group interaction for rumination about weight, affect, and tension (for cross-level effects of time and
day, see Table 2, for effect sizes see Table S7). These effects were not present for the arousal regulation score or the neural regulation score of the amygdala (see Supplementary Tables S8
and S9). Slope analysis revealed that the VS neural regulation score predicted rumination about weight in the AN sample only (Fig. 3a): the higher the regulation score, the higher the
subsequent rumination measured over 2 weeks post scanning (_p_ = 0.004). The interaction between neural regulation and diagnostic group for affect was driven by a larger neural regulation
score being associated with higher negative affect in the AN sample (_p_ = 0.034; Fig. 3b) and a larger regulation score being associated with higher positive affect (_p_ = 0.018; Fig. 3b)
and lower tension (_p_ = 0.015; Fig. S4) in HC over 2 weeks post scanning. DISCUSSION The goal of this combined fMRI/EMA study was to investigate whether regulation of positive emotions as
gauged by the reduction of hemodynamic activity in a reward-related brain region (VS) was associated with negative consequences for AN patients in their everyday lives as measured by EMA.
Indeed, the imaging data were predictive of patients’ momentary ratings of affect and rumination. Specifically, we found the neural correlates of downregulation of positive emotions in the
VS to be closely related with real-life measures of disorder-relevant rumination as well as negative affect and tension scores measured several times a day over 2 weeks after the fMRI scan.
Furthermore, the more VS activity was downregulated during distancing from positive emotions, the less weight AN patients gained until follow-up time points at 60 and 90 days into therapy.
As discussed below in light of the ironic processes theory68 and accounts of limited neural control capacities69, we propose disorder-relevant negative outcomes to be the result of a
maladaptive self-control mechanism closely related to AN symptomatology. In the framework of their ironic processes theory, Wenzlaff and Wegner70 have conducted various studies showing that
frequently suppressing thoughts or emotions can be maladaptive and ultimately ineffective as it often results in a paradoxical increase or rebound of the unwanted thoughts68,71,72,73,74.
This rebound is often characterized by an increased accessibility of the suppressed thought72 and heightened emotional arousal75. It has also been suggested that chronic suppression might
prevent habituation to emotional stimuli, and as such results in hypersensitivity to depression and anxiety-related thoughts and symptoms70,73. This is in line with previous findings of
increased rumination in AN patients35,36,37 and might also explain the association between neural correlates of emotion regulation (as measured in the VS in the current study) and increased
post-scan rumination about weight-related and body-related content. Although the detachment strategy applied in our task belongs to the group of reappraisal strategies28, it seems possible
that detachment and suppression share some common mechanisms26,76 or are used interchangeably by some patients. Indeed, the instructions of emotion regulation experiments in some studies
reflect these similarities, with some stating to induce “suppression” of emotions via detachment77, while others instruct participants to “distance” themselves from upcoming emotions during
a condition that is labeled “suppression”78. Others have also highlighted the importance of distinguishing between the reappraisal strategies of reappraisal as reinterpretation and
reappraisal as detachment/distancing, with the latter being conceptually more similar to thought suppression76,78,79,80. The assumption that especially patients might tend to use both
strategies interchangeably is also supported by the found association between the neural regulation score (acquired during “distancing”) and the suppression subscale of the ERQ in our AN
group only. A possible interpretation of this finding might be that patients, who appear to have less access to adaptive strategies like acceptance, problem-solving or strategies involving
reappraisal, might use suppression as an alternative in order to cope with their emotions. Studies have shown that reappraisal, in contrast to suppression, is not associated with negative
consequences like rebound effects such as increased ruminative thinking and more negative affect81. This rather speculative interpretation is supported by our finding in HC, who seemingly
executed a reappraisal strategy (as requested) and therefore benefit from emotion regulation. In this group, a higher neural regulation score during the instructed reappraisal was associated
with more positive affect and less tension post scan. Affect plays a major role in eating-related behaviors82 and previous studies have found links between the reliance on suppression as an
emotion regulation strategy and ED symptoms. Emotion suppression seems not only to result in increased rumination about food and eating-related content, but also be related to binge eating
and food cravings among restrained eaters and individuals high in both restraint and disinhibition41,43,71,83. In clinical populations, it has been reported that thought suppression was
associated with bulimic symptoms42,84. Moreover, studies and clinical observations suggest that AN patients are characterized by a general mode of avoidance toward intense emotional
states35,85–87, even positive ones, which may also require constant monitoring and suppression of upcoming emotions. This is in line with evidence that the avoidance of various psychological
experiences (thoughts, emotions, memories, or urges) are associated with ED symptoms such as binge eating and general psychological symptoms cross-sectionally and at
follow-up35,85,88,89,90. Further, emotional avoidance in AN has been closely tied to negative affect and ruminative processes35,86. Considering the constant downregulation of hunger and food
intake, as well as emotional avoidance typically found in AN36,37,86, a possible interpretation might be that these regulation strategies “backfire” by making the “over-controlled” stimuli
even more accessible, which is reflected in heightened rumination, negative affect, and increased tension in AN as found with our EMA measures. A similar interpretation of our findings comes
from theories of self-control failure and “ego depletion”69,91,92. These theories have emphasized the costs and negative consequences of self-control within a limited capacity model69.
Several experimental studies have yielded empirical evidence supporting this model by showing that performance on other cognitive tasks is worse after an emotion regulation condition, or
that emotion regulation ability decreases with cognitive load93,94. This converges with results from studies in cohorts characterized by high dietary restraint. Participants of these studies
have been shown to ruminate, crave, and overeat when asked to regulate emotions while cognitive resources are depleted84,95,96. Consequently, it has been suggested that emotion regulation
depletes control resources96, which facilitates the emergence of ED-associated symptoms such as ruminative thinking or negative affect, which, as we were able to show, might even influence
treatment outcome. The current findings, suggestive of over-regulation over reward-related processing in AN, are generally in line with recent findings of altered neural correlates of
self-control in the disorder97 and may also be related to abnormal processing of positive socioemotional stimuli98,99. Although the current study did not explore potential evidence in the
respect that the cortical regions typically involved in distancing (temporal parietal junction, inferior parietal lobule, and inferior frontal gyrus)30,31, directly modulated VS activity in
AN, recent investigations of effective connectivity have shown altered top–down control within cognitive-emotional frontostriatal circuitry in AN16,100.” The current study has to be
considered in the light of a number of limitations: first of all, in order to avoid anxious mood states, we only investigated emotion regulation to a strictly filtered stimulus set. Emotions
that are elicited through food pictures could be associated with even more regulation effort and therefore costs. Our analysis also does not address the association between regulation of
negative stimuli and AN symptomatology. Second, although inter-individual differences in VS regulation during emotion regulation predicted disorder-relevant measures such as rumination,
affect, and BMI outcome, when looking at the sample as a whole, we did not observe robust main effects of emotion and regulation. However, perceived emotion induction and regulation was
successful for both groups. Third, the current results do not answer the question whether alterations in reward-associated brain areas represent a state-like response during the acute state
of AN or a stable, trait-like difference in reward processing, and whether it changes with recovery and during therapy. Fourth, scanning after an overnight fast may have differential effects
on AN patients, and HC thus may have modulated VS activation. Fifth, although we used a pairwise matching procedure regarding age, given the relatively large age range of the participants,
age might have biased our results. Additional analyses with age as a covariate confirmed our initial results, but other possible confounders such as pubertal status or hormonal changes need
to be taken into account by future studies and when interpreting the current findings. Last but not least, the majority of patients were treated as inpatients, which might have biased the
naturalistic elements of data collection, mainly by preventing them to use restrictive eating as an emotion regulation strategy101,102. The current investigation constitutes a first attempt
to link underlying neural processes with real-life measures in AN. Because behavior and affect in laboratory studies may have little relevance to everyday life, establishing links between
brain activity during controlled tasks and disorder-relevant cognition and affective states in natural settings is important when trying to elucidate the neural underpinnings of AN. Taken
together, we established associations between neural responses of a reward-associated brain region during an emotion regulation task and rumination and negative affect measured in real life.
Most therapeutic strategies deal with the acceptance of negative affect103. In contrast, our results seem to indicate the importance of a balanced way to deal with positive emotions. This
could, for example, include the reintroduction of pleasurable feelings and joy during therapeutic approaches such as a meditation-based loving kindness intervention as part of the “Radically
open-dialectical behavior therapy”, which has been recommended for AN104 as well as disorders of over-control105. Our results are compatible with the view that AN patients constantly
downregulate, maybe suppress, pleasurable emotions, which seems to be associated with adverse therapy outcome. Allowing such responses (even if in response to food) or, if appropriate, using
healthier emotion regulation strategies, could be an element that needs to be integrated into modern psychotherapeutic approaches to AN. Although our findings need to be replicated, they
seem to suggest that focusing on more adaptive emotion regulation strategies that rely less on control aspects that deplete cognitive resources, such as emotional acceptance106, may foster
better overall psychological well-being and outcome success in patients with AN. REFERENCES * Gross J. J. _Handbook of Emotion Regulation_ 2nd edn (Guilford Publications, New York, 2013). *
Gross, J. J. & Muñoz, R. F. Emotion regulation and mental health. _Clin. Psychol. Sci. Pract._ 2, 151–164 (1995). Article Google Scholar * Quinn, P. D. & Fromme, K. Self-regulation
as a protective factor against risky drinking and sexual behavior. _Psychol. Addict. Behav. J. Soc. Psychol. Addict. Behav._ 24, 376–385 (2010). Article Google Scholar * Tangney, J. P.,
Baumeister, R. F. & Boone, A. L. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. _J. Pers._ 72, 271–324 (2004). Article PubMed
Google Scholar * Baumeister R. F., Heatherton T. F., Tice D. M. _Losing Control: How and Why People Fail at Self-regulation_ (Academic Press, New York, 1994). * Heatherton, T. F. &
Wagner, D. D. Cognitive neuroscience of self-regulation failure. _Trends Cogn. Sci._ 15, 132–139 (2011). Article PubMed PubMed Central Google Scholar * Ehrlich, S. et al. The role of
leptin and cortisol in hyperactivity in patients with acute and weight-recovered anorexia nervosa. _Prog. Neuropsychopharmacol. Biol. Psychiatry_ 33, 658–662 (2009). Article CAS PubMed
Google Scholar * Merle, J. V. et al. Agouti-related protein in patients with acute and weight-restored anorexia nervosa. _Psychol. Med._ 41, 2183–2192 (2011). Article CAS PubMed Google
Scholar * Raboch, J. & Faltus, F. Sexuality of women with anorexia nervosa. _Acta Psychiatr. Scand._ 84, 9–11 (1991). Article CAS PubMed Google Scholar * Klein, D. A., Schebendach,
J. E., Gershkovich, M., Smith, G. P. & Walsh, B. T. Modified sham feeding of sweet solutions in women with anorexia nervosa. _Physiol. Behav._ 101, 132–140 (2010). Article CAS PubMed
PubMed Central Google Scholar * Davis, C., Strachan, S. & Berkson, M. Sensitivity to reward: implications for overeating and overweight. _Appetite_ 42, 131–138 (2004). Article PubMed
Google Scholar * Giel, K. E. et al. Attentional processing of food pictures in individuals with anorexia nervosa—an eye-tracking study. _Biol. Psychiatry_ 69, 661–667 (2011). Article
PubMed Google Scholar * Kaye, W. H., Fudge, J. L. & Paulus, M. New insights into symptoms and neurocircuit function of anorexia nervosa. _Nat. Rev. Neurosci._ 10, 573–584 (2009).
Article CAS PubMed Google Scholar * Wierenga, C. et al. Altered BOLD Response during Inhibitory and Error Processing in Adolescents with Anorexia Nervosa. _PLoS. ONE_ 9, e92017 (2014).
Article PubMed PubMed Central Google Scholar * O’Hara, C. B., Campbell, I. C. & Schmidt, U. A reward-centred model of anorexia nervosa: a focussed narrative review of the
neurological and psychophysiological literature. _Neurosci. Biobehav. Rev._ 52, 131–152 (2015). Article PubMed Google Scholar * Frank, G. K. W., Shott, M. E., Riederer, J. & Pryor, T.
L. Altered structural and effective connectivity in anorexia and bulimia nervosa in circuits that regulate energy and reward homeostasis. _Transl. Psychiatry_ 6, e932 (2016). Article CAS
PubMed PubMed Central Google Scholar * Fladung, A.-K. et al. A neural signature of anorexia nervosa in the ventral striatal reward system. _Am. J. Psychiatry_ 167, 206–212 (2010). Article
PubMed Google Scholar * Via, E. et al. Abnormal social reward responses in anorexia nervosa: an fMRI study. _PLoS. ONE_ 10, e0133539 (2015). Article PubMed PubMed Central Google
Scholar * Boehm, I. et al. Increased resting state functional connectivity in the fronto-parietal and default mode network in anorexia nervosa. _Front. Behav. Neurosci._ 8, 346 (2014).
Article PubMed PubMed Central Google Scholar * Lock, J., Garrett, A., Beenhakker, J. & Reiss, A. L. Aberrant brain activation during a response inhibition task in adolescent eating
disorder subtypes. _Am. J. Psychiatry_ 168, 55–64 (2011). Article PubMed Google Scholar * Oberndorfer, T. A., Kaye, W. H., Simmons, A. N., Strigo, I. A. & Matthews, S. C.
Demand-specific alteration of medial prefrontal cortex response during an inhibition task in recovered anorexic women. _Int. J. Eat. Disord._ 44, 1–8 (2011). Article PubMed Google Scholar
* Ehrlich, S. et al. Elevated cognitive control over reward processing in recovered female patients with anorexia nervosa. _J. Psychiatry Neurosci. JPN_ 40, 307 (2015). Article PubMed
Google Scholar * King J. A. et al. Altered neural efficiency of decision making during temporal reward discounting in anorexia nervosa. _J. Am. Acad. Child Adolesc. Psychiatry_
https://doi.org/10.1016/j.jaac.2016.08.005. * Goschke, T. Dysfunctions of decision-making and cognitive control as transdiagnostic mechanisms of mental disorders: advances, gaps, and needs
in current research. _Int. J. Methods Psychiatr. Res._ 23, 41–57 (2014). Article PubMed Google Scholar * Kool, W., McGuire, J. T., Wang, G. J. & Botvinick, M. M. Neural and behavioral
evidence for an intrinsic cost of self-control. _PLoS. ONE_ 8, e72626 (2013). Article CAS PubMed PubMed Central Google Scholar * Walter, H. et al. The temporal dynamics of voluntary
emotion regulation. _PLoS. ONE_ 4, 1–8 (2009). Article Google Scholar * Ochsner, K. N. et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of
negative emotion. _Neuroimage_ 23, 483–499 (2004). Article PubMed Google Scholar * Gross, J. J. & Thompson, R. A. In: Handbook of Emotion Regulation. James J. Gross (ed.). 3–24
(Guilford Press, New York, 2007). * Winecoff, A., LaBar, K. S., Madden, D. J., Cabeza, R. & Huettel, S. A. Cognitive and neural contributors to emotion regulation in aging. _Soc. Cogn.
Affect. Neurosci._ 6, 165–176 (2011). Article PubMed Google Scholar * Staudinger, M. R., Erk, S. & Walter, H. Dorsolateral prefrontal cortex modulates striatal reward encoding during
reappraisal of reward anticipation. _Cereb. Cortex_ 21, 2578–2588 (2011). Article PubMed Google Scholar * Hollmann, M. et al. Neural correlates of the volitional regulation of the desire
for food. _Int. J. Obes._ 36, 648–655 (2012). Article CAS Google Scholar * Delgado, M. R., Gillis, M. M. & Phelps, E. A. Regulating the expectation of reward via cognitive strategies.
_Nat. Neurosci._ 11, 880–881 (2008). Article CAS PubMed PubMed Central Google Scholar * Hare, T. A., Camerer, C. F. & Rangel, A. Self-control in decision-making involves modulation
of the vmPFC valuation system. _Science_ 324, 646–648 (2009). Article CAS PubMed Google Scholar * Nederkoorn, C., Houben, K., Hofmann, W., Roefs, A. & Jansen, A. Control yourself or
just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. _Health Psychol._ 29, 389–393 (2010).
Article PubMed Google Scholar * Cowdrey, F. A. & Park, R. J. The role of experiential avoidance, rumination and mindfulness in eating disorders. _Eat. Behav._ 13, 100–105 (2012).
Article PubMed Google Scholar * Seidel M. _et al_. A naturalistic examination of negative affect and disorder-related rumination in anorexia nervosa. _Eur. Child Adolesc. Psychiatry_
https://doi.org/10.1007/s00787-016-0844-3 (2016).. * Startup, H. et al. Worry and rumination in anorexia nervosa. _Behav. Cogn. Psychother._ 41, 301–316 (2013). Article PubMed Google
Scholar * Herman, C. P., Janet, P., Patricia, P., Joyce, T. & Donna, M. Distractibility in dieters and nondieters: an alternative view of ‘externality. _J. Pers. Soc. Psychol._ 36,
536–548 (1978). Article CAS PubMed Google Scholar * Keys A. et al. _The Biology of Human Starvation_. Vol. 1 and 2 (University of Minnesota Press, Minneapolis, 1950). * Polivy, J.
Psychological consequences of food restriction. _J. Am. Diet. Assoc._ 96, 589–592 (1996). Article CAS PubMed Google Scholar * Barnes, R. D. & Tantleff-Dunn, S. Food for thought:
examining the relationship between food thought suppression and weight-related outcomes. _Eat. Behav._ 11, 175–179 (2010). Article PubMed Google Scholar * Lavender, J. M., Jardin, B. F.
& Anderson, D. A. Bulimic symptoms in undergraduate men and women: contributions of mindfulness and thought suppression. _Eat. Behav._ 10, 228–231 (2009). Article PubMed Google Scholar
* Svaldi, J., Tuschen-Caffier, B., Lackner, H. K., Zimmermann, S. & Naumann, E. The effects of emotion regulation on the desire to overeat in restrained eaters. _Appetite_ 59, 256–263
(2012). Article PubMed Google Scholar * Lavender, J. M. et al Associations between retrospective versus ecological momentary assessment measures of emotion and eating disorder symptoms in
anorexia nervosa. _J. Psychiatr. Res._ 47, 1514–1520 (2013). Article PubMed PubMed Central Google Scholar * Harenski, C. L. & Hamann, S. Neural correlates of regulating negative
emotions related to moral violations. _Neuroimage_ 30, 313–324 (2006). Article PubMed Google Scholar * Kanske, P., Heissler, J., Schönfelder, S., Bongers, A. & Wessa, M. How to
regulate emotion? Neural networks for reappraisal and distraction. _Cereb. Cortex_ 21, 1379–1388 (2011). Article PubMed Google Scholar * Koenigsberg, H. W. et al. Neural correlates of
using distancing to regulate emotional responses to social situations. _Neuropsychologia_ 48, 1813–1822 (2010). Article PubMed PubMed Central Google Scholar * Harris, P. A. et al.
Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. _J. Biomed. Inform._ 42, 377–381
(2009). Article PubMed Google Scholar * Thiel, A. et al. Eine deutschsprachige version des eating disorder inventory EDI-2. [german translation of the eating disorder inventory EDI-2.].
_Psychother. Psychosom. Med. Psychol._ 47, 365–376 (1997). CAS PubMed Google Scholar * Hautzinger M., Keller F., Kühner C. _Beck Depressions Inventar: Revised_ (Huber, Bern, Swizerland,
2009). * Gross, J. J. & John, O. P. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. _J. Pers. Soc. Psychol._ 85,
348–362 (2003). Article PubMed Google Scholar * Kromeyer-Hauschild, K. et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher
Stichproben. _Mon. Kinderheilkd._ 149, 807–818 (2001). Article Google Scholar * Lang P., Bradley M., Cuthbert B. _International Affective Picture System (IAPS): Affective Ratings of
Pictures and Instruction Manual_ Technical Report A-8. (University of Florida: Gainesville, FL, 2008). * Wessa M. et al. _EmoPicS: Subjektive und psychophysiologische Evaluation neuen
Bildmaterials für die klinisch-biopsychologische Forschung_ (2010). * Steyer R., Schwenkmezger P., Notz P., Eid M. _Der mehrdimensionale Befindlichkeitsfragebogen (MDBF). Handanweisung [The
multidimensional affect rating scale (MDBF). Manual]_ (Hogrefe, Göttingen, Germany, 1997). * Wilhelm, P. & Schoebi, D. Assessing mood in daily life: structural validity, sensitivity to
change, and reliability of a short-scale to measure three basic dimensions of mood. _Eur. J. Psychol. Assess._ 23, 258–267 (2007). Article Google Scholar * Maldjian, J. A., Laurienti, P.
J., Kraft, R. A. & Burdette, J. H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. _Neuroimage_ 19, 1233–1239 (2003). Article
PubMed Google Scholar * Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in spm using a macroscopic anatomical parcellation of the MNI MRI single-subject brain.
_Neuroimage_ 15, 273–289 (2002). Article CAS PubMed Google Scholar * Worsley, K. J., Evans, A. C., Marrett, S. & Neelin, P. A three-dimensional statistical analysis for CBF
activation studies in human brain. _J. Cereb. Blood Flow. Metab._ 12, 900–918 (1992). Article CAS PubMed Google Scholar * Brett, M., Anton, J.-L., Valabregue, R. & Poline, J.-B.
Region of interest analysis using the MarsBar toolbox for SPM 99. _Neuroimage_ 16, S497 (2002). Google Scholar * Raudenbush S. W., Bryk A. S. _Hierarchical Linear Models: Applications and
Data Analysis Methods_ (Sage Publications, Thousand Oaks California, 2002). * Bolger N., Laurenceau J. P. _Intensive Longitudinal Methods: an Introduction to Diary and Experience Sampling
Research_ (N Y Guilford Press, New York, 2013). * Preacher, K. J., Curran, P. J. & Bauer, D. J. Computational tools for probing interactions in multiple linear regression, multilevel
modeling, and latent curve analysis. _J. Educ. Behav. Stat._ 31, 437–448 (2006). Article Google Scholar * Phelps, E. A. Emotion and cognition: insights from studies of the human amygdala.
_Annu. Rev. Psychol._ 57, 27–53 (2006). Article PubMed Google Scholar * Baxter, M. G. & Murray, E. A. The amygdala and reward. _Nat. Rev. Neurosci._ 3, 563–573 (2002). Article CAS
PubMed Google Scholar * Joos, A. A. et al. Amygdala hyperreactivity in restrictive anorexia nervosa. _Psychiatr. Res. Neuroimaging_ 191, 189–195 (2011). Article Google Scholar * Vocks,
S. et al. Neural correlates of viewing photographs of one’s own body and another woman’s body in anorexia and bulimia nervosa: an fMRI study. _J. Psychiatry Neurosci. JPN_ 35, 163–176
(2010). Article PubMed Google Scholar * Wegner, D. M. Ironic processes of mental control. _Psychol. Rev._ 101, 34–52 (1994). Article CAS PubMed Google Scholar * Muraven, M., Tice, D.
M. & Baumeister, R. F. Self-control as a limited resource: regulatory depletion patterns. _J. Pers. Soc. Psychol._ 74, 774–789 (1998). Article CAS PubMed Google Scholar * Wenzlaff,
R. M. & Wegner, D. M. Thought suppression. _Annu. Rev. Psychol._ 51, 59–91 (2000). Article CAS PubMed Google Scholar * Soetens, B. ‘When suppression backfires’: the ironic effects of
suppressing eating-related thoughts. _J. Health Psychol._ 11, 655–668 (2006). Article PubMed Google Scholar * Wegner, D. M. & Erber, R. The hyperaccessibility of suppressed thoughts.
_J. Pers. Soc. Psychol._ 63, 903–912 (1992). Article Google Scholar * Wegner, D. M. & Zanakos, S. Chronic thought suppression. _J. Pers._ 62, 615–640 (1994). Article Google Scholar
* Abramowitz, J. S., Tolin, D. F. & Street, G. P. Paradoxical effects of thought suppression: a meta-analysis of controlled studies. _Clin. Psychol. Rev._ 21, 683–703 (2001). Article
CAS PubMed Google Scholar * Wegner, D. M., Broome, A. & Blumberg, S. J. Ironic effects of trying to relax under stress. _Behav. Res. Ther._ 35, 11–21 (1997). Article CAS PubMed
Google Scholar * Dörfel, D. et al. Common and differential neural networks of emotion regulation by detachment, reinterpretation, distraction, and expressive suppression: a comparative fMRI
investigation. _Neuroimage_ 101, 298–309 (2014). Article PubMed Google Scholar * Phan, K. L. et al. Neural substrates for voluntary suppression of negative affect: a functional magnetic
resonance imaging study. _Biol. Psychiatry_ 57, 210–219 (2005). Article PubMed Google Scholar * Dunn, B. D., Billotti, D., Murphy, V. & Dalgleish, T. The consequences of effortful
emotion regulation when processing distressing material: a comparison of suppression and acceptance. _Behav. Res. Ther._ 47, 761–773 (2009). Article PubMed PubMed Central Google Scholar
* Ochsner, K. N., Silvers, J. A. & Buhle, J. T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. _Ann. N. Y.
Acad. Sci._ 1251, E1–24 (2012). Article PubMed PubMed Central Google Scholar * Webb, T. L., Miles, E. & Sheeran, P. Dealing with feeling: A meta-analysis of the effectiveness of
strategies derived from the process model of emotion regulation. _Psychol. Bull._ 138, 775–808 (2012). Article PubMed Google Scholar * Gross, J. J. Antecedent- and response-focused
emotion regulation: divergent consequences for experience, expression, and physiology. _J. Pers. Soc. Psychol._ 74, 224–237 (1998). Article CAS PubMed Google Scholar * Cardi, V.,
Leppanen, J. & Treasure, J. The effects of negative and positive mood induction on eating behaviour: a meta-analysis of laboratory studies in the healthy population and eating and weight
disorders. _Neurosci. Biobehav. Rev._ 57, 299–309 (2015). Article PubMed Google Scholar * O’Connell, C., Larkin, K., Mizes, J. S. & Fremouw, W. The Impact of caloric preloading on
attempts at food and eating-related thought suppression in restrained and unrestrained eaters. _Int. J. Eat. Disord._ 38, 42–48 (2005). Article PubMed Google Scholar * Ward, T., Bulik, C.
M. & Johnston, L. Return of the suppressed: mental control and bulimia nervosa. _Behav. Chang._ 13, 79–90 (1996). Article Google Scholar * Lampard, A. M., Byrne, S. M., McLean, N.
& Fursland, A. Avoidance of affect in the eating disorders. _Eat. Behav._ 12, 90–93 (2011). Article PubMed Google Scholar * Wildes, J. E., & Ringham, R. M. & Marcus, M. D.
Emotion avoidance in patients with anorexia nervosa: Initial test of a functional model. _Int. J. Eat. Disord._ 43, 398–404 (2010). PubMed PubMed Central Google Scholar * Corstorphine,
E., Mountford, V., Tomlinson, S., Waller, G. & Meyer, C. Distress tolerance in the eating disorders. _Eat. Behav._ 8, 91–97 (2007). Article PubMed Google Scholar * Heatherton, T. F.
& Baumeister, R. F. Binge eating as escape from self-awareness. _Psychol. Bull._ 110, 86 (1991). Article CAS PubMed Google Scholar * Polivy, J. & Herman, C. P. Causes of eating
disorders. _Annu. Rev. Psychol._ 53, 187–213 (2002). Article PubMed Google Scholar * Rawal, A., Park, R. J. & Williams, J. M. G. Rumination, experiential avoidance, and dysfunctional
thinking in eating disorders. _Behav. Res. Ther._ 48, 851–859 (2010). Article PubMed PubMed Central Google Scholar * Boon, B., Stroebe, W., Schut, H. & IJntema, R. Ironic processes
in the eating behaviour of restrained eaters. _Br. J. Health Psychol._ 7, 1–10 (2002). Article PubMed Google Scholar * Muraven, M. & Baumeister, R. F. Self-regulation and depletion of
limited resources: does self-control resemble a muscle? _Psychol. Bull._ 126, 247 (2000). Article CAS PubMed Google Scholar * Richards, J. M. & Gross, J. J. Emotion regulation and
memory: the cognitive costs of keeping one’s cool. _J. Pers. Soc. Psychol._ 79, 410–424 (2000). Article CAS PubMed Google Scholar * Sheppes, G. & Meiran, N. Divergent cognitive costs
for online forms of reappraisal and distraction. _Emotion_ 8, 870–874 (2008). Article PubMed Google Scholar * Jones, N. & Rogers, P. J. Preoccupation, food, and failure: an
investigation of cognitive performance deficits in dieters. _Int. J. Eat. Disord._ 33, 185–192 (2003). Article PubMed Google Scholar * Vohs, K. D. & Heatherton, T. F. Self-regulatory
failure: a resource-depletion approach. _Psychol. Sci._ 11, 249–254 (2000). Article CAS PubMed Google Scholar * Decker, J. H., Figner, B. & Steinglass, J. E. On weight and waiting:
delay discounting in anorexia nervosa pretreatment and posttreatment. _Biol. Psychiatry_ 78, 606–614 (2015). Article PubMed Google Scholar * McAdams, C. J., Lohrenz, T. & Montague, P.
R. Neural responses to kindness and malevolence differ in illness and recovery in women with anorexia nervosa. _Hum. Brain Mapp._ 36, 5207–5219 (2015). Article PubMed PubMed Central
Google Scholar * Dapelo, M. M. et al. Facial expression of positive emotions in individuals with eating disorders. _Psychiatry Res._ 230, 70–77 (2015). Article PubMed Google Scholar *
Cha, J. et al. Abnormal reward circuitry in anorexia nervosa: a longitudinal, multimodal MRI study. _Hum. Brain Mapp._ 37, 3835–3846 (2016). Article PubMed PubMed Central Google Scholar
* Haynos, A. F. & Fruzzetti, A. E. Anorexia nervosa as a disorder of emotion dysregulation: evidence and treatment implications: emotion dysregulation in anorexia nervosa. _Clin.
Psychol. Sci. Pract._ 18, 183–202 (2011). Article Google Scholar * Lavender, J. M. et al. Dimensions of emotion dysregulation in anorexia nervosa and bulimia nervosa: a conceptual review
of the empirical literature. _Clin. Psychol. Rev._ 40, 111–122 (2015). Article PubMed PubMed Central Google Scholar * Hayes S. C., Strosahl K. D., Wilson K. G. _Acceptance and Commitment
Therapy: An Experiential Approach to Behavior Change_ (Guilford Press, 1999). http://psycnet.apa.org/psycinfo/1999-04037-000 (accessed 14 September 2015). * Lynch, T. R. et al. Radically
open-dialectical behavior therapy for adult anorexia nervosa: feasibility and outcomes from an inpatient program. _BMC Psychiatry_ 13, 293 (2013). Article PubMed PubMed Central Google
Scholar * Lynch, T. R., Hempel, R. J. & Dunkley, C. Radically open-dialectical behavior therapy for disorders of over-control: signaling matters. _Am. J. Psychother._ 69, 141–162
(2015). PubMed Google Scholar * Wildes, J. E., Marcus, M. D., Cheng, Y., McCabe, E. B. & Gaskill, J. A. Emotion acceptance behavior therapy for anorexia nervosa: a pilot study. _Int.
J. Eat. Disord._ 47, 870–873 (2014). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGMENTS The authors would like to express their gratitude to Laura
Soltwedel, Benjamin Roschinski, Sabine Clas, Juliane Petermann, Anne Schulze, Stefanie Huber, Matthias Beck, Pascal Friebel, Lea Scheuvens, Nadja Schutkowski, and Johannes Zwipp for their
assistance with participant recruitment and data collection and thank all participants for their time and cooperation. We thank the Centre for Information Services and High Performance
Computing (ZIH) at TU Dresden for generous allocations of computer time. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Psychological and Social Medicine and Developmental
Neuroscience, Faculty of Medicine, Technische Universität Dresden, Dresden, Germany Maria Seidel, Joseph A. King, Franziska Ritschel, Ilka Boehm, Daniel Geisler, Fabio Bernardoni, Larissa
Holzapfel & Stefan Ehrlich * Translational Developmental Neuroscience Section, Eating Disorder Research and Treatment Center, Department of Child and Adolescent Psychiatry, Faculty of
Medicine, Technische Universität Dresden, Dresden, Germany Franziska Ritschel & Stefan Ehrlich * International School of Management and Technical University of Dortmund, Dortmund,
Germany Stefan Diestel * Department of Psychology, Technische Universität Dresden, Dresden, Germany Kersten Diers, Alexander Strobel & Thomas Goschke * Department of Psychology, Charité
Berlin, Berlin, Germany Henrik Walter * Department of Child and Adolescent Psychiatry, Faculty of Medicine, Technische Universität Dresden, Dresden, Germany Veit Roessner Authors * Maria
Seidel View author publications You can also search for this author inPubMed Google Scholar * Joseph A. King View author publications You can also search for this author inPubMed Google
Scholar * Franziska Ritschel View author publications You can also search for this author inPubMed Google Scholar * Ilka Boehm View author publications You can also search for this author
inPubMed Google Scholar * Daniel Geisler View author publications You can also search for this author inPubMed Google Scholar * Fabio Bernardoni View author publications You can also search
for this author inPubMed Google Scholar * Larissa Holzapfel View author publications You can also search for this author inPubMed Google Scholar * Stefan Diestel View author publications You
can also search for this author inPubMed Google Scholar * Kersten Diers View author publications You can also search for this author inPubMed Google Scholar * Alexander Strobel View author
publications You can also search for this author inPubMed Google Scholar * Thomas Goschke View author publications You can also search for this author inPubMed Google Scholar * Henrik Walter
View author publications You can also search for this author inPubMed Google Scholar * Veit Roessner View author publications You can also search for this author inPubMed Google Scholar *
Stefan Ehrlich View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Stefan Ehrlich. ETHICS DECLARATIONS CONFLICT OF
INTEREST This work was supported by the Deutsche Forschungsgemeinschaft (EH 367/5-1 & SFB 940) and the Swiss Anorexia Nervosa Foundation. In the last two years, Dr. Roessner has received
payment for consulting and writing activities from Lilly, Novartis, and Shire Pharmaceuticals, lecture honoraria from Lilly, Novartis, Shire Pharmaceuticals, and Medice Pharma, and support
for research from Shire and Novartis. He has carried out (and is currently carrying out) clinical trials in cooperation with the Novartis, Shire, and Otsuka companies. Henrik Walter has
received a speaker honorarium from Servier. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Seidel, M., King, J.A., Ritschel, F. _et al._ The real-life costs of emotion
regulation in anorexia nervosa: a combined ecological momentary assessment and fMRI study. _Transl Psychiatry_ 8, 28 (2018). https://doi.org/10.1038/s41398-017-0004-7 Download citation *
Received: 15 January 2017 * Revised: 29 June 2017 * Accepted: 14 July 2017 * Published: 24 January 2018 * DOI: https://doi.org/10.1038/s41398-017-0004-7 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative