Dendritic cell-derived IL-27 p28 regulates T cell program in pathogenicity and alleviates acute graft-versus-host disease
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Interleukin 27 (IL-27), a heterodimeric cytokine composed of Epstein-Barr virus-induced 3 and p28, is a pleiotropic cytokine with both pro-and anti-inflammatory properties. However, the
precise role of IL-27 in acute graft-versus-host disease is not yet fully understood. In this study, utilizing mice with IL-27 p28 deficiency in dendritic cells (DCs), we demonstrated that
IL-27 p28 deficiency resulted in impaired Treg cell function and enhanced effector T cell responses, corresponding to aggravated aGVHD in mice. In addition, using single-cell RNA sequencing,
we found that loss of IL-27 p28 impaired Treg cell generation and promoted IL-1R2+TIGIT+ pathogenic CD4+ T cells in the thymus at a steady state. Mechanistically, IL-27 p28 deficiency
promoted STAT1 phosphorylation and Th1 cell responses, leading to the inhibition of Treg cell differentiation and function. Finally, patients with high levels of IL-27 p28 in serum showed a
substantially decreased occurrence of grade II-IV aGVHD and more favorable overall survival than those with low levels of IL-27 p28. Thus, our results suggest a protective role of DC-derived
IL-27 p28 in the pathogenesis of aGVHD through modulation of the Treg/Teff cell balance during thymic development. IL-27 p28 may be a valuable marker for predicting aGVHD development after
transplantation in humans.
Acute graft-versus-host disease (aGVHD), one of the major complications early after allogeneic hematopoietic stem cell transplantation (allo-HSCT), is characterized by host tissue injury
mediated mainly by donor T cells following interaction with either donor- or host-derived antigen-presenting cells (APCs).1,2 Preconditioning induces tissue damage and leads to the secretion
of massive pro-inflammatory cytokines by the donor or host APCs, including IL-1β, IL-6, IL-12, and TNF-α, which then trigger the activation and proliferation of alloreactive T cells and
subsequently promote donor T cell polarization toward Th1, Th2, or Th17 phenotypes.3 These cells further contribute to the pathogenesis of aGVHD by their effector cytokines, such as IFN-γ,
IL-4, or IL-17A.4 Of note, several therapeutic approaches targeting cytokines have already shown promising outcomes for aGVHD prevention.5,6 Therefore, a better understanding of the
pathophysiology of aGVHD could help to develop novel strategies for the prevention and treatment of aGVHD.
IL-27, a heterodimeric cytokine composed of EBI3 and p28, signals through binding with IL-27 receptor formed by IL-27Rα (also named as WSX1) and gp130.7 It has been reported that p28 subunit
could be secreted independently of EBI3 and conversely antagonize the IL-27 signaling.8 IL-27 is predominantly produced by activated APCs, including DCs, monocytes, and macrophages.7
IL-27Rα is constitutively expressed among a range of cell types including T cells, B cells, intestinal epithelial cells as well as hematopoietic stem cells.9,10 Engagement of IL-27 and IL-27
receptor transduces cellular signals via JAK1/2 and STAT1/3 pathways.11 IL-27 was recognized as an inflammatory cytokine with potent immune regulatory properties. On the one hand, IL-27 was
early characterized by the promotion of IFN-γ production by NK cells and naive CD4+ T cells.12 IL-27 also induced T-bet expression to facilitate Th1 cell function.13 Likewise, IL-27Rα was
essential for Th1 cell differentiation in infectious diseases.14 Endogenous IL-27 p28 produced by DCs and monocytes increased antigen-specific CD8+ T cells expansion and their effector
function in vaccine-elicited cellular immunity.15 On the other hand, IL-27 exhibited immune inhibitory roles by promoting IL-10 release in Treg, T regulatory type 1 (Tr1), IFN-γ+T-bet+Foxp3−
Th1, Th2, and Th17 cells.8,16,17,18 T cells deficient in IL-27Rα failed to produce IL-10 by TCR stimulation.19 Mice with IL-27Rα ablation had a high susceptibility to experimental
autoimmune encephalomyelitis (EAE), which was associated with enhanced IL-17A production.20 Concordantly, Treg cell-specific depletion of IL-27Rα argumented EAE development by impairing Treg
cell stability.21 Moreover, IL-27 upregulated the expression of a diversity of inhibitory molecules on T cells, including programmed death ligand 1 (PD-L1), lymphocyte activation gene-3
(Lag3), T cell immunoglobulin domain and mucin domain 3 (Tim3) and stem cell antigen-1 (Sca-1).8 IL-27 produced by innate-like natural regulatory B1-a cells (i27-Breg) prevents
neuroinflammation through upregulating PD-1 and Lag3 and thereby suppressing Th1/17 responses.22 A subset of antigen-specific Foxp3−CD4+T cells could also produce IL-27 upon malaria parasite
infections.23 A very recent study showed that CX3CR1+ cells produced IL-27 and restrained obesity in mice.24 However, the exact cellular source of IL-27 during the process of aGVHD
development has not been fully addressed. Recent studies indicated that IL-27 p28 deficiency aggravated, whereas blockage of IL-27 p28 signaling alleviated aGVHD in murine models.25,26
However, a lack of IL-27Rα expression on donor T cells reduced Th1 responses and mitigated aGVHD.25,27 Therefore, the role of IL-27 signaling in aGVHD remains controversial. Moreover, the
expression pattern and the clinical significance of IL-27 in aGVHD patients remain unknown.
Treg cells are central regulators of immune response and tolerance, which potently suppress both acute and chronic GVHD.28,29 It has been well accepted that IL-27 regulates Treg cell
differentiation and functions in malignancy, allergic pathologies and autoimmune disorders. IL-27 gene therapy-induced melanoma rejection by inhibiting Treg cell function in mice .30
Accordingly, IL-27 restrained Treg cell generation and promoted colitis induced by CD4+CD45Rbhi T cells.31 However, IL-27 promoted Lag3 expression on Tregs and attenuated antigen-induced
allergic inflammatory response.32 IL-27 also drived T-bet+CXCR3+ Treg cell expansion and limited Th1 cell type infections.33 IL-27 or IL-27Rα deficient mice have no changes in Treg
development, suggesting IL-27 does not affect Treg homeostasis at a steady state.20 As in the context of aGVHD, IL-27 pre-stimulation enforced iTreg function, while IL-27Rα expression
inhibited Treg development after allo-BMT.27,34 Thus, the complicated role of IL-27 signal in Treg regulation upon aGVHD remains to be investigated in detail.
In this study, we found that IL-27 p28 was predominantly produced by DCs in aGVHD and DC-specific deficiency of IL-27 p28 mitigated aGVHD by regulating T cell program in pathogenicity in a
cell-intrinsic manner. IL-27 p28 deficiency inhibited Th1 responses and promoted the differentiation and inhibitory ability of Treg cells via the IFN-γ/STAT1 signaling pathway. IL-27 p28
deficiency impaired Treg generation and promoted IL-1R2+TIGIT+ pathogenic CD4+ T cells in the thymus at a steady state. We also observed that high serum levels of IL-27 p28 were associated
with a lower incidence of severe aGVHD in patients who underwent allo-HSCT, suggesting that DC-derived IL-27 p28 protects against aGVHD development and might be a potential prognostic marker
for severe aGVHD after allo-HSCT.
To address the physiological role of IL-27 p28 in aGVHD pathogenesis, we first assessed the cellular source of IL-27 p28 during aGVHD. Results showed that DCs, rather than T cells,
neutrophils, or monocytes, were the predominant source of IL-27 p28 in both murine aGVHD models and patients at the onset of aGVHD (Supplementary Fig. 1a, b). We therefore generated IL-27
p28 conditional knockout (p28 cKO) mice in DCs by crossing p28f/f mice with CD11c Cre mice.35 IL-27 p28 expression in the serum was markedly reduced in CD11c-p28f/f mice compared with WT
mice at a steady-state or on day 7 after allogenic-bone marrow transplantation (BMT) (Supplementary Fig. 1c, d). Moreover, the levels of IL-27 heterodimer were also reduced in CD11c-p28f/f
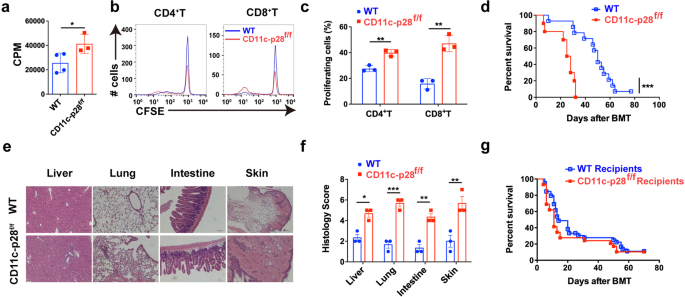
mice compared with WT mice (Supplementary Fig. 1e, f). In vitro mixed lymphocyte reaction (MLR) revealed that T cells from p28 cKO mice displayed aggravated alloreactivity than those from WT
mice (Fig. 1a), as evidenced by enhanced cell proliferation (Fig. 1b, c). We then established an MHC-mismatched murine aGVHD model using CD11c-p28f/f or WT mice as donors. Compared with WT
recipients, mice that received CD11c-p28f/f splenocytes showed accelerated aGVHD mortality (Fig. 1d). Histological analysis also revealed that recipients of CD11c-p28f/f splenocytes showed
much more severe tissue damage (Fig. 1e, f). However, the mortality showed no significant difference when CD11c-p28f/f mice were used as recipients (Fig. 1g). Collectively, these results
demonstrate that donor-derived IL-27 p28 protects from aGVHD development in mice.
IL-27 p28 deficiency aggravates aGVHD in mice. a–c BALB/c DCs were cocultured with CFSE-labeled T cells (ratio 1:10) from CD11c-p28f/f mice or control littermates, respectively.
Proliferations were assessed by a 3H-TdR or b, c flow cytometry 5 days post coculture. d–f BALB/c recipients were transplanted with 1 × 107 WT BMs and 5 × 106 splenocytes from either WT or
CD11c-p28f/f mice (n = 10–14 per group). Overall survival curve is depicted (d). Representative H&E stained sections and histological scores of aGVHD tissues from recipients 14 days
post-transplantation are shown (e, f). g C57BL/6 or CD11c-p28f/f recipients were lethal irradiation and received 1 × 107 BMs and 7.5 × 107 splenocytes from BALB/c mice (n = 15 per group).
The overall survival curve is depicted. Data are representative of three independent experiments and presented as mean ± SD. *P