Maximal detrusor pressure can be predicted using technetium-99m-mertcaptoacetyltriglycine renal scintigraphy in the early stages of spinal cord injury
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT STUDY DESIGN Retrospective cohort study. OBJECTIVE To investigate the potential of technetium-99m-mercaptoacetyltriglycine (99mTc-MAG-3) renal scintigraphy for predicting maximal
detrusor pressure in the early stages of spinal cord injury (SCI). SETTING Tertiary rehabilitation facility. METHODS Medical records of individuals with SCI admitted between January 2020 and
April 2023 who underwent both 99mTc-MAG-3 renal scintigraphy and urodynamic study within 90 days of SCI onset were retrospectively reviewed. Pearson’s coefficient analysis was performed to
determine the relationship between 99mTc-MAG-3 renal scintigraphy findings and urodynamic study findings. A multivariate linear regression analysis was performed to determine the best
predictors of maximal detrusor pressure. A multivariate logistic regression analysis was performed to determine risk factors for high detrusor pressure. RESULTS Ninety-four participants were
enrolled in this study. Pearson’s correlation analysis showed that effective renal plasma flow (ERPF) and ERPF (% predicted) were significantly correlated with maximal detrusor pressure.
The multivariate linear regression analysis demonstrated that ERPF (% predicted) was a significant predictor of maximal detrusor pressure. The multivariate logistic regression analysis
showed that ERPF (% predicted) was significantly associated with high detrusor pressure. The receiver operating characteristic curve demonstrated that the predictive model had an area under
the curve of 0.725, with an ERPF (% predicted) cut-off of 64.05%, sensitivity 1.000, and specificity 0.429. CONCLUSIONS These results suggest that 99mTc-MAG-3 renal scintigraphy may be
useful for predicting high detrusor pressure in early SCI and may guide the timing of urodynamic studies in individuals with early SCI for appropriate management of neurogenic lower urinary
tract dysfunction. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS WHAT DO X-RAY IMAGES OF THE BLADDER DURING VIDEO URODYNAMICS
SHOW US IN PATIENTS WITH SPINAL CORD INJURY? Article 23 February 2022 DURATION OF DETRUSOR OVERACTIVITY AS AN INDEPENDENT PREDICTIVE FACTOR OF UPPER URINARY TRACT DETERIORATION IN PATIENTS
WITH TRAUMATIC SPINAL CORD INJURY: RESULTS OF A RETROSPECTIVE COHORT STUDY Article 04 April 2024 USE OF THE ICE WATER TEST AS AN EARLY PREDICTOR OF RECOVERY OF ERECTILE FUNCTION IN PATIENTS
WITH SPINAL CORD INJURY Article 29 June 2020 INTRODUCTION Neurogenic lower urinary tract dysfunction is a common secondary complication following spinal cord injury (SCI). The incidence of
neurogenic lower urinary tract dysfunction in individuals with SCI ranges between 70–84% [1, 2]. Neurogenic lower urinary tract dysfunction requires careful consideration because it can
cause upper urinary tract deterioration and decrease renal function [3]. In severe cases, it can cause renal failure [4]. Thus, it is essential to prevent upper urinary tract deterioration
and maintain renal function when treating neurogenic lower urinary tract dysfunction in SCI [5]. The gold standard for assessing lower urinary tract function is currently a urodynamic study
(UDS) [6], and it has been emphasized that follow-up UDS should be conducted on a regular basis to maintain renal function [7]. During spinal shock, the bladder is in a silent state with an
areflexic detrusor [6]. Most clinical consensuses recommend a baseline UDS evaluation after the return of bladder function [8]. According to previous literature, the period is usually
considered to be approximately 3–6 months after injury [9, 10]. However, there is controversy over the timing of UDS in the early stages of SCI [11, 12]. Detrusor overactivity has been
observed in the first 53 days after SCI [12]. Over 70% of the patients already showed unfavorable urodynamic parameters within the first month after acute SCI, in contradiction to the
assumption of an acontractile detrusor during this period [13]. This suggests the necessity for UDS evaluation during the early stage of SCI. Since UDS itself is invasive and carries the
risk of urinary tract infection, it is a concern to perform UDS at an early stage within three months after injury. Research on neurogenic lower urinary tract dysfunction in the very early
stages of SCI is still insufficient, and no studies have suggested predicting neurogenic lower urinary tract dysfunction using non-invasive examinations other than UDS. Therefore, it is
questionable which parameter indicates that clinicians should perform UDS in the very early stage of SCI and whether non-invasive technetium-99m-mercaptoacetyltriglycine (99mTc-MAG-3) renal
scintigraphy is a possible indicator for UDS. Renal scintigraphy has been used as a tool for monitoring renal function in SCI [14,15,16]. Recently, 99mTc-MAG-3 was introduced as a
99mTc-labeled replacement for iodine-131 o-iodohippurate. Although 99mTc-MAG-3 cannot be used to measure the glomerular filtration rate, it has been introduced as the most promising tubular
function agent to date and has replaced iodine-131 o-iodohippurate and 99mTc-diethylenetriaminepentaacetic acid in a number of institutions [17]. Among individuals with SCI, 99mTc-MAG-3
renal scintigraphy is a sensitive indicator for early renal deterioration and is recommended as the initial modality for monitoring renal function [18, 19]. Many studies have investigated
the risk factors for upper urinary tract deterioration in individuals with SCI. In particular, high detrusor pressure is an independent factor for upper urinary tract deterioration in
individuals with SCI [20,21,22,23]. However, no previous study has investigated the relationship between 99mTc-MAG-3 renal scintigraphy findings and detrusor pressure. This study aimed to
determine whether 99mTc-MAG-3 renal scintigraphy could be used to predict elevated detrusor pressure in advance in the early stages of SCI, and to establish new guidelines for early UDS
implementation. In this study, we attempted to predict maximal detrusor pressure by utilizing 99mTc-MAG-3 renal scintigraphy parameters in individuals with SCI. MATERIALS AND METHODS
PARTICIPANTS Medical records of individuals with SCI who visited the Department of Rehabilitation Medicine, Severance Hospital, Yonsei University College of Medicine between January 2020 and
April 2023 were retrospectively reviewed. This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System, Seoul, Korea (4-2022-1490).
Inclusion criteria were as follows: (1) individuals aged ≥ 18 years, and (2) individuals who underwent both 99mTc-MAG-3 renal scintigraphy and UDS. Exclusion criteria were: (1) age <18
years; (2) time between 99mTc-MAG-3 renal scintigraphy and UDS > 28 days; (3) time between 99mTc-MAG-3 renal scintigraphy and other laboratory tests (cystatin C and creatinine clearance)
>28 days; (4) presence of renal disease or injury (i.e., preexisting chronic kidney disease, kidney contusion, renal infarction, renal agenesis, nephrectomy status, etc.); and (5)
ambiguous onset date of SCI. DATA ASSESSMENT Technetium-99m-mercaptoacetyltriglycin renal scintigraphy was performed using QuantEM Expert (General Electric Healthcare, Milwaukee, WI, USA).
The time to peak activity (Tmax), half-time excretion (T1/2), and effective renal plasma flow (ERPF) of the right and left kidneys were collected. For the Tmax, T1/2, and ERPF obtained from
the right and left kidneys, the larger value between the two sides was labeled as ‘max,’ the smaller as ‘min,’ and the average value of both sides was labeled as ‘average.’ The ERPF of the
predicted normal was automatically calculated using the QuantEM Expert system. The percentage of the ERPF to the predicted normal (ERPF (% predicted)) was then obtained. A UDS was performed
using the Duet Logic G2 (Mediwatch, Rugby, UK). During the UDS, normal saline was infused into the bladder at a rate of 30 mL/min at room temperature. Bladder filling ended at 450 mL, except
if there was leakage or autonomic dysreflexia [24]. In this study, maximal bladder capacity, compliance, maximal detrusor pressure, and the presence of involuntary detrusor contractions
were obtained. STATISTICAL ANALYSIS Statistical analyses were performed using R Statistical Software version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria). Simple
descriptive statistics were used to characterize the participants and distribution of the variables. For continuous variables, data are presented as mean (standard deviation). The
correlation of either ERPF or ERPF (% predicted) with UDS findings was analyzed using the Pearson’s correlation analysis. Multivariate linear regression analysis was performed to determine
the best predictors of maximal detrusor pressure. Multivariate logistic regression analysis was performed to identify factors associated with high detrusor pressure. For high detrusor
pressure, we defined thresholds of 50 for males and 30 for females [25]. Specifically, a maximum detrusor pressure equal to or greater than these values for males and females, respectively,
was considered indicative of high detrusor pressure. The model performance was evaluated by constructing receiver operating characteristic (ROC) curves and computing the area under the curve
(AUC). The threshold for statistical significance was set at _P_ < 0.05. RESULTS DEMOGRAPHICS AND CLINICAL FEATURES OF PARTICIPANTS The general demographics and clinical characteristics
of the participants are shown in Table 1. A total of 94 individuals with SCI participated in this study, of whom 75 were males (79.79%) and 19 were females (20.21%). The mean age was 47.44
(15.89) years. The neurological levels of injury were classified as cervical or thoracic/lumbar; 61 (64.89%) cases were cervical and 33 (35.11%) were thoracic/lumbar. Seventeen (18.09%)
participants had traumatic SCI and 77 (81.91%) had non-traumatic SCI. There were 25 (26.60%), 7 (7.45%), 36 (38.30%), and 26 (27.66%) participants with the American Spinal Injury Association
Impairment Scales (AIS) grades A, B, C, and D, respectively. TECHNETIUM-99M-MERCAPTOACETYLTRIGLYCINE RENAL SCINTIGRAPHY FINDINGS OF PARTICIPANTS The parameters obtained using 99 mTc-MAG-3
renal scintigraphy are listed in Table 2. The mean time from injury to renal scintigraphy was 55.96 (17.75) days. The mean Tmax (average), Tmax (max), and Tmax (min) were 2.80 (0.90) min,
2.97 (1.07) min, and 2.63 (0.80) min, respectively. The mean T1/2 (average), T1/2 (max), and T1/2 (min) were 5.48 (1.88) min, 6.09 (2.50) min, and 4.88 (1.57) min, respectively. The mean
ERPF (average), ERPF (max), and ERPF (min) were 176.45 (37.20) mL/min, 187.76 (38.57) mL/min, and 165.14 (38.28) mL/min, respectively. The mean ERPF (% predicted) (average), ERPF (%
predicted) (max), and ERPF (% predicted) (min) were 67.32 (20.24) (%), 71.69 (21.48) (%), and 62.96 (19.73) (%) respectively. The mean cystatin C level was 0.94 (0.18) mg/L. The mean eGFR
was 90.41 (18.74) mL/min/1.73 m2, and the mean creatinine clearance was 129.16 (29.65) mL/min/1.73 m2. URODYNAMIC STUDY FINDINGS OF PARTICIPANTS The results of the UDS are shown in Table 3.
The mean time from injury to UDS was 60.45 (17.57) days. The mean maximal bladder capacity was 480.61 (66.07) mL. The mean maximal detrusor pressure was 16.02 (18.89) cmH2O. The mean
compliance was 95.02 (88.48) mL/cmH2O. High detrusor pressure was observed in 10 (10.64%) participants. Involuntary detrusor contractions were observed in 10 (10.64%) participants. PEARSON’S
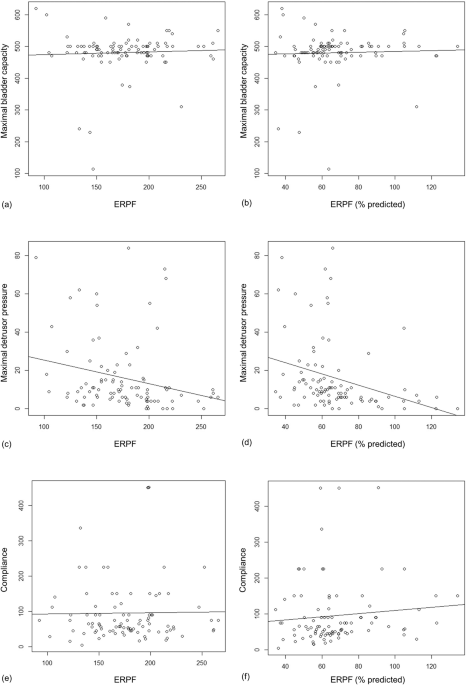
CORRELATION ANALYSIS OF ERPF AND ERPF (% PREDICTED) TO UDS FINDINGS Fig. 1 shows the results of the Pearson’s correlation analysis. Each figure shows the correlation between (a) ERPF and
maximal bladder capacity; (b) ERPF (% predicted) and maximal bladder capacity; (c) ERPF and maximal detrusor pressure; (d) ERPF (% predicted) and maximal detrusor pressure; (e) ERPF and
compliance; and (f) ERPF (% predicted) and compliance. The correlation coefficients of ERPF and ERPF (% predicted) with maximal bladder capacity were 0.046 (95% confidence interval (CI),
−0.148 to 0.246; _P_ = 0.661) and 0.039 (95% CI, −0.165 to 0.239; _P_ = 0.712), respectively. The correlation coefficient of ERPF and ERPF (% predicted) with maximal detrusor pressure were
−0.240 (95% CI, −0.422 to −0.039; _P_ = 0.020) and −0.312 (95% CI, −0.585 to −0.112; _P_ = 0.002), respectively. The correlation coefficients of ERPF and ERPF (% predicted) with compliance
were 0.015 (95% CI, −0.188 to 0.217; _P_ = 0.884) and 0.101 (95% CI, −0.104 to 0.297; _P_ = 0.333), respectively. MULTIVARIATE LINEAR REGRESSION ANALYSIS FOR PREDICTION OF MAXIMAL DETRUSOR
PRESSURE Table 4 shows the results of the multivariate linear regression analysis. Univariate analysis was initially performed, and variables, including the time of renal scintigraphy from
injury, ERPF (average), ERPF (max), ERPF (min), ERPF (% predicted) (average), ERPF (% predicted) (max), and ERPF (% predicted) (min), were significantly correlated with maximal detrusor
pressure (all _P_ < 0.05). Owing to multicollinearity, the time of renal scintigraphy from injury, ERPF (min), and ERPF (% predicted) (min) were included in the multivariate analysis;
ERPF (average), ERPF (max), ERPF (% predicted) (average), and ERPF (% predicted) (max) were excluded. The multivariate linear regression analysis showed a positive correlation with ERPF (%
predicted) (min) (_P_ = 0.033). MULTIVARIATE LOGISTIC REGRESSION ANALYSIS FOR PREDICTION OF THE PRESENCE OF HIGH DETRUSOR PRESSURE Table 5 shows the results of the multivariate logistic
regression. Univariate analysis was initially performed, and variables, including the time of renal scintigraphy from injury, ERPF (% predicted) (average), ERPF (% predicted) (max), and ERPF
(% predicted) (min), were found to be significant (all _P_ < 0.05). Owing to multicollinearity, the time of renal scintigraphy from injury and ERPF (% predicted) (min) were included in
the multivariate analysis; ERPF (% predicted) (average) and ERPF (% predicted) (max) were excluded. The multivariate logistic regression analysis showed that the risk of high detrusor
pressure was significantly correlated with ERPF (% predicted) (min) (odds ratio, 0.941; 95% CI, 0.887 to 0.985; _P_ = 0.021). The AUC obtained from the ROC curve (Fig. 2) for ERPF (%
predicted) (min) was 0.725 (95% CI, 0.556 to 0.884) for predicting the presence of high detrusor pressure. The optimal value was determined to be 64.05%, with a sensitivity of 1.000 and
specificity of 0.429. DISCUSSION Neurogenic lower urinary tract dysfunction is a common complication in individuals with SCI and requires careful management to prevent upper urinary tract
deterioration and maintain renal function [1,2,3,4]. The gold standard for assessing lower urinary tract function is UDS, but controversy exists over the timing of its use in the early
stages of SCI [6]. This study aimed to investigate the use of 99 mTc-MAG-3 renal scintigraphy to predict elevated detrusor pressure and determine whether it can be used as a guideline for
early UDS implementation. Elevated detrusor pressure is a well-known risk factor for upper urinary tract deterioration in individuals with SCI [20,21,22,23]. The intramural ureters traverse
between the bladder submucosa and the detrusor muscle before opening into the bladder. High pressure in the bladder leads to compression of these ureters, causing urinary stasis, which in
turn limits urine flow from the kidneys into the bladder [26]. If left untreated, this stasis can cause direct renal damage, first in the renal tubules, and then in the glomeruli [27].
Technetium-99m-mercaptoacetyltriglycine is a radioisotope that is largely eliminated by tubular secretion and allows direct measurement of renal plasma filtration in each kidney [28]. A
strong correlation was observed when renal ultrasonography and 99 mTc-MAG-3 renal scintigraphy were compared for upper urinary stasis [28]. In our study, we analyzed the associations between
Tmax, T1/2, ERPF, ERPF (% predicted), and UDS findings. There was a negative correlation between ERPF, ERPF (% predicted), and maximal detrusor pressure. In the multivariate linear
regression analysis, ERPF (% predicted) (min) showed a significant correlation, even after adjusting for other confounders. Additionally, in multivariate logistic regression analysis, ERPF
(% predicted) was a significant factor for the presence of abnormally elevated detrusor pressure, and the cut-off value was confirmed to be 64.05%. As a result, ERPF (% predicted) may
predict high detrusor pressure, and if the value is ≤64.05%, it may be considered that early UDS is helpful for early detection of elevated detrusor pressure. In this study, the specificity
of ERPF (% predicted) (min) was 0.429, indicating the difficulty in using this value alone to determine normal detrusor pressure. However, on the other hand, a sensitivity of 1.000 was
observed, indicating that all individuals displaying high detrusor pressure had ERPF (% predicted) (min) values below the cut-off. As the aim of utilizing 99 mTc-MAG-3 renal scintigraphy in
this study was to promptly identify individuals with high detrusor pressure, the high sensitivity suggests the potential utility of 99 mTc-MAG-3 renal scintigraphy. The EAU guidelines
suggest that early diagnosis of neuro-urological disorders is essential to prevent irreversible changes [29]. As shown by a previous study, even within 40 days of acute SCI, 63% exhibited
unfavorable UDS findings, including detrusor overactivity and low compliance, necessitating timely urodynamic investigation for patient-tailored therapy [30]. Although multiple literature
sources highlight the necessity of early urodynamic investigation, determining the timing of UDS involves considering various factors, such as clinical symptoms and signs and renal function.
In this study, we propose that utilizing 99mTc-MAG-3 renal scintigraphy could assist in determining the timing for UDS. This study has several limitations. First, it was designed as a
retrospective review of previous medical records with a small number of participants. Distinct features of the neurogenic lower urinary tract dysfunction in suprasacral, sacral, and
infrasacral lesions were not considered. Additionally, in the ROC analysis, the specificity of the ERPF cut-off value was insufficient. Furthermore, the analysis was limited to individuals
within 90 days of SCI. Therefore, we are planning to conduct a prospective study to further analyze with various onset durations and to examine how the relationship between the parameters of
99mTc-MAG-3 renal scintigraphy and UDS changes over time. In conclusion, neurogenic lower urinary tract dysfunction is a common complication in individuals with SCI that requires careful
consideration to prevent upper urinary tract deterioration and maintain renal function. The gold standard for assessing neurogenic lower urinary tract dysfunction is UDS; however,
controversy exists over the timing of its use in the early stages of SCI. This study aimed to investigate whether 99mTc-MAG-3 renal scintigraphy could be used to predict elevated detrusor
pressure and establish new guidelines for early UDS implementation. These results suggest that 99mTc-MAG-3 renal scintigraphy could potentially be used to predict detrusor pressure and guide
the timing of UDS in individuals with SCI within 90 days of SCI. Further studies are needed to confirm these findings and to develop a standardized protocol for utilizing 99mTc-MG-3 renal
scintigraphy in clinical practice. DATA AVAILABILITY The authors will freely share the unfiltered raw data underlying the results of this study. REFERENCES * Manack A, Motsko SP,
Haag-Molkenteller C, Dmochowski RR, Goehring EL Jr, Nguyen-Khoa BA, et al. Epidemiology and healthcare utilization of neurogenic bladder patients in a US claims database. Neurourol Urodyn.
2011;30:395–401. Article PubMed Google Scholar * Anderson CE, Birkhäuser V, Jordan X, Liechti MD, Luca E, Möhr S, et al. Urological management at discharge from acute spinal cord injury
rehabilitation: a descriptive analysis from a population-based prospective cohort. Eur Urol Open Sci. 2022;38:1–9. Article PubMed PubMed Central Google Scholar * Ginsberg DA, Boone TB,
Cameron AP, Gousse A, Kaufman MR, Keays E, et al. The AUA/SUFU guideline on adult neurogenic lower urinary tract dysfunction: treatment and follow-up. J Urol. 2021;206:1106–13. Article
PubMed Google Scholar * Lawrenson R, Wyndaele JJ, Vlachonikolis I, Farmer C, Glickman S. Renal failure in patients with neurogenic lower urinary tract dysfunction. Neuroepidemiology.
2001;20:138–43. Article CAS PubMed Google Scholar * Danforth TL, Ginsberg DA. Neurogenic lower urinary tract dysfunction: how, when, and with which patients do we use urodynamics? Urol
Clin North Am. 2014;41:445–52. Article PubMed Google Scholar * Watanabe T, Rivas DA, Chancellor MB. Urodynamics of spinal cord injury. Urol Clin North Am. 1996;23:459–73. Article CAS
PubMed Google Scholar * Nosseir M, Hinkel A, Pannek J. Clinical usefulness of urodynamic assessment for maintenance of bladder function in patients with spinal cord injury. Neurourol
Urodyn. 2007;26:228–33. Article PubMed Google Scholar * Harris CJ, Lemack GE. Neurourologic dysfunction: evaluation, surveillance and therapy. Curr Opin Urol. 2016;26:290–4. Article
PubMed Google Scholar * Kavanagh A, Baverstock R, Campeau L, Carlson K, Cox A, Hickling D, et al. Canadian Urological Association guideline: Diagnosis, management, and surveillance of
neurogenic lower urinary tract dysfunction - Full text. Can Urol Assoc J. 2019;13:E157–E76. PubMed PubMed Central Google Scholar * Welk B, Schneider MP, Thavaseelan J, Traini LR, Curt A,
Kessler TM. Early urological care of patients with spinal cord injury. World J Urol. 2018;36:1537–44. Article PubMed Google Scholar * Alsulihem A, Corcos J. Evaluation, treatment, and
surveillance of neurogenic detrusor overactivity in spinal cord injury patients. Neurosciences. 2019;6:13. CAS Google Scholar * Bywater M, Tornic J, Mehnert U, Kessler TM. Detrusor
acontractility after acute spinal cord injury-myth or reality? J Urol. 2018;199:1565–70. Article PubMed Google Scholar * Kozomara M, Birkhäuser V, Anderson CE, Bywater M, Gross O, Kiss S,
et al. Neurogenic lower urinary tract dysfunction in the first year after spinal cord injury: A descriptive study of urodynamic findings. J Urol. 2023;209:225–32. Article PubMed Google
Scholar * Tuna H, Cermik TF, Tuna F. Monitoring of renal function using 99mTc-DMSA and 99mTc-DTPA scintigraphy in patients with spinal cord injury. Rev Esp Med Nucl Imagen Mol.
2012;31:322–7. CAS PubMed Google Scholar * Klingensmith WC 3rd, Lammertse DP, Briggs DE, Smith WI, Roberts JF, Froelich JW, et al. Technetium-99m-MAG3 renal studies in spinal cord injury
patients: normal range, reproducibility, and change as a function of duration and level of injury. Spinal Cord. 1996;34:338–45. Article PubMed Google Scholar * Phillips JR, Jadvar H,
Sullivan G, Lin VW, Segall GM. Effect of radionuclide renograms on treatment of patients with spinal cord injuries. AJR Am J Roentgenol. 1997;169:1045–7. Article CAS PubMed Google Scholar
* Eshima D, Taylor A Jr. Technetium-99m (99mTc) mercaptoacetyltriglycine: update on the new 99mTc renal tubular function agent. Semin Nucl Med. 1992;22:61–73. Article CAS PubMed Google
Scholar * Bih LI, Changlai SP, Ho CC, Lee SP. Application of radioisotope renography with technetium-99m mercaptoacetyltriglycine on patients with spinal cord injuries. Arch Phys Med
Rehabil. 1994;75:982–6. Article CAS PubMed Google Scholar * Linsenmeyer TA. Update on bladder evaluation recommendations and bladder management guideline in patients with spinal cord
injury. Current Bladder Dysfunction Reports. 2007;2:134–40. Article Google Scholar * Çetinel B, Önal B, Can G, Talat Z, Erhan B, Gündüz B. Risk factors predicting upper urinary tract
deterioration in patients with spinal cord injury: A retrospective study. Neurourol Urodyn. 2017;36:653–8. Article PubMed Google Scholar * Ozkan B, Demirkesen O, Durak H, Uygun N,
Ismailoglu V, Çetinel B. Which factors predict upper urinary tract deterioration in overactive neurogenic bladder dysfunction? Urology. 2005;66:99–104. Article PubMed Google Scholar *
Önal B, Kırlı EA, Selçuk B, Buğdaycı D, Can G, Çetinel B. Risk factors predicting upper urinary tract deterioration in children with spinal cord injury. Neurourol Urodyn. 2021;40:435–42.
Article PubMed Google Scholar * Swatesutipun V, Tangpaitoon T. The safety cutoff storage pressure for preventing upper urinary tract damage in neurogenic bladder from spinal cord
pathology and risk factor analysis. Neurourol Urodyn. 2022;41:991–1001. Article PubMed Google Scholar * Caramel R, Corcos J Normal urodynamic parameters in adults. In: Corcos J, Ginsberg
D, Karsenty G Textbook of the Neurogenic Bladder. 3rd ed. CRC Press, New York, 2016. pp 411-23. * Linsenmeyer TA Urologic management and renal disease in spinal cord injury. In: Kirshblum S,
Lin VW Spinal Cord Medicine. 3rd ed. Demos Medical Publishing, New York, 2019. 332-86. * Staskin DR. Hydroureteronephrosis after spinal cord injury. Effects of lower urinary tract
dysfunction on upper tract anatomy. Urol Clin North Am. 1991;18:309–16. Article CAS PubMed Google Scholar * Kao CH, Changlai SP. 99Tcm-MAG3 renal studies: effective renal plasma flow in
patients with spinal cord injuries. Nucl Med Commun. 1996;17:1068–71. Article CAS PubMed Google Scholar * Solinsky R, Garstang SV, Linsenmeyer TA. Comparing the role of renal ultrasound
vs MAG3 renal scans for evaluation of neurogenic bladder after spinal cord injury. J Spinal Cord Med. https://doi.org/10.1080/10790268.2022.2088504:1-5 2022. * Block B, Castro-Díaz DM, Del
Popolo G, Groen J, Hamid R, Karsenty G et al. EAU guidelines on neuro-urology. European Association of Urology. 2023. https://uroweb.org/guidelines/neuro-urology/. * Bywater M, Tornic J,
Mehnert U, Kessler TM. Detrusor acontractility after acute spinal cord injury: Myth or reality? J Urol. 2018;199:1565–70. Article PubMed Google Scholar Download references FUNDING This
study is supported by a research grant of Research Institute of Rehabilitation Medicine, Yonsei University College of Medicine. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department and
Research Institute of Rehabilitation Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea Su Ji Lee & Ji Cheol Shin Authors * Su Ji Lee View
author publications You can also search for this author inPubMed Google Scholar * Ji Cheol Shin View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS This paper was co-authored by SJL and JCS. JCS contributed as a corresponding author. SJL and JCS contributed equally to data analysis and interpretation. All authors
participated in the writing and editing of the manuscript. CORRESPONDING AUTHOR Correspondence to Ji Cheol Shin. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. ETHICAL APPROVAL The study was approved by the institutional review board of Severance Hospital, Yonsei University Health System, Seoul, Korea (4-2022-1490). PATIENT CONSENT Due
to the retrospective nature of the study, the review board waived the need for informed consent. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION REPRODUCIBILITY CHECKLIST RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a
society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript
version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lee, S.J., Shin, J.C.
Maximal detrusor pressure can be predicted using technetium-99m-mertcaptoacetyltriglycine renal scintigraphy in the early stages of spinal cord injury. _Spinal Cord_ 62, 207–213 (2024).
https://doi.org/10.1038/s41393-024-00967-w Download citation * Received: 02 May 2023 * Revised: 07 February 2024 * Accepted: 14 February 2024 * Published: 07 March 2024 * Issue Date: May
2024 * DOI: https://doi.org/10.1038/s41393-024-00967-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative