Linking il-10 signaling with lipid metabolic programs in macrophages: dysregulated ceramide homeostasis drives colitis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
In a recent study published in _Nature_, York and coworkers identified the immunometabolic regulation of mono-unsaturated fatty acid (MUFA) pools and the subsequent increase in sphingolipid
biosynthesis in TLR2-activated macrophages as an important link between impaired IL-10 signaling and the development of intestinal inflammation.1 Thus, targeted interference with the newly
identified IL-10/MUFA/sphingolipid axis appears as a promising strategy to restore immunometabolic homeostasis in the intestine of patients with inflammatory bowel diseases (IBD). The
critical dependence of gut health on the anti-inflammatory cytokine IL-10 is well established.2 To mention some key evidence, knockout (KO) mice lacking IL-10 or one of the IL-10 receptor
subunits develop spontaneous colitis, while mutations within the coding region of the human IL-10 or IL-10 receptor genes account for a relevant percentage of patients diagnosed with very
early-onset IBD. Although IL-10 can be sensed by various immune cells, a macrophage-restricted loss of the IL-10RA receptor subunit was sufficient to induce spontaneous colitis, implicating
mucosal macrophages as central mediators of anti-inflammatory IL-10 signaling.2 Despite the well-described colitis-promoting consequence of in vivo IL-10 blockade/neutralization, the
intracellular cascade linking IL-10 receptor ligation to the regulation of inflammatory macrophage function remains incompletely understood. Interestingly, a previous study suggested that
the anti-inflammatory function of IL-10 in macrophages involves its ability to block activation-induced metabolic reprogramming, thus describing its cellular effects as an interplay between
immunological signaling and metabolic regulation.3 From a translational perspective, there is a growing awareness of the relevance of metabolic processes for immune cell function in the
context of IBD. This awareness arises in particular from the clinical need to offer optimized treatment strategies to more than 30% of IBD patients who do not show a satisfying response to
the currently available therapeutic regimens. New targets that allow modulation of the metabolic response of immune cells to inflammatory triggers, rather than exclusively blocking classical
immune mediators upstream or downstream of cytokine receptors, may be particularly beneficial for those IBD patients who do not respond to biological agents such as anti-TNF therapy,
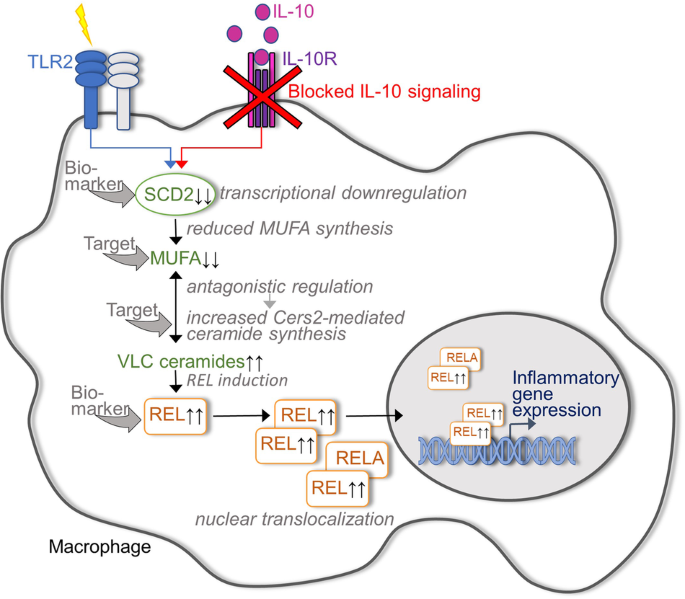
vedolizumab, p19/p40 blockers and JAK inhibitors. Since the lipid pool in macrophages is known to be altered during TLR-mediated activation, York et al.1 first focused on whether this
reprogramming of lipid metabolism depends on IL-10, whose secretion by macrophages is also induced by TLR ligation. Indeed, the lipid metabolomic data indicated that TLR2-induced IL-10
signaling significantly affects sphingolipid metabolism of macrophages. Based on direct infusion mass spectrometry, TLR2-activated IL-10-deficient macrophages could be characterized by
increased levels of ceramides and hexosyl ceramides compared to IL-10-positive control macrophages, whereas all unsaturated and some saturated sphingomyelins were decreased in the absence of
IL-10. The accumulation of ceramides in TLR2-activated IL-10 KO macrophages appeared to be driven by increased de novo ceramide synthesis. Accordingly, genetic deletion of ceramide synthase
Cers2, an enzyme that controls very long chain (VLC) ceramide synthesis, prevented the induction of inflammatory genes in in vitro activated macrophages by IL-10R blockade and, under in
vivo conditions, IL-10 receptor/Cers2 double knockout bone marrow chimeric mice showed milder signs of intestinal inflammation than cohoused IL-10 receptor knockout chimeric mice. The next
step was to elucidate the cascade underlying the regulation of ceramide biosynthesis and the accumulation of VLC ceramides in IL-10 KO macrophages. Somewhat unexpectedly, the expression
levels of all genes directly involved in sphingolipid metabolism turned out to be comparable between IL-10 KO and control macrophages. However, IL-10 deficiency in macrophages caused a
significant downregulation of the enzyme stearoyl-CoA desaturase 2 (SCD2), which catalyzes the formation of MUFAs. These findings led to the hypothesis that MUFA synthesis links inflammation
and VLC ceramide synthesis in IL-10 KO macrophages. Indeed, stable-isotope tracer analysis indicated that the reduced SCD2 expression in TLR2-stimulated IL-10 KO macrophages was associated
with reduced MUFA synthesis, whereas the inflammatory gene expression profile of IL-10 KO macrophages and their increased VLC ceramide biosynthesis could be attenuated by exogenous MUFA
supplementation. Furthermore, SCD2-deficient macrophages phenocopied IL-10 KO macrophages in terms of VLC ceramide accumulation and increased inflammatory gene expression. Taken together,
York et al. were able to identify the insufficient capacity of IL-10-deficient macrophages to upregulate MUFA synthesis as the underlying cause of their pathologically enhanced inflammatory
activity (Fig. 1). But how does the IL-10-controlled availability of VLC ceramides affect the regulation of inflammatory genes? York et al.1 interestingly demonstrated that the NF-kappaB
family transcription factor REL is required for the ability of VLC ceramides to induce inflammatory gene expression in TLR2-activated macrophages and, accordingly, for the full development
of intestinal inflammation in IL-10-deficient mice. Interestingly, in vitro analyses of activated IL-10/c-Rel double knockout macrophages implicated that the ceramide-induced activation of
REL predominantly supports the prolonged maintenance of inflammatory gene expression rather than enhancing its early initiation. Regarding the therapeutic targetability of IL-10 signaling,
previous data from experimental mouse models suggested that direct administration of recombinant IL-10 may ameliorate colitis,2 but clinical IBD trials have been disappointing overall. The
new insights into the immunometabolic cascade that is initiated when macrophages are activated in the absence of IL-10 signaling may pave the way for more clinically successful strategies to
elicit IL-10-triggered anti-inflammatory effects, which may even be beneficial for IBD patients with IL-10 receptor dysfunction. Nutritional MUFA supplementation or targeted blockade of the
VLC ceramide synthesis via inhibition of Cers2 appear, at least on the first view, as two attractive strategies in this context (Fig. 1). However, assuming that both these approaches cannot
be implemented in a macrophage-restricted way, their expected impact on other cells involved in the maintenance of intestinal homeostasis must be considered. One potential limitation of the
study is the lack of validation in other IL-10 receptor-expressing intestinal immune cells, including those of humans. Cers2 represents a tumor metastasis suppressor gene,4 and defined
MUFAs have been associated with enhanced T effector cell function5 underlining potential risk factors when targeting this pathway. Thus, the validation of the findings on the IL-10/MUFA/VLC
ceramide/REL axis in other IL-10 receptor-expressing intestinal immune cells, in addition to macrophages, is essential. Moreover, the validation of this axis in human immune cells in the
inflamed intestine of IBD patients is of paramount importance. The more complete the molecular consequences of insufficient IL-10 signaling in innate and adaptive immune cells and in the
intestinal epithelium can be deciphered, the better the chances are to fully exploit the high translational potential of this study. In terms of personalized medicine, rationally designed
biomarker strategies based on the intestinal expression profile of genes/proteins that regulate MUFA synthesis and/or REL activation could reliably identify individual IBD patients with
relevant impaired IL-10 signaling who could best benefit from targeted local corrections of MUFA/VLC ceramide homeostasis. REFERENCES * York, A. G. et al. IL-10 constrains sphingolipid
metabolism to limit inflammation. _Nature_ 627, 628–635 (2024). Article CAS PubMed PubMed Central Google Scholar * Zigmond, E. et al. Macrophage-restricted interleukin-10 receptor
deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. _Immunity_ 40, 720–733 (2014). Article CAS PubMed Google Scholar * Ip, W. K. E. et al. Anti-inflammatory effect
of IL-10 mediated by metabolic reprogramming of macrophages. _Science_ 356, 513–519 (2017). Article CAS PubMed PubMed Central Google Scholar * Zhang, Q. et al. Clinical and pathological
significance of Homo sapiens ceramide synthase 2 (CerS-2) in diverse human cancers. _Biosci. Rep._ 39, BSR20181743 (2019). Article CAS PubMed PubMed Central Google Scholar * Fan, H. et
al. Trans-vaccenic acid reprograms CD8(+) T cells and anti-tumour immunity. _Nature_ 623, 1034–1043 (2023). Article CAS PubMed PubMed Central Google Scholar Download references
ACKNOWLEDGEMENTS The work was supported by the following grants from the DFG; German Research Foundation: TRR241 (project number 375876048) and AT 122/2-1 (project number 511510453). In
addition, this work was supported by the Interdisciplinary Center for Clinical Research Erlangen: IZKF D37. FUNDING Open Access funding enabled and organized by Projekt DEAL. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Medicine 1, University Hospital of Erlangen, Friedrich-Alexander University Erlangen-Nürnberg, Erlangen, Germany Imke Atreya & Markus
F. Neurath * Deutsches Zentrum Immuntherapie (DZI), Erlangen, Germany Imke Atreya & Markus F. Neurath Authors * Imke Atreya View author publications You can also search for this author
inPubMed Google Scholar * Markus F. Neurath View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.F.N. and I.A. drafted the manuscript and the
figure. All authors critically revised the manuscript for important intellectual content. All authors have read and approved the article. CORRESPONDING AUTHOR Correspondence to Markus F.
Neurath. ETHICS DECLARATIONS COMPETING INTERESTS M.F.N. has served as an advisor for Pentax, Giuliani, PPM, MSD, Abbvie, Janssen, Takeda and Boehringer. IA discloses no conflicts. RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Atreya, I.,
Neurath, M.F. Linking IL-10 signaling with lipid metabolic programs in macrophages: dysregulated ceramide homeostasis drives colitis. _Sig Transduct Target Ther_ 9, 156 (2024).
https://doi.org/10.1038/s41392-024-01864-7 Download citation * Received: 15 March 2024 * Revised: 26 April 2024 * Accepted: 06 May 2024 * Published: 08 June 2024 * DOI:
https://doi.org/10.1038/s41392-024-01864-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative