Bacterial vitamin b12 production enhances nematode predatory behavior
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Although the microbiota is known to affect host development, metabolism, and immunity, its impact on host behavior is only beginning to be understood. In order to better
characterize behavior modulation by host-associated microorganisms, we investigated how bacteria modulate complex behaviors in the nematode model organism _Pristionchus pacificus_. This
nematode is a predator that feeds on the larvae of other nematodes, including _Caenorhabditis elegans_. By growing _P_. _pacificus_ on different bacteria and testing their ability to kill
_C. elegans_, we reveal large differences in killing efficiencies, with a _Novosphingobium_ species showing the strongest enhancement. This enhanced killing was not accompanied by an
increase in feeding, which is a phenomenon known as surplus killing, whereby predators kill more prey than necessary for sustenance. Our RNA-seq data demonstrate widespread metabolic
rewiring upon exposure to _Novosphingobium_, which facilitated screening of bacterial mutants with altered transcriptional responses. We identified bacterial production of vitamin B12 as an
important cause of such enhanced predatory behavior. Although vitamin B12 is an essential cofactor for detoxification and metabolite biosynthesis, shown previously to accelerate development
in _C. elegans_, supplementation with this enzyme cofactor amplified surplus killing in _P. pacificus_, whereas mutants in vitamin B12-dependent pathways reduced surplus killing. By
demonstrating that production of vitamin B12 by host-associated microbiota can affect complex host behaviors, we reveal new connections between animal diet, microbiota, and nervous system.
SIMILAR CONTENT BEING VIEWED BY OTHERS A NEUROTRANSMITTER PRODUCED BY GUT BACTERIA MODULATES HOST SENSORY BEHAVIOUR Article 17 June 2020 VITAMIN B12 PRODUCED BY GUT BACTERIA MODULATES
CHOLINERGIC SIGNALLING Article 02 January 2024 RAPID EVOLUTION OF A NOVEL PROTECTIVE SYMBIONT INTO KEYSTONE TAXON IN _CAENORHABDITIS ELEGANS_ MICROBIOTA Article Open access 18 August 2022
INTRODUCTION Organisms harbor and interact with diverse microbial communities depending on their own ecology and environment. Furthermore, the microbiota are considered a fundamental aspect
of a host’s biology and are known to provide developmental cues, effect metabolism, and alter immunity [1,2,3], However, the microbiota constitutes a complex network of microorganisms and
disentangling specific interactions at a mechanistic level is challenging. Bacterial-feeding nematodes therefore provide a highly attractive system to study the influence of the microbiota
as the specific interactions can be investigated in monoxenic cultures where the microbiota and diet are indistinguishable from one another and easily controlled. Remarkably, despite the
abundance of nematode species, often little is known of their ecology and the bacterial associations found naturally between these organisms. For instance, the microbiota of the model
nematode _Caenorhabditis elegans_ was only recently characterized [4,5,6] and shown to influence host fitness and response to pathogens [5, 7]. Furthermore, its microbiota is thought capable
of synthesizing all the essential nutrients _C. elegans_ may require [8]. Another well-characterized nematode species in addition to _C. elegans_ is _Pristionchus pacificus_, which shows
novel ecological, morphological, and behavioral traits not observed in _C. elegans_. _P. pacificus_ is a soil nematode frequently found associated with scarab beetles upon which it shares a
necromenic association [9]. Here, _P. pacificus_ exploits the distinct microbial habitat found on the decaying beetle carcass to complete its life cycle [10]. In addition, _P. pacificus_ is
an omnivorous nematode capable of feeding on bacteria, fungi, and also predating on other nematodes [11,12,13]. Predation is dependent on morphological and behavioral novelties, involving
the formation of teeth-like denticles and a self-recognition mechanism [14,15,16,17]. The ability to form teeth-like denticles is an example of developmental plasticity with two discrete
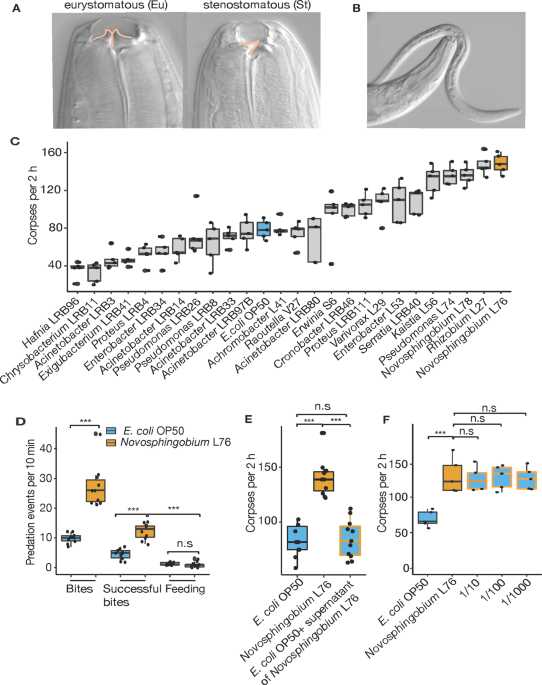
mouth forms [18]. The stenostomatous morph has a single blunt tooth, whereas the eurystomatous morph has two large teeth with only the latter capable of predation (Fig. 1a, b) [12, 14].
Predation may confer a selective advantage in certain environmental settings with previous studies indicating that different culture conditions, including microbial diet, are able to
modulate the ratio of the two mouth forms [19, 20]. Furthermore, _P. pacificus_ predation under laboratory conditions is also an example of a phenomenon known as surplus-killing behavior
[12]. Surplus killing is a well-documented complex behavior observed in many predators across the animal kingdom, in which more prey are killed than nutritionally required
[21,22,23,24,25,26,27]. Theoretical and experimental studies considered surplus killing a potentially context-dependent, adaptive foraging strategy or alternatively, a context-general
syndrome of high aggression [21, 22, 26]. However, the full impact of diet on predation is currently poorly understood. Here, utilizing previously isolated bacteria from soil, scarab
beetles, and figs that are found naturally associated with _Pristionchus_ nematodes [28], we investigate their influence on its predatory behavior and predatory associated traits. We analyze
25 different bacterial species and establish their ability to modulate the predatory feeding behaviors including surplus killing. Subsequently, by focusing on one bacterial species in which
surplus killing is strongly enhanced, _Novosphingobium_, we conduct a mutant screen and identify bacterial derived vitamin B12 as a major component involved in enhancing the predatory
behaviors. Furthermore, we demonstrate that the addition of exogenous vitamin B12 is sufficient to recapitulate the heightened predatory behaviors, whereas mutations in B12-dependent
pathways had an opposing effect. Therefore, ecologically relevant bacteria found naturally associated with _P. pacificus_ influence behavioral traits. MATERIALS AND METHODS NEMATODE AND
BACTERIAL STRAINS A list of all nematode and bacterial species and strains can be found in Supplementary Table 1. BACTERIAL CULTURE CONDITIONS All bacterial strains and mutants were grown
overnight in LB (Lysogeny broth) supplemented with 50 μg/ml kanamycin where required. Bacteria were grown at 30 °C or 37 °C depending on the species and 6 cm nematode growth medium (NGM)
plates were seeded with 50 μl bacterial overnight cultures and were incubated for 2 days. NEMATODE CULTURE CONDITIONS _P. pacificus_, _C. elegans_, _Rhabditophanes_ sp. KR302, and _A.
sudhausi_ were grown under standard nematode growth conditions on NGM plates seeded with _Escherichia coli_ OP50. Egg cultures were obtained by treating healthy gravid adults with alkaline
hypochlorite (bleaching) and were maintained and raised at 20 °C on NGM plates. The free-living generation of _Parastrongyloides trichosuri_ was cultured as described in Grant et al. [29].
Briefly, to maintain the _P. trichosuri_ free-living generation in culture, _E. coli_ OP50-spotted NGM plates were incubated for 2 days at room temperature (RT). Autoclaved rabbit feces were
lightly broken and placed on the spotted NGM plate along with _P. trichosuri_ animals. Additional _E. coli_ OP50 (supplemented with/without vitamin B12) was subsequently added to the dry
rabbit feces. The entomopathogenic nematode _Steinernema carpocapsae_ was grown on its symbiotic bacterium _Xenorhabdus nematophila_. Symbiotic bacteria were inoculated in LB and incubated
at 25 °C overnight, 300 μl from overnight cultures were spotted to NGM plates (supplemented with/without vitamin B12) and incubated for 1 day at RT. _S. carpocapsae_ nematodes were
transferred to their respective symbiotic bacterial plates and subsequently grown at 20 °C. MOUTH-FORM PHENOTYPING Mouth-form phenotyping was performed as previously reported [12, 13]. In
brief, axenic worm eggs were obtained by treating healthy gravid _P. pacificus_ adults with alkaline hypochlorite, which were subsequently maintained on the test bacteria strains or mutants
for at least two generations. Synchronized J4 stage juvenile larvae were picked onto NGM plates with the same test bacteria and roughly 12 h later, worms became young adults. NGM plates with
synchronized young adults were placed onto a stereomicroscope with high magnification (×150). The eurystomatous (Eu) mouth form was determined by the presence of a wide mouth, whereas the
stenostomatous (St) forms were determined by a narrow mouth. Eu young adult worms were picked for predation assays. PREDATION ASSAYS We used two types of predation assays as described below.
CORPSE ASSAYS Corpse assays facilitated rapid quantification of predatory behavior and were conducted as previously described [12, 13, 17]. Briefly, in order to generate substantial _C.
elegans_ larvae for use as prey, cultures were maintained on _E. coli_ OP50 bacteria until freshly starved resulting in an abundance of young larvae. These plates were washed with M9 buffer,
passed through two Millipore 20 μm filters and centrifuged at 377 × _g_ to form a concentrated larval pellet of juvenile animals. Excess buffer was removed and 1 μl of worm pellet was
deposited onto a 6 cm NGM unseeded assay plates. This resulted in roughly 3000 prey larvae on each assay plate. Assay plates were left for a minimum of 1 h to allow larvae to distribute
evenly over the plate. Young adult _P. pacificus_ predators were screened for the predatory Eu mouth form and transferred to empty NGM plates for 30 min to remove any excess bacteria from
their bodies. Subsequently, five _P. pacificus_ nematodes were added to each assay plate. Predators were permitted to feed on the prey for 2 h before removal and the plate was subsequently
screened for the presence of larval corpses, which were identified by the absence of motility coinciding with obvious morphological defects including leaking innards or missing worm
fragments. Each assay was replicated ≥5 times. When post-feeding size measurement was required, predatory animals were picked to NGM plates containing no bacteria and measurements were taken
using the Wormsizer plug in for Image J/Fiji [30]. See below for Wormsizer experimental details. BITE ASSAYS Bite assays provide a more detailed and thorough analysis of the specific
interactions associated with predatory behaviors. Bite assays were conducted as previously described [12, 13, 17]. Briefly, substantial _C. elegans_ prey was generated by maintaining _C.
elegans_ cultures on _E. coli_ OP50 bacteria until freshly starved resulting in an abundance of young larvae. These plates were washed with M9 buffer, passed through two Millipore 20 μm
filters and centrifuged at 377 × _g_ to form a concentrated larval pellet of juvenile animals. Excess buffer was removed and 1 μl of worm pellet was deposited onto a 6 cm NGM unseeded assay
plate. This resulted in roughly 3000 prey larvae on each assay plate. Assay plates were left for a minimum of 1 h to allow larvae to distribute evenly over the plate. Young adult _P.
pacificus_ predators were screened for the appropriate predatory Eu mouth morph and transferred to empty NGM plates for 30 min to remove any excess bacteria from their bodies. A single
predator was placed on to the assay plate and allowed to recover for 20 min. After recovery, the predatory animal was directly observed under a light stereomicroscope for 10 min and the
number of bites, successful bites and feeding events quantified. “Bites” were characterized by a switch to the slower predatory pharyngeal pumping rhythms previously described [12, 13, 17]
coinciding with a restriction in movement of the prey. “Successful bites” were characterized by successful rupturing of the prey cuticle resulting in sufficient damage to cause the innards
to leak from the wound. “Feeding” was characterized by consumption of the prey through either the observation of prolonged predatory feeding rhythms once the predator had successful grasped
its prey, or alternatively, observation of the faster bacterial associated feeding rhythms at the site of a puncture wound. In these assays, no distinction was made as to whether the
predatory behavior events were against live prey or against recently killed or wounded animals. Indeed, predators were occasionally observed repeatedly biting the same dying or dead larvae
and each contact was quantified as a distinct predatory event. Each assay was conducted with ten different animals. PHARYNGEAL PUMPING ANALYSIS _P. pacificus_ worms were maintained on 6 cm
NGM agar plates and fed on the appropriate test bacterial strains prior to assaying. Young adults were transferred onto assay plates and allowed to recover for 15 min from the stress of
being transferred. Worms were observed on a Zeiss microscope at ×40–×63 magnifications, with a high-speed camera and pharyngeal pumping was recorded for 15 s, at 50 Hz in at least 20 animals
to ensure accurate quantification. The recorded movies were replayed at the desired speed to count individual pumps as previously described [6]. _E. COLI_ OP50 SUPPLEMENTATION WITH
_NOVOSPHINGOBIUM_ L76 SUPERNATANT _E. coli_ OP50 and _Novosphingobium_ L76 were grown overnight in LB at 37 °C and 30 °C, respectively. Five milliliters overnight cultures of each bacteria
were grown until they measured an OD600 1. Bacterial cultures were centrifuged at 10,000 rpm, RT for 5 min and supernatants were isolated by filtering with 5 μm filters. The _E. coli_ OP50
pellet was re-suspended with 5 ml _Novosphingobium_ L76 supernatant. Three hundred microliters of the _E. coli_ OP50 with _Novosphingobium_ L76 supernatant was subsequently spotted to 6 cm
NGM plates. OP50 pellet with OP50 supernatant and additionally, _Novosphingobium_ L76 were also spotted to 6 cm NGM plates as controls. Spotted NGM plates were ready for assay after 2 days
of incubation. Freshly bleached eggs from well-grown _P. pacificus_ cultures were then transferred onto assay plates and worms were transferred to new assay plates 2 days later. Worms were
grown until young adult stage and synchronized young adults were picked and assessed via corpse assays. MIXING BACTERIAL DIETS Liquid cultures of _E. coli_ OP50 and _Novosphingobium_ L76
were grown in LB at 37 °C and 30 °C, respectively. Bacterial cultures were diluted to the same OD600 and mixed in ratios 1/10, 1/100, and 1/1000. Bacterial suspensions were spread onto
peptone-free NGM plates to minimize bacterial growth and plates were briefly air dried in a sterile hood. Bleached _P. pacificus_ eggs were added to the plates and worms were allowed to grow
until young adult stage; synchronized young adults were then picked and assessed via corpse assays. SWITCHING BACTERIAL DIET Overnight cultures of _E. coli_ OP50 and _Novosphingobium_ L76
were spread to NGM plates and incubated at RT for 2 days. Subsequently, bleached _P. pacificus_ eggs were added to the _E. coli_ OP50 plates. Worms were transferred from these _E. coli_ OP50
plates to _Novosphingobium_ L76 at specific developmental stages, L2, L3, and L4, respectively, and were allowed to develop into young adult stage on _Novosphingobium_ L76. Worms fed with
_E. coli_ OP50 or _Novosphingobium_ L76 from egg to young adult stage were used as controls. Synchronized young adults were then picked and assessed via corpse assays. RNA SEQUENCING
Bacterial strains were grown in LB overnight and spotted to 6 cm NGM plates. Starting from bleached eggs _P. pacificus_ nematodes were grown on bacteria for at least two generations and 50
young adults were picked for RNA isolation. Total RNA was extracted using Direct-Zol RNA Mini prep kit (Zymo Research) according to the manufacturer’s guidelines. RNA libraries were prepared
by following Truseq RNA library prep kit according to the manufacturer’s guidelines from 1 μg of total RNA in each sample (Illumina Company). Libraries were quantified using a combination
of Qubit and Bioanalyzer (Agilent Technologies) and normalized to 2.5 nM. Samples were subsequently sequenced as 150 bp paired end reads on multiplexed lanes of an Illumina HiSeq3000
(IIlumina Inc). Raw reads have been uploaded to the European Nucleotide archive under the study accession PRJEB33410. ANALYSIS OF RNA-SEQ DATA The software TopHat (version:2.0.14) was used
to align raw reads against the _P. pacificus_ reference genome (pristionchus.org, version: Hybrid1) and tests for differential expression were performed by Cuffdiff (version: 2.2.1) [31].
Genes with an FDR-corrected _p_ value < 0.05 were considered as significantly differentially expressed. For upregulated and downregulated genes, the most significantly enriched metabolic
pathways were identified as described previously [19]. GENERATION OF TRANSGENIC LINES We selected the genes _Ppa-stdh-1_ and _Ppa-acs-19.1_ to generate transcriptional reporters and
established transgenic lines necessary for their use as dietary sensors. For _Ppa-stdh-1_, a 2.3 kb interval encompassing the upstream region and the first two exons was amplified. For
_Ppa-acs-19.1_, a 1.4 kb region upstream of the first predicted exon was amplified. These promoters were fused to TurboRFP (Evrogen), together with the 3′ UTR sequence of the gene
_Ppa-rpl-23_ using the primers listed in Supplementary Table 1. PCR fragments were assembled using Gibson assembly kit (NEB) and verified by Sanger sequencing. The _Ppa-stdh-1_::RFP and
_Ppa-acs-19.1_::RFP constructs were amplified with the addition of restriction sites (XmaI and PstI) for subsequent digestion. To form stable lines via the formation of complex arrays, the
expression construct _Ppa-stdh-1_::RFP was digested with PstI and 5 ng/μl of this, co-injected into the germlines of young adult _P. pacificus_ worms with the marker _Ppa-egl-20_::Venus (10
ng/μl), and genomic carrier DNA (60 ng/μl), also digested with PstI [32]. For the _Ppa-acs-19.1_::RFP construct, 10 ng/μl of the construct cut with PstI, was injected with the marker
_Ppa-egl-20_::RFP (10 ng/μl), and genomic carrier DNA (60 ng/μl) also cut with PstI. At least two independent lines were obtained from microinjections for both transgenes. TRANSPOSON
MUTAGENESIS OF BACTERIA To generate electro-competent cells of _N. lindaniclasticum_ LE124 for electroporation, _N. lindaniclasticum_ LE124 cells were grown in LB overnight at 30 °C. These
overnight cultures were diluted (1:10 vol/vol) and incubated for ≅6 h to reach early log phase (optical density [OD] at 600 nm of 0.3). The culture was centrifuged at 4 °C, 10,000 rpm for 10
min before being washed once with ice-cold distillated water and two times with ice-cold 10% glycerol. After the final washing step, cells were centrifuged and the pellet re-suspended with
≅1 ml 10% glycerol before 50 μl aliquots were distributed to 1.5 ml Eppendorf tubes. The cells in glycerol were electroporated with the EZ-Tn_5_ R6Kγ_ori_/KAN-2>Tnp transposon (Epicentre,
Madison WI) using an Eppendorf Electroporator 2510 at 2.5 kV yielding around 5 ms. After electroporation, the sample was immediately mixed with SOC (super optimal broth with catabolite
repression) medium and incubated at 30 °C for 2 h, the culture was then plated on LB agar medium supplemented with 50 μg/ml of kanamycin. BACTERIAL TRANSPOSON MUTAGENESIS LIBRARY PREPARATION
After 2 days incubation of the bacteria at 30 °C, ten colonies were randomly selected, picked and a PCR carried out together with Sanger sequencing to confirm the integration of the
transposon into the _N. lindaniclasticum_ LE124 genome using the primers KAN-2 FP-1-F (5′-ACCTACAACAAAGCTCTCATCAACC-3′) and R6KAN-2 RP-1 -R (5′-CTACCCTGTGGAACACCTACATCT-3′). After successful
confirmation of the bacterial transposon mutagenesis, around 4500 single mutant colonies were picked and inoculated to 96-well plates in 160 μl LB supplemented with 50 μg/ml of kanamycin.
Overnight cultures of all mutants were mixed with 160 μl 50% glycerol and frozen at −80 °C. TRANSPOSON MUTANT LIBRARY SCREENING USING DIETARY SENSORS Transposon mutants were inoculated into
96-well plates in 180 μl LB supplemented with 50 μg/ml of kanamycin. After overnight growth at 30 °C, 20 μl from the mutant cultures were spotted to 24-well NGM plates. Bacterial mutant
strains were incubated for 2 days and eggs of _P. pacificus_ RS3271 (_Ppa-stdh-1::_RFP_)_ or _P. pacificus_ RS3379 (_Ppa-acs-19.1_::RFP) were bleached and filtered with Millipore 120 μm
filters to reduce the amount of adult worm carcasses. Around 50–100 bleached eggs were spotted to each well with mutant bacteria; _E. coli OP50_ and _N. lindaniclasticum_ LE124 wild-type
strain were used as controls. Fluorescent worms were grown on the bacterial strains until they became young adults. The _Ppa-stdh-1::_RFP line was screened for decreased RFP expression,
whereas the _Ppa-acs-19.1_::RFP line was screened for increased RFP expression. Initial positive results were re-screened at least three times to confirm changes in gene expression. ANALYSIS
OF TRANSPOSON MUTANT SEQUENCING DATA Raw reads were aligned against _N. lindaniclasticum_ LE124 reference genome and transposon sequence by the BWA aln and samse programs (version
0.7.12-r1039) [33]. The generated sam files were screened for read pairs where one read aligned to the transposon sequence and the second read was unmapped. For each mutant line a single
gene harboring a transposon insertion site was identified by realignment of the unmapped second read against the _N. lindaniclasticum_ LE124 reference with the help of blastn (version:
2.6.0) [34]. Raw whole-genome sequencing data of these mutant lines is available at the European Nucleotide archive under the study accession PRJEB33410. GENERATION OF CRISPR-INDUCED MUTANTS
OF _PPA-METR-1_ AND _PPA-MCE-1_ We generated mutant alleles for _Ppa-metr-1_ and _Ppa-mce-1_ using the CRISPR/Cas9 technique following the protocol described previously [35]. crRNAs were
synthesized by Integrated DNA Technologies and fused to tracrRNA (also Integrated DNA Technologies) at 95 °C for 5 min before the addition of the Cas9 endonuclease (New England Biolab).
After a further 5 min incubation at RT, TE buffer was added to a final concentration of 18.1 μM for the sgRNA and 2.5 μM for Cas9. Around 20 young adults were injected; eggs from injected
P0s were recovered up to 16 h post injection. After hatching and 2 days of growth these F1 were picked onto individual plates until they had also developed and laid eggs. The genotype of the
F1 animals was subsequently analyzed via Sanger sequencing and mutations identified and isolated in homozygosity. PHYLOGENETIC ANALYSIS For two fatty acid metabolism related genes with
differential expression between the bacterial diets, we retrieved homologs by BLASTP searches against WormBase (version: WS270) and pristionchus.org (version: TAU2011). Homologous protein
sequences from _C. elegans_ and _P. pacificus_ were aligned by MUSCLE (version: 3.8.31) [36] and maximum likelihood trees were generated with the help of the phangorn package in R (version:
3.5.3, parameters: model = “LG”, optNni = TRUE, optBf = TRUE, optInv = TRUE) [37]. To assess the robustness of the resulting trees, 100 bootstrap pseudoreplicates were calculated. For two
_C. elegans_ candidate genes involved in the Vitamin B12 pathway, one-to-one orthologs in _P. pacificus_ could directly be retrieved from BLASTP searches against WormBase (version: WS270):
_Ppa-metr-1_ (PPA25255) and _Ppa-mce-1_ (PPA39850). One-to-one orthology was confirmed by phylogenetic analysis. METABOLITE SUPPLEMENTATION Methylcobalamin (Vitamin B12 CAS Number
13422–55–4) and l-methionine (CAS Number 63–68–3) were purchased from Sigma and dissolved in water at the highest possible soluble concentrations to prepare stock solution. A methylcobalamin
stock was prepared fresh before use in each experiment. Metabolite solutions were mixed with NGM agar at the required concentration just before pouring the 6 cm plates. Plates were allowed
to dry at RT for 2 days and then spotted with _E. coli_ OP50. We first tested different concentrations of vitamin B12 and found the strongest and most reliable effect with a concentration of
500 nM, which is most likely un-physiological. In _C. elegans_, similar dose-dependent effects have been seen for vitamin B12 [38]. _PPA_-_ACS-19.1_::RFP GENE EXPRESSION SCREENING ON
METABOLITE-SUPPLEMENTED PLATES We used _Ppa_-_acs-19.1_::RFP transgenic animals to determine working concentrations of metabolite supplementations. Bleached _Ppa_-_acs-19.1_::RFP transgenic
eggs were transferred to metabolite-supplemented plates, which were prepared as described above. _Ppa_-_acs-19.1_::RFP positive young adults were screened for differences in gene expression
in comparison to control animals grown on a _E. coli_ OP50 and _N. lindaniclasticum_ LE124 diet without metabolite supplementation. IMAGING TRANSGENIC REPORTER LINES Eggs of transgenic
reporter lines _Ppa_-_acs-19.1_::RFP and _Ppa-stdh-1_::RFP were bleached and transferred to bacteria plates that were prepared as described. Three milliliters of 2% agar was prepared and a
drop (150 μl) of 1 M sodium azide (NaN3) was added and mixed with agar to immobilize the worms. Around 200 μl agar was dropped on microscope slide and young adult transgenic worms were
placed on the agar. Images of the worms were taken with 10× objective of ZEISS Imager Z1 equipped with the AxioCam camera using ZEN imaging software. The same exposure time was applied to
all images. VITAMIN B12 (METHYLCOBALAMIN) SUPPLEMENTATION ASSAYS Vitamin B12-supplemented plates were prepared as described above. _P. pacificus_, _C. elegans_, _Rhabditophanes_ sp. KR3021_,
A. sudhausi_ SB413, as well as _Ppa-metr-1_ (_tu1436, tu1436_) and _Ppa-mce-1_(_tu1433, tu1434_ and _tu1435_) mutant animals were grown on supplemented plates from egg to young adult stage
and subsequently used for (i) predatory assays, (ii) worm size measurements, and (iii) developmental assays. For supplementation experiments with free-living _P. trichosuri_, juvenile larvae
(J2 stage) were washed five times with M9 medium and filtered with Millipore 20 μm filters before being soaked in PBS supplemented with 100 μg/ml penicillin and ampicillin for 1 h to avoid
contamination. juvenile larvae (J2 stage) were washed a final time with PBS containing no antibiotics and transferred to assay plates. For _S. carpocapsae_, juvenile larvae (J2 stage) were
washed with M9 medium and filtered with Millipore 20 μm filters before transferring to NGM plates supplemented with/without 500 nM vitamin B12. WORM SIZE MEASUREMENT _P. pacificus_, _C.
elegans_, _Rhabditophanes_ sp., _P. trichosuri, A. sudhausi_, and _S. carpocapsae_ synchronized young adults were transferred from assay plates to NGM plates without bacteria. Bright field
images of the worms were taken using 0.63× objective of ZEISS SteREO Discovery V12 using the AxioCam camera. Images were analyzed using the Wormsizer plug in for Image J/Fiji [30]. Wormsizer
detects and measures the volume of the worms. DEVELOPMENT RATE ASSAYS For development rate assays, _P. pacificus_, _C. elegans, Rhabditophanes sp_., and _A. sudhausi_ were grown on OP50 at
20 °C. Nematode eggs were bleached, washed with M9 several times and allowed to hatch in M9 medium for 20 h in the absence of food to cause juvenile arrest at the J2 development stage. Once
synchronized, juvenile larvae were filtered through two Millipore 20 μm filters and around 30–60 juvenile (J2) animals were transferred to NGM plates (supplemented with/without 500 nM
vitamin B12) spotted in 50 μl of the desired test bacterial strain. Nematodes were subsequently allowed to develop on test bacteria for the following time periods: _P. pacificus_ 57 h at 20
°C, _C. elegans_ and _Rhabditophanes sp_. 45 h at 20 °C and _A. sudhausi_ for 144 h at RT. Following this, worms were categorized into groups based on the development of the vulva and germ
line using 0.63× objective of ZEISS SteREO Discovery V12 following previously established protocols [38]. STATISTICAL ANALYSIS Statistical calculations (mean, SEM, and t test) were performed
by using R studio software. Pairwise _t_-tests with Benjamini–Hochberg multiple testing correction were applied when testing the effect of a single treatment or mutant against one single
control sample. For tests across different groups (e.g., treatments, mutants, behaviors), Tukey-HSD test was applied. Significance is designated between two samples according to the
following scale: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘n.s’ 0.1 ‘n.s’ 1. RESULTS BACTERIAL DIET MODULATES COMPLEX BEHAVIORS IN NEMATODES We tested the effect of 25 different bacteria recently
isolated from _Pristionchus_-associated environments [28] on various predation associated traits (Table S1). Specifically, we grew _P. pacificus_ for two generations on monoxenic cultures
and investigated the effect on mouth-form ratio, pharyngeal pumping, and killing behavior by comparing them to standard laboratory cultures grown on _Escherichia coli_ OP50. While diet had a
limited effect on mouth-form ratios, we found up to a fourfold difference in killing efficiency and pharyngeal pumping depending on microbial diet (Figs. 1c and S1A, B). The strongest
effect on killing efficiency was observed when _P. pacificus_ was fed upon three alpha-proteobacteria of the genera _Novosphingobium_ and _Rhizobium_, resulting in up to 160 corpses of dead
prey in standardized corpse assays (Fig. 1c). We therefore focused on one bacterium of this group, _Novosphingobium_ L76, and its effect on killing efficiency. Stronger killing efficiency
translated into higher rates of surplus killing. Specifically, we performed bite assays to observe individual predators for 10 min to distinguish specific predatory events including biting,
successful biting that results in penetration of the cuticle, and feeding on prey larvae (see “Method” section for exact description of terms). When grown on _E. coli_ OP50, _P. pacificus_
only kills 50% of its prey after biting, and subsequent feeding was only observed in roughly 10% of all cases (Fig. 1d, Movie S1). Using _Novosphingobium_ L76, we found that the number of
_P. pacificus_ bites and successful biting events indeed doubled relative to _E. coli_ OP50 grown predators (Fig. 1d). However, we found no increase in feeding on the dead prey (Fig. 1d).
Instead, predators rapidly moved over agar plates searching for new prey items. In addition, this behavior required live bacterial food, as worms do not grow on heat killed
_Novosphingobium_. Thus, a _Novosphingobium_ diet enhances predation and surplus killing. A _NOVOSPHINGOBIUM_ DIET ALTERS THE EXPRESSION OF _P. PACIFICUS_ GENES INVOLVED IN FATTY ACID
METABOLISM Next, we established the necessary bacterial exposure time required to influence predatory behavior and additionally, wanted to know whether the increase in killing was mediated
by factors secreted by the bacteria or solely by their ingestion. Only a limited exposure to a diet of _Novosphingobium_ L76 during development was sufficient for _P. pacificus_ nematodes to
exhibit increased predatory behavior (Fig. S1C). In contrast, _Novosphingobium_ L76 culture supernatants alone were unable to recapitulate this effect (Fig. 1e). When _Novosphingobium_ was
diluted with _E. coli_ OP50, the effect still persisted suggesting that the response to _Novosphingobium_ L76 is unlikely due to differences in caloric intake (Fig. 1f). Instead, the
behavioral change is likely a result of physiological alterations caused by the different nutritional composition of _Novosphingobium_ L76. Therefore, we analyzed the transcriptomic response
of young _P. pacificus_ adults grown on _Novosphingobium_ in comparison with _E. coli_. We identified a total of 2677 (9%) genes with significant differential expression (FDR-corrected _p_
value < 0.05) between the two bacterial diets (Table S2). Most strikingly, more than half of all genes that are predicted to be involved in fatty acid metabolism are significantly
differentially expressed between the two diets (Fig. 2a, b). A MUTANT SCREEN IDENTIFIED BACTERIAL DERIVED VITAMIN B12 AS A METABOLITE ABLE TO ENHANCE SURPLUS KILLING To study the mechanisms
by which _Novosphingobium_ alters fatty acid metabolism and induces behavioral changes, we used an unbiased bacterial mutagenesis approach. We replaced _Novosphingobium_ L76 with
_Novosphingobium lindaniclasticum_ LE124 (_N. lin_. LE124 thereafter), as the latter can easily be manipulated by transposon mutagenesis, has an available genome [39], and induces similar
behavioral effects in _P. pacificus_ (Fig. S1D). In addition, to detect any physiological changes in _P. pacificus_ caused by mutations in the bacteria, two dietary sensors were generated
using _P. pacificus_ fatty acid metabolism genes that showed differential expression on different bacteria (Fig. 2a, b) [40]. Specifically, we used homologs of the acyl-CoA synthetase enzyme
_Ppa-acs-19.1_, which was upregulated on _E. coli_ OP50 and downregulated on _Novosphingobium_, as well as the short-chain dehydrogenase reductase enzyme _Ppa-stdh-1_, which has the
opposite expression profile (Fig. 2c, d). Both reporter lines confirmed the differential expression that was detected by RNA-seq (Fig. 2c, d). Subsequently, we used these dietary sensors to
screen for bacterial mutants that fail to differentially regulate these genes. From a library of 4320 _N. lin_. LE124 mutants, three affected the expression of _Ppa-stdh-1_ and 21 altered
the expression of _Ppa-acs-19.1_. Whole-genome sequencing of these bacterial mutants identified transposon insertions in genes corresponding to four biological pathways: purine and
pyrimidine metabolism, nitrogen metabolism, and vitamin B12 (Fig. 2e; Fig. S2A, key resources table). Importantly, in mutants of all four pathways, the change of transcriptomic response
coincided with a reduction in predatory behavior including surplus-killing relative to wild-type _N. lin_. LE124 (Figs. 2f and S2B, C). Thus, the dietary sensor allows the identification of
factors regulating complex behavioral traits. Vitamin B12 has been shown to be a crucial cofactor involved in growth, development and behavior in several animals, including mice and human.
Therefore, we focus on vitamin B12, which was recently also found to affect growth and development of _C. elegans_ [38], whereas nothing is known about vitamin B12 affecting _C. elegans_
behavior. We first analyzed if vitamin B12 supplementation was sufficient to affect the expression of the _Ppa-acs-19.1_ sensor and determined the required concentration for this.
Supplementation of an _E. coli_ diet with 500 nM vitamin B12 resulted in the absence of _Ppa-acs-19.1_ expression with no adverse effects to the health of wild-type animals (Fig. S3A). In
addition, this vitamin B12 concentration abolished _Ppa-acs-19.1_ expression on _N. lin_. LE124 _CbiQ::Tn5_ mutants (Fig. S3B). Subsequently, we analyzed if this supplementation was also
sufficient to enhance the predatory behaviors. Indeed, supplementation with 500 nM vitamin B12 rescued the vitamin B12-deficient _N. lin_. LE124 _CbiQ_ mutant and similarly, increased
surplus-killing behavior on an _E. coli_ diet (Fig. 3a, b). These results demonstrate that vitamin B12 is an important micronutrient involved in complex behaviors in nematodes. VITAMIN B12
AFFECTS _P. PACIFICUS_ DEVELOPMENT AND GROWTH SIMILAR TO _C. ELEGANS_ Studies by Walhout and co-workers in _C. elegans_ showed that developmental acceleration under a _Comamonas aq_. DA1877
diet was also due to vitamin B12 [31]. Given the similarities of the _C. elegans_ developmental response to _Comamonas_ DA1877 and the behavioral response of _P. pacificus_ to _N. lin_.
LE124, we compared the effect of both bacteria on development and behavior. Indeed, _Comamonas_ DA1877 as well as _N. lin_. LE124 induced developmental acceleration of _C. elegans_ and _P.
pacificus_ (Fig. 3c). Similarly, both bacteria enhanced predatory behaviors of _P. pacificus_ (Fig. 3d). Thus, the differential effect of bacterial diet on nematode development and behavior
might often be due to the uneven distribution of vitamin B12 biosynthesis capabilities of bacteria, but this remains to be tested. TWO VITAMIN B12-DEPENDENT PATHWAYS ARE REQUIRED FOR
ENHANCED SURPLUS KILLING In many animals and humans, vitamin B12 is a cofactor for two enzymes in different pathways (Fig. S4A). Methionine-synthase (MS) converts homocysteine to methionine
in the cytosolic methionine/S-adenosylmethionine (SAM) cycle and in _C. elegans_ is encoded by the _metr-1_ gene. The second enzyme, methylmalonyl coenzyme A (CoA) mutase, converts
methylmalonyl-CoA to succinyl-CoA in mitochondria and is encoded by the _mce-1_ gene in _C. elegans_. In humans, vitamin B12 deficiency causes methylmalonic aciduria and homocysteinemia
resulting in devastating diseases. To test if both pathways are required for increased killing behavior in _P. pacificus_, we generated CRISPR/Cas9-derived mutants in _Ppa-metr-1_ and
_Ppa-mce-1_ (Fig. S4B, C, D). Both mutants failed to respond to the supplementation of an _E. coli_ diet with vitamin B12 (Fig. 4a). Given that SAM is a donor of methyl-groups for many
different substrates including RNA, DNA, and proteins, we supplemented an _E. coli_ diet of _P. pacificus_ wild type and _Ppa-metr-1_ mutant animals with methionine. In both cases,
methionine supplementation resulted in enhanced killing behavior when predators were fed _E. coli_ bacteria (Fig. 4b). Thus, both vitamin B12-dependent pathways seem to be involved in _P.
pacificus_ predatory behaviors. VITAMIN B12 DEPENDENT DEVELOPMENTAL ACCELERATION IS CONSERVED ACROSS NEMATODES The experiments described above indicate crucial roles of bacterial derived
vitamin B12 for the development and behavior of both _P. pacificus_ and _C. elegans_. As these nematodes are estimated to have diverged roughly 100 Mya [41], we next tested how prevalent the
effects of vitamin B12 are on the development and physiology of other nematodes, including more distantly related species and representatives that live in diverse ecological settings
(Supplementary Table 1). We grew six nematode species of four major taxonomic clades on a vitamin B12 supplemented diet and measured the effects on their development and growth by
quantifying the total worm volume of young adults. In all species tested, we found a significant increase in worm volume (Fig. 4c, d). This included the facultative parasite
_Parastrongyloides trichosuri_ and the entomopathogenic nematode _Steinernema carpocapsae_. We found the strongest effect on the large free-living nematode _Allodiplogaster sudhausi_ that
nearly doubled its volume on a vitamin B12 supplemented diet (Fig. 4d). Where possible, we also investigated the effects on developmental speed. Similar to the increase in body size, vitamin
B12 supplementation accelerated the development of _Rhabditophanes_ and _Allodiplogaster_ (Fig. S4E, F). Taken together, these results demonstrate important physiological and developmental
functions of vitamin B12 that are shared across many nematode species. DISCUSSION Here, we identified a novel role for nematode-associated microbiota in modulating the complex behavioral
trait of predation and therefore, demonstrates a connection between the microbial diet and the nervous system in nematodes. Diverse bacterial species, which have previously been found
naturally associated with _Pristionchus_ nematodes [28], elicit different effects on the predatory behavioral state after feeding. Some adversely influence predation, whereas others enhance
the predatory behaviors. The greatest enhancement in predatory behaviors was observed when _P. pacificus_ was fed upon _Novosphingobium_ with the increase in killing influenced by bacterial
derived vitamin B12. In addition, we have revealed a more general, conserved role for vitamin B12 in nematode development and growth. Previous studies have shown vitamin B12 to be essential
for _C. elegans_ development with infertility, growth retardation, and a reduction in life-span observed in animals deficient in vitamin B12 [38, 42, 43]. In contrast, behavioral effects
have not been reported and similarly, mechanisms of vitamin B12 deficiency in humans that result in neuropathies are currently unknown. It is important to note that the modulation of
predation and surplus killing in _P. pacificus_ requires both vitamin B12-dependent pathways. Therefore, we speculate that the influence of vitamin B12 on these behaviors is multifactorial
and might well involve several factors. Specifically, the SAM pathway feeds into the methylation of DNA, RNA, and proteins, but also lipids and neurotransmitters (Fig. S4a). Indeed, both the
purine and pyrimidine synthesis pathways were also isolated in our bacterial mutant screen with mutants negatively influencing the predatory behaviors and both are biochemically related to
SAM. Thus, the presence of vitamin B12 might act through multiple downstream factors, but how it stimulates these effects has yet to be discovered. Most importantly, the neuronal circuits
that are directly or indirectly affected by vitamin B12 have to be identified in future research. Notably, several neural circuits and neurotransmitter systems of _P. pacificus_ have been
determined and investigated in detail [12, 13, 44, 45]. Therefore, future studies can reveal the cellular and molecular foci of vitamin B12-dependence and the influence of the microbiota on
nematode predation. This study complements previous work [10], which explored the succession and dynamics of the nematode-microbiota environment associated with the decaying beetle carcass
on which _Pristionchus_ nematodes are frequently found. Whereas our previous omics approach identified the larger scale ecological communities and their changing composition, by focusing on
individual bacterial species we have begun to discover the potential complex interactions influencing these environments. However, much of the influence of the microbiota-nematode
interactions within this community still remains to be elucidated. This includes how the complex microbial community contributes to the nematode life cycle and how the abundance and
composition of the microbiota may drive the dispersal of the nematodes in order to seek new beetle hosts on which to colonize. DATA AVAILABILITY RNA-seq data has been deposited at the
European Nucleotide Archive under the study accession PRJEB33410. All other data is available in the main text or the supplementary materials. CHANGE HISTORY * _ 03 APRIL 2020 A Correction
to this paper has been published: https://doi.org/10.1038/s41396-020-0644-0 _ REFERENCES * Kamareddine L, Robins WP, Berkey CD, Mekalanos JJ, Watnick PI. The drosophila immune deficiency
pathway modulates enteroendocrine function and host metabolism. Cell Metab. 2018;28:449–.e5. Article CAS PubMed PubMed Central Google Scholar * Thaiss CA, Zmora N, Levy M, Elinav E. The
microbiome and innate immunity. Nature. 2016;535:65–74. Article CAS PubMed Google Scholar * Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and
the immune system. Nature. 2011;474:327–36. Article CAS PubMed PubMed Central Google Scholar * Berg M, Stenuit B, Ho J, Wang A, Parke C, Knight M, et al. Assembly of the Caenorhabditis
elegans gut microbiota from diverse soil microbial environments. ISME J. 2016;10:1998–2009. Article PubMed PubMed Central Google Scholar * Dirksen P, Marsh SA, Braker I, Heitland N,
Wagner S, Nakad R, et al. The native microbiome of the nematode Caenorhabditis elegans: gateway to a new host-microbiome model. BMC Biol. 2016;14:38. Article PubMed PubMed Central CAS
Google Scholar * Samuel BS, Rowedder H, Braendle C, Félix M, Ruvkun G. Caenorhabditis elegans responses to bacteria from its natural habitats. Proc Natl Acad Sci. 2016;113:E3941–E3949. CAS
PubMed PubMed Central Google Scholar * Kissoyan KAB, Drechsler M, Stange E, Zimmermann J, Kaleta C, Bode HB. Natural C. elegans microbiota protects against infection via production of a
cyclic lipopeptide of the viscosin group. Curr Biol. 2019;29:1030–.e5. Article CAS PubMed Google Scholar * Zimmermann J, Obeng N, Yang W, Pees B, Petersen C, Waschina S, et al. The
functional repertoire contained within the native microbiota of the model nematode Caenorhabditis elegans. ISME J. 2020;14:26–38. Article CAS PubMed Google Scholar * Herrmann M, Mayer
WE, Sommer RJ. Nematodes of the genus Pristionchus are closely associated with scarab beetles and the Colorado potato beetle in Western Europe. Zoology. 2006;109:96–108. Article CAS PubMed
Google Scholar * Meyer JM, Baskaran P, Quast C, Susoy V, Rödelsperger C, Glöckner FO, et al. Succession and dynamics of Pristionchus nematodes and their microbiome during decomposition of
Oryctes borbonicus on La Réunion Island. Environ Microbiol. 2017;19:1476–89. Article CAS PubMed Google Scholar * Sommer RJ, Carta LK, Kim SY, Sternberg PW. Morphological, genetic and
molecular description of pristionchus pacificus sp. n. (nematoda: neodiplogastridae). Fundam Appl Nematol. 1996;19:511–21. Google Scholar * Wilecki M, Lightfoot JW, Susoy V, Sommer RJ.
Predatory feeding behaviour in Pristionchus nematodes is dependent on phenotypic plasticity and induced by serotonin. J Exp Biol. 2015;218:1306–13. PubMed Google Scholar * Okumura M,
Wilecki M, Sommer RJ. Serotonin drives predatory feeding behavior via synchronous feeding rhythms in the nematode pristionchus pacificus. G3 (Bethesda). 2017;7:3745–55. Article CAS Google
Scholar * Ragsdale EJ, Müller MR, Rödelsperger C, Sommer RJ. A developmental switch coupled to the evolution of plasticity acts through a sulfatase. Cell. 2013;155:922–33. Article CAS
PubMed Google Scholar * Serobyan V, Xiao H, Namdeo S, Rödelsperger C, Sieriebriennikov B, Witte H, et al. Chromatin remodelling and antisense-mediated up-regulation of the developmental
switch gene eud-1 control predatory feeding plasticity. Nat Commun. 2016;7:12337. Article CAS PubMed PubMed Central Google Scholar * Sieriebriennikov B, Prabh N, Dardiry M, Witte H,
Röseler W, Kieninger MR, et al. A developmental switch generating phenotypic plasticity is part of a conserved multi-gene locus. Cell Rep. 2018;23:2835–.e4. Article CAS PubMed Google
Scholar * Lightfoot JW, Wilecki M, Rödelsperger C, Moreno E, Susoy V, Witte H, et al. Small peptide–mediated self-recognition prevents cannibalism in predatory nematodes. Science.
2019;364:86–89. Article CAS PubMed Google Scholar * Bento G, Ogawa A, Sommer RJ. Co-option of the hormone-signalling module dafachronic acid-DAF-12 in nematode evolution. Nature.
2010;466:494–7. Article CAS PubMed Google Scholar * Sanghvi GV, Baskaran P, Röseler W, Sieriebriennikov B, Rödelsperger C, Sommer RJ. Life history responses and gene expression profiles
of the nematode pristionchus pacificus cultured on cryptococcus yeasts. PLoS ONE. 2016;11:1–13. Article CAS Google Scholar * Werner MS, Sieriebriennikov B, Loschko T, Namdeo S, Lenuzzi M,
Dardiry M, et al. Environmental influence on Pristionchus pacificus mouth form through different culture methods. Sci Rep. 2017;7:7207. Article PubMed PubMed Central CAS Google Scholar
* Zimmermann B, Sand H, Wabakken P, Liberg O, Andreassen HP. Predator-dependent functional response in wolves: from food limitation to surplus killing. J Anim Ecol. 2015;84:102–12. Article
PubMed Google Scholar * Veselý L, Boukal DS, Buřič M, Kozák P, Kouba A, Sentis A. Effects of prey density, temperature and predator diversity on nonconsumptive predator-driven mortality
in a freshwater food web. Sci Rep. 2017;7:1–9. Article CAS Google Scholar * Maupin JL. Superfluous killing in spiders: a consequence of adaptation to food-limited environments? Behav
Ecol. 2001;12:569–76. Article Google Scholar * Lounibos LP, Makhni S, Alto BW, Kesavaraju B. Surplus killing by predatory larvae of corethrella appendiculata: prepupal timing and
site-specific attack on mosquito prey. J Insect Behav. 2008;21:47–54. Article CAS PubMed PubMed Central Google Scholar * Kruuk H. Surplus killing by carnivores. J Zool. 2009;166:233–44.
Article Google Scholar * Trubl P, Blackmore V, Chadwick Johnson J. Wasteful killing in urban black widows: gluttony in response to food abundance. Ethology. 2011;117:236–45. Article
Google Scholar * Gaydos JK, Raverty S, Baird RW, Osborne RW. Suspected surplus killing of harbor seal pups (Phoca Vitulina) by killer whales (Orcinus Orca). Northwest Nat. 2005;86:150–4.
Article Google Scholar * Akduman N, Rödelsperger C, Sommer RJ. Culture-based analysis of Pristionchus-associated microbiota from beetles and figs for studying nematode-bacterial
interactions. PLoS ONE. 2018;13:1–13. Article CAS Google Scholar * Grant WN, Stasiuk S, Newton-Howes J, Ralston M, Bisset SA, Heath DD, et al. Parastrongyloides trichosuri, a nematode
parasite of mammals that is uniquely suited to genetic analysis. Int J Parasitol. 2006;36:453–66. Article CAS PubMed Google Scholar * Moore BT, Jordan JM, Baugh LR. WormSizer:
high-throughput analysis of nematode size and shape. PLoS ONE. 2013;8:1–13. Google Scholar * Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and
transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–78. Article CAS PubMed PubMed Central Google Scholar * Schlager B, Wang X, Braach
G, Sommer RJ. Molecular cloning of a dominant roller mutant and establishment of DNA-mediated transformation in the nematode Pristionchus pacificus. Genesis. 2009;47:300–4. Article CAS
PubMed Google Scholar * Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–95. Article PubMed PubMed Central CAS Google
Scholar * Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. Article CAS PubMed Google Scholar * Witte H, Moreno E,
Rödelsperger C, Kim J, Kim JS, Streit A, et al. Gene inactivation using the CRISPR/Cas9 systemin the nematode Pristionchus pacificus. Dev Genes Evol. 2014;225:55–62. Article PubMed CAS
Google Scholar * Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7. Article CAS PubMed PubMed Central Google
Scholar * Schliep KP. phangorn: phylogenetic analysis in R. Bioinformatics. 2011;27:592–3. Article CAS PubMed Google Scholar * Watson E, Macneil LT, Ritter AD, Yilmaz LS, Rosebrock AP,
Caudy AA, et al. Interspecies systems biology uncovers metabolites affecting C. elegans gene expression and life history traits. Cell. 2014;156:759–70. Article CAS PubMed PubMed Central
Google Scholar * Saxena A, Nayyar N, Sangwan N, Kumari R, Khurana JP, Lal R. Genome sequence of Novosphingobium lindaniclasticum LE124T, Isolated from a Hexachlorocyclohexane Dumpsite.
Genome Announc. 2013;1:1–2. Article Google Scholar * MacNeil LT, Watson E, Arda HE, Zhu LJ, Walhout AJM. Diet-induced developmental acceleration independent of TOR and insulin in C.
elegans. Cell. 2013;153:240–52. Article CAS PubMed Google Scholar * Prabh N, Roeseler W, Witte H, Eberhardt G, Sommer RJ, Rödelsperger C. Deep taxon sampling reveals the evolutionary
dynamics of novel gene families in Pristionchus nematodes. Genome Res. 2018;28:1664–74. Article CAS PubMed PubMed Central Google Scholar * Bito T, Matsunaga Y, Yabuta Y, Kawano T,
Watanabe F. Vitamin B12 deficiency in Caenorhabditis elegans results in loss of fertility, extended life cycle, and reduced lifespan. FEBS Open Bio. 2013;3:112–7. Article CAS PubMed
PubMed Central Google Scholar * Na H, Ponomarova O, Giese GE, Walhout AJM. C. elegans MRP-5 exports vitamin B12 from mother to offspring to support embryonic development. Cell Rep.
2018;22:3126–33. Article CAS PubMed PubMed Central Google Scholar * Bumbarger DJ, Riebesell M, Rödelsperger C, Sommer RJ. System-wide rewiring underlies behavioral differences in
predatory and bacterial-feeding nematodes. Cell. 2013;152:109–19. Article CAS PubMed Google Scholar * Hong RL, Riebesell M, Bumbarger DJ, Cook SJ, Carstensen HR, Sarpolaki T, et al.
Evolution of neuronal anatomy and circuitry in two highly divergent nematode species. Elife. 2019;8:1–34. Google Scholar Download references ACKNOWLEDGEMENTS We thank Drs A. Streit and R.
Ehlers for _Parastrongyloides_ and _Steinernema_ material, respectively, and members of the Sommer lab for discussion. This work was funded by the Max Planck Society. Open access funding
provided by Projekt DEAL. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department for Evolutionary Biology, Max Planck Institute for Developmental Biology, Max Planck Ring 9, 72076,
Tübingen, Germany Nermin Akduman, James W. Lightfoot, Waltraud Röseler, Hanh Witte, Wen-Sui Lo, Christian Rödelsperger & Ralf J. Sommer Authors * Nermin Akduman View author publications
You can also search for this author inPubMed Google Scholar * James W. Lightfoot View author publications You can also search for this author inPubMed Google Scholar * Waltraud Röseler View
author publications You can also search for this author inPubMed Google Scholar * Hanh Witte View author publications You can also search for this author inPubMed Google Scholar * Wen-Sui Lo
View author publications You can also search for this author inPubMed Google Scholar * Christian Rödelsperger View author publications You can also search for this author inPubMed Google
Scholar * Ralf J. Sommer View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS N.A. and J.W.L. performed all behavioral experiments. W.R.
performed the RNA-seq experiments. H.W., N.A., and J.W.L. generated dietary sensor lines and CRISPR-induced mutants. Bioinformatic analysis was performed by W-S.L. and C.R. All experiments
were designed by N.A., C.R., J.W.L. and R.J.S. CORRESPONDING AUTHOR Correspondence to Ralf J. Sommer. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict
of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY
INFORMATION SUPPL MATERIAL TABLE S2 MOVIE S1 SURPLUS KILLING RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which
permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to
the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Akduman, N., Lightfoot, J.W., Röseler, W. _et al._ Bacterial vitamin B12 production enhances nematode predatory behavior. _ISME J_ 14,
1494–1507 (2020). https://doi.org/10.1038/s41396-020-0626-2 Download citation * Received: 03 November 2019 * Accepted: 26 February 2020 * Published: 09 March 2020 * Issue Date: 01 June 2020
* DOI: https://doi.org/10.1038/s41396-020-0626-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative