Novel approaches to capturing and using continuous cardiorespiratory physiological data in hospitalized children
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Continuous cardiorespiratory physiological monitoring is a cornerstone of care in hospitalized children. The data generated by monitoring devices coupled with machine learning could
transform the way we provide care. This scoping review summarizes existing evidence on novel approaches to continuous cardiorespiratory monitoring in hospitalized children. We aimed to
identify opportunities for the development of monitoring technology and the use of machine learning to analyze continuous physiological data to improve the outcomes of hospitalized children.
We included original research articles published on or after January 1, 2001, involving novel approaches to collect and use continuous cardiorespiratory physiological data in hospitalized
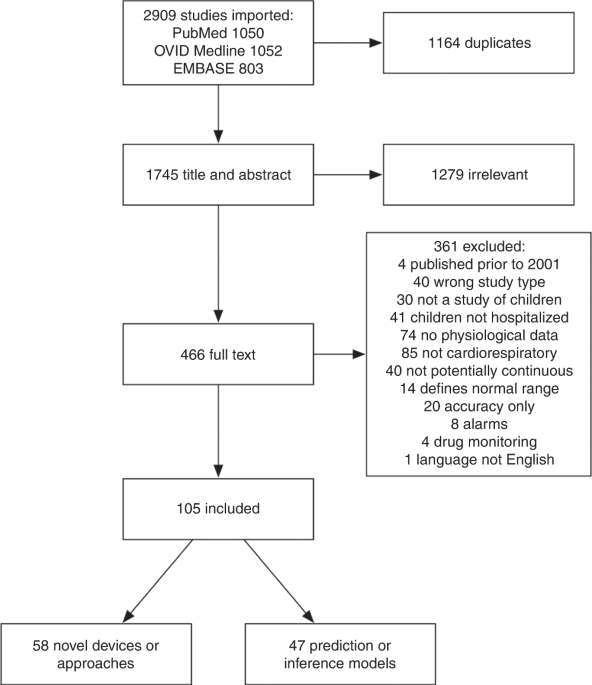
children. OVID Medline, PubMed, and Embase databases were searched. We screened 2909 articles and performed full-text extraction of 105 articles. We identified 58 articles describing novel
devices or approaches, which were generally small and single-center. In addition, we identified 47 articles that described the use of continuous physiological data in prediction models, but
only 7 integrated multidimensional data (e.g., demographics, laboratory results). We identified three areas for development: (1) further validation of promising novel devices; (2) more
studies of models integrating multidimensional data with continuous cardiorespiratory data; and (3) further dissemination, implementation, and validation of prediction models using
continuous cardiorespiratory data. IMPACT * We performed a comprehensive scoping review of novel approaches to capture and use continuous cardiorespiratory physiological data for monitoring,
diagnosis, providing care, and predicting events in hospitalized infants and children, from novel devices to machine learning-based prediction models. * We identified three key areas for
future development: (1) further validation of promising novel devices; (2) more studies of models integrating multidimensional data with continuous cardiorespiratory data; and (3) further
dissemination, implementation, and validation of prediction models using cardiorespiratory data. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING
VIEWED BY OTHERS THE PRINCIPLES OF WHOLE-HOSPITAL PREDICTIVE ANALYTICS MONITORING FOR CLINICAL MEDICINE ORIGINATED IN THE NEONATAL ICU Article Open access 31 March 2022 CARDIORESPIRATORY
SIGNATURE OF NEONATAL SEPSIS: DEVELOPMENT AND VALIDATION OF PREDICTION MODELS IN 3 NICUS Article 02 January 2023 ARTIFICIAL INTELLIGENCE-DRIVEN WEARABLE TECHNOLOGIES FOR NEONATAL
CARDIORESPIRATORY MONITORING: PART 1 WEARABLE TECHNOLOGY Article 02 January 2023 INTRODUCTION Continuous cardiorespiratory monitoring using bedside devices and sensor-based technology is
pervasive in modern healthcare and a cornerstone of care in hospitalized children. Though in widespread use, the current state-of-the-art bedside monitoring would benefit from novel
wireless, noninvasive, and non-contact sensing methods that could protect fragile skin, reduce interference in care, and promote caregiver-child interactions.1,2 In addition, the wealth of
data generated by these devices needs to be saved and utilized in prediction models or clinical decision support tools, rather than automatically deleted from vendor servers soon after
patient discharge. Preliminary data indicate that these continuous physiological cardiorespiratory data coupled with machine learning techniques could fundamentally transform the way we
provide care.3 Machine learning models using continuous physiological data in children already show promise in early identification of sepsis,4,5,6,7,8,9,10 cardiac arrest,11,12 and risk of
readmission.13 As we look to the future of continuous cardiorespiratory monitoring, we must improve monitoring technology, identify those patients who would benefit from beat-to-beat and
breath-to-breath bedside monitoring data, and find new ways to use these data to improve the care and outcomes of hospitalized children. In this scoping review, we summarize existing
evidence on (1) the use of novel approaches to capture continuous cardiorespiratory physiological data in hospitalized children in order to identify key opportunities for the development of
continuous cardiorespiratory monitoring technology; and (2) the use of machine learning to analyze continuous cardiorespiratory physiological data to improve the care of hospitalized
children. METHODS We conducted a scoping review of original research articles published on or after January 1, 2001, involving novel approaches to the capture and use of continuous
cardiovascular and/or respiratory data in hospitalized neonates, children, and adolescents. “Novel” devices were defined as those not currently part of the standard of care. “Continuous”
devices were those which acquired data in an automated way at a frequency of greater than one sample per minute for a duration of at least 5 min. “Physiological” data were defined as an
observed variable measuring functioning of the human body. We excluded studies focused on: continuous biochemical information (e.g., glucose), intermittently collected imaging devices (e.g.,
ultrasound), alarm fatigue, drug monitoring, measurement accuracy in previously validated devices, and normal reference range definitions. OVID Medline, PubMed, and Embase databases were
searched. Abstract and full-text screening was completed in Covidence (Veritas Health Innovation Ltd, Melbourne, Australia) by six investigators (S.B.W., M.S.C., K.S.H., C.M.B., D.E.W.-M.,
L.N.S.-P.), with two reviewers required per article. Further details can be found in the Supplementary materials. RESULTS We screened 2909 articles and performed full-text extraction of 105:
58 describing novel continuous cardiorespiratory monitoring devices or measurement approaches and 47 describing prediction or inference models using continuous cardiorespiratory
physiological data (Fig. 1). Studies describing novel devices or measurement approaches were primarily small (<50 patients), single-center, observational trials or proof-of-concept
studies in an intensive care unit (ICU) setting (Table 1 and Supplementary Tables 1 and 2). Articles describing prediction or inference models using continuous cardiorespiratory
physiological data mostly involved a single physiological variable (31 of 47) and the majority of these studied heart rate variability (14 of 31). Sample size for these studies varied widely
(<10 to >7000 patients). All studies took place at least partially in the ICU and only three were randomized control trials (Table 2 and Supplementary Tables 3 and 4). NOVEL DEVICES
OR MEASUREMENT APPROACHES TO CARDIOVASCULAR MONITORING BLOOD PRESSURE (BP) Six articles described novel methods to measure BP in hospitalized children. These aimed to provide data that are
continuous, noninvasive, and usable to reconstruct features of arterial line waveforms (e.g., pulse pressure variation). All were prospective observational trials of 10–35 patients, all but
one in pediatric ICU (PICU) patients, and all were compared to gold standard invasive arterial line measurements (Table 1 and Supplementary Table 1). Three articles described different
proprietary noninvasive BP cuffs that are attached to fingers.14,15,16 Finger BP cuff use was feasible and well tolerated but noted erroneous values due to placement. None were specifically
tested in hemodynamically unstable children. One method of BP prediction based on heart sounds resulted in predictions with high standard deviation.17 The most recent article described a
noninvasive wireless skin interface sensor that had a good correlation with invasive arterial BP including after pharmacologic interventions known to cause hemodynamic change.18 Finally, a
study comparing pulse oximeter waveforms to invasive arterial line waveforms in mechanically ventilated patients found a good correlation with waveform features.19 HEART RATE (HR) Novel
methods to measure HR in hospitalized children are mostly neonatal ICU (NICU) based. These alternative methods were developed to: (1) avoid adhesive-based sensors to capture ECG data, which
can be obtrusive and potentially harmful to the skin of neonates1 and (2) reduce motion artifacts that can affect sensor accuracy.20 Most of the 14 articles identified are small
proof-of-concept or prospective observational cohorts of less than 80 patients (Table 1 and Supplementary Table 1). Five studies presented results of the use of cameras to detect HR, in some
cases using machine learning techniques to identify and suppress artifacts.21,22,23,24,25 The correlation with standard measurements was generally good, although problems with artifacts
were common, particularly during hands-on care. Two articles presented results of HR measurements based on the detection of chest movement, one using a radar signal26 and the other using
laser Doppler.26,27 Two studies presented results from sensors embedded in the blankets or mattress of neonates: a capacitive sensor1 and a piezoelectric sensor.28 Four articles presented
results of novel methods to reduce artifacts in HR captured in neonates, including forehead reflectance photoplethysmography,29 transesophageal measurement,30 transcutaneous diaphragm
electromyography sensors,31 and spectrogram analysis of ECG data, instead of RR detection.32 While these approaches were generally successful at reducing artifacts, few considered
implementation strategies that would make them feasible alternatives to traditional ECG. Finally, one study compared methods for forecasting neonatal HR and found that an autoregressive
moving average model could predict future HR values from historical values with reasonable accuracy.33 HEART RATE VARIABILITY (HRV) HRV is defined as the fluctuation in time between
consecutive heartbeats and may be altered when there is an imbalance in autonomic nervous system inputs to the heart.34 There are a variety of ways to look at these fluctuations, but most
require complex waveform analysis.35 Many measures have shown promise in clinical prediction or inference models as described later, but none have been compared to other measurements of
autonomic dysfunction (and thus are not included in Table 1). A simplified proxy of HRV using the standard deviation of HR, termed integer HRV (HRVi), has also shown promise in prediction
models, although it has not been directly compared to traditional methods for deriving HRV. One article, however, did show that HRVi estimated using the pulse rate from photoplethysmography
correlated well with HRVi calculated from ECG (Table 1 and Supplementary Table 1).36 CARDIAC OUTPUT (CO) We found ten articles describing the estimation of CO using five different strategies
(Table 1 and Supplementary Table 1). Four studies of <25 patients each studied the pulse index continuous cardiac output (PiCCO) device. PiCCO uses pulse contour analysis calibrated by
thermodilution in order to estimate CO as well as ten additional proprietary measurements.37 The first article published showed poor agreement between markers of clinical assessment and
measurements of hemodynamic status.38 However, it is unclear how much of this discrepancy results from variability in clinical assessment, as subsequent articles have shown estimates of
PiCCO to have good agreement with thermodilution39 and transthoracic ultrasound measurements of cardiac index,40 as well as global perfusion.41 Two additional articles explored uncalibrated
pulse contour analysis using the pressure recording analytical method (PRAM) with the Mostcare® device and compared it to transthoracic echocardiogram (TTE)42 or transpulmonary ultrasound
dilution using COstatus®.43 PRAM had an excellent agreement with TTE measurements but lower agreement with COstatus® measurements. Given COstatus® has not been validated in hospitalized
children, more studies are necessary to determine the usefulness of PRAM in this population. Three articles described the use of two forms of bioimpedance to estimate CO. One study described
the use of electrical cardiometry bioimpedance in two children with hypoplastic left heart syndrome, showing some correlation between estimated CO and clinical change.44 Two studies
described the use of electrical velocimetry bioimpedance in critically ill children (pediatric, cardiac, and neonatal) using the AESCULON®.45,46 Generally, the AESCULON® had a good
correlation with CO based on TTE but was associated with a large margin of error. Bioreactance, the most recently developed form of bioimpedance, has not been compared to a gold standard but
has been used in a prediction model as described later.47 Finally, one article studied CO estimations using partial rebreathing of carbon dioxide measured via the noninvasive cardiac output
(NICO) device (Novametrix Medical Systems Inc., Wallingford, CT) on hemodynamically stable, mechanically ventilated patients >15 kg and was found to have good agreement with TTE.48
SYSTEMIC VASCULAR RESISTANCE (SVR) No novel device or approach was specifically targeted toward measuring SVR alone; however, four of the five strategies used to measure CO also provide
measurements of SVR (NICO cannot; AESCULON® requires a central venous pressure estimate). Despite the ability to measure SVR, measurements of SVR were only reported in three articles, all
using thermodilution via the PiCCO device. These three articles found no association when comparing PiCCO-measured SVR to clinician assessment38 or markers of oxygen balance;41 but did
demonstrate significantly higher SVR between low and normal-to-high cardiac index states.49 No article compared these novel SVR continuous measurements to a gold standard. PERFUSION We found
five articles describing the use of near-infrared spectroscopy (NIRS), a common noninvasive continuous monitoring device that estimates regional tissue saturation. In critically ill
children, NIRS showed a good correlation with peripheral and central measurements of venous oxygenation saturation (SvO2),50 as well as weak but statistically significant correlations with
BP.51 Among post-operative cardiac ICU (CICU) patients, NIRS demonstrated not only significant correlations with central SvO2 but also highly correlated with adverse events after Norwood
procedure.52 NIRS has also been used as a proxy for intestinal perfusion in neonates at high risk for intestinal failure (Table 1 and Supplementary Table 1).53 Despite its promise in healthy
newborn54 and adult populations,55 we did not find any articles comparing perfusion index, a measurement of the ratio of pulsatile flow to non-pulsatile flow derived from pulse oximeters,
to a gold standard of perfusion. We did find one clinical prediction or inference model that will be described later. NOVEL DEVICES OR MEASUREMENT APPROACHES TO RESPIRATORY MONITORING
RESPIRATORY RATE (RR) Similar to novel methods for HR measurement previously discussed, the ten articles describing novel methods for RR detection come from the NICU and are focused on
reducing artifacts (Table 1 and Supplementary Table 2). Seven studies presented data of simultaneous HR and RR detection using: chest wall movement detected via a radar signal26 or laser
Doppler27 image analysis of video signal,21,23,25 piezoelectric sensor in the bedding of the crib,28 or leveraging transcutaneous diaphragm electromyography sensors.31 While the correlation
with RR via airway flow or impedance was good, motion artifacts and implementation barriers limited the feasibility of these devices. Two studies demonstrated the challenges of measuring RR
in neonates when compared to adults, one using a fiber-optical monitoring device integrated into a nasal cannula56 and another using a video-based method.57 In both cases, the performance of
the devices was suboptimal compared to adults. Finally, a small proof-of-concept study in neonates studied the use of infrared thermography of the nares to detect changes in temperature
related to breathing.58 While the results are promising, implementation would have to be validated in children with various respiratory devices and nasal pathology. OXYGENATION Three
articles described noninvasive tools to measure oxygenation. A large PICU study used machine learning approaches to demonstrate the potential use of noninvasive pulse oximetry and
ECG-derived HR measurements to reliably estimate PaO2 and the oxygenation index.59 A NICU study examined continuous positive airway pressure for respiratory distress and demonstrated a
correlation between a novel index, the saturation oxygen pressure index, and alveolar-arteriolar oxygen difference.60 Finally, a neonatal conjunctival pulse oximetry monitor was found to be
effective, although it caused conjunctival edema in one neonate.61 Both oxygen saturation index and ratio of SpO2 to FiO2 are well studied, noninvasive ways to assess hypoxemia in children
with acute lung injury.62 Although not considered novel for the purposes of this review, these methods could be transformed into continuous, noninvasive measures. CARBON DIOXIDE (CO2) Twelve
articles described technology to estimate CO2 measures, primarily in observational trials of <75 PICU patients. Several studies correlated PaCO2 to end-tidal CO2 (EtCO2), transcutaneous
CO2, or novel microstream capnometer devices.63,64,65,66,67 Three articles focused on the use of continuous measures of CO2 during mechanical ventilation. One study used bedside monitor data
and ventilator measurements to identify deviations from pre-defined “trend templates” and made suggestions for the management of ventilator settings.68 Another study demonstrated strong
correlations between EtCO2 and PaCO2 in a range of dead space to tidal volume ratios with the EtCO2-PaCO2 difference increasing predictably with increasing dead space to tidal volume
ratio.69 Two studies used capnography to measure dead space, finding success with a novel EtCO2 monitor (CO2SMO Plus!™),70 but not with conventional time-based capnography.71 Investigators
also studied measures of CO2 for applications outside of mechanical ventilation. One study demonstrated noninvasive EtCO2 monitoring to be a reliable tool to monitor acidosis in diabetic
ketoacidosis.72 Another study demonstrated that cerebral NIRS measurements were a useful indicator of hypercapnia, though with low sensitivity.73 Finally, during apnea testing for the
determination of neurologic death, transcutaneous CO2 monitoring demonstrated high correlation, accuracy, and minimal bias when compared with PaCO2.74 REGIONAL LUNG VENTILATION MEASUREMENTS
Measurement of regional lung ventilation using electrical impedance tomography has been studied in children for over two decades.75 Electrical impedance tomography is a radiation-free
functional modality that allows for bedside imaging and real-time monitoring of lung expansion, particularly useful for PEEP titration with avoidance of overdistension and collapse.76
Electrical impedance tomography has proven useful in both PICU and NICU patients, but most validation studies have limited sample sizes.77 Further studies are needed to determine the
usefulness and cost-effectiveness of this method. PREDICTION AND/OR INFERENCE MODELS USING CARDIORESPIRATORY PHYSIOLOGICAL DATA MODELS USING HRV AS A SINGLE PHYSIOLOGICAL VARIABLE Fourteen
articles used HRV as a single continuous variable physiological variable within a clinical prediction or inference model. HRV has been studied as a single variable to predict clinical
deterioration across a range of pathologies, most extensively in sepsis (Table 2 and Supplementary Table 3).4,7,78,79,80,81 HRV has also been used to identify neonates at risk for
bradycardia,82 predict the duration of respiratory support,83 trace recovery during noninvasive respiratory support,84 and correlate with adverse outcomes at a 15-month follow-up in neonates
after hypoxic-ischemic encephalopathy.85 Continuous bedside display of trended HRV and other HR characteristics integrated into a single score (the HeRO score) was associated with mortality
reduction in a randomized trial of low birth weight neonates,86 mostly driven by patients with late-onset sepsis,87 and is now available as a commercial device. Subsequent validation of
this device has shown a limited ability to predict bloodstream infections.88 The simplified proxy of HRV (HRVi) has also shown promise in predicting new or worsening organ dysfunction in
PICU13 and oncology ward patients.36 MODELS USING OTHER SINGLE PHYSIOLOGICAL VARIABLES Seventeen studies described a prediction or inference model using a single physiological variable other
than HRV, mostly pertaining to cardiac function (Table 2 and Supplementary Table 3). Variation in stroke volume with breathing measured through bioreactance using the NICOM device predicted
an increase in stroke volume after fluid administration better than central venous pressure, but less accurately than intermittent ultrasound measurement.47 CO estimations using PRAM were
associated with the duration of mechanical ventilation, tissue perfusion, vasoactive and diuretic drug requirements in children post-cardiac surgery.89 In PICU patients with viral
respiratory failure, management of fluid status through PiCCO was associated with fewer ventilator days, although without a significant change in length of stay.90 The compensatory reserve
index, a machine learning-based measure that continuously compares the arterial line waveform to a store of “normal” arterial line waveforms in order to predict cardiac deterioration, was
found to be feasible and safe, with early changes in the index associated with length of stay.91 A study of CICU patients found estimations of normal cardiac index via PiCCO to be associated
with shorter duration of mechanical ventilation and length of stay.49 Finally, EtCO2 was studied as a proxy of CO and CPR quality during in-hospital cardiac arrest, but was not associated
with the return of circulation or survival to hospital discharge.92 Five articles described perfusion as a clinical predictor. One case report described how splanchnic NIRS measurements
precipitously dropped and remained low until the time of necrotizing enterocolitis diagnosis.93 Cardiac conditions affecting systemic perfusion have also been discovered through NIRS
changes, including large pericardial effusion94 and hemodynamically significant patent ductus arteriosus.95 These observational studies and case reports suggest that NIRS can alert
clinicians to underlying changes in central and peripheral SvO2 and oxygen extraction –in some cases as the first signal that the patient is beginning to deteriorate (Table 2 and
Supplementary Table 3). Although this trend in small studies is encouraging, the quality of evidence is low, and NIRS would benefit from larger, multicenter, randomized control trials.96 In
addition, perfusion index was investigated in a single study of PICU patients, where it showed high sensitivity and specificity in diagnosing shock as defined by tachycardia with poor
peripheral perfusion, but poor ability to predict hypotension.97 Four studies investigated respiratory variables. The ventilatory ratio, a variable dependent on CO2, body weight, and minute
ventilation, was compared to alveolar dead space fraction, and only alveolar dead space fraction was associated with mortality.98 Various measures of time-based capnography were associated
with extubation success in infants.99 Another study created an age-dependent index of the pulse oximeter waveform to predict episodic clinical deterioration.100 The pulse oximeter-based
index had a number of false positives equal to the number of events, but performed better than clinical prediction alone.100 Finally, a study of critically ill children investigated the use
of noninvasive thermal imaging to detect and/or predict shock 0–12 h before onset, and was found to have fair performance in critically ill children.101 MODELS USING MULTIPLE CONTINUOUS
PHYSIOLOGICAL VARIABLES Most studies of prediction or inference using multivariable models only included continuously collected physiological variables rather than a combination of
continuously collected physiological variables with intermittently collected multidimensional variables (e.g., electronic health record (EHR) or demographic data). Six articles presented
models to predict cardiorespiratory deterioration or arrest. Four of these articles were in the CICU and presented models using commonly monitored cardiorespiratory variables combined with
novel variables, including beat-to-beat ST segment morphology,11,102 HRV,12 and systemic vascular resistance,103 with variable performance. Two PICU studies of models using commonly
monitored cardiorespiratory variables alone, either from conventional means104 or through novel wireless sensors,105 reported varying sensitivity and specificity to predict cardiorespiratory
deterioration and arrest. All models required an hour or longer of continuous physiological input for model training. Four articles presented models of sepsis prediction using multiple
cardiorespiratory physiological variables. Three were derived in NICU patients and despite slightly different features of physiological variables used, all had similar modest predictive
value.5,9,106 Notably, two of these studies did not include HRV,9,106 one finding that HRV was not as useful for the subset of adults in their cohort.106 A PICU article included HRV in its
model but likewise found it less important than other features.6 Two studies presented models predicting unplanned intubation in the NICU. The first found good prediction of urgent unplanned
intubation, notably performing only slightly better than the HRV-based HeRO score alone.107 The second noted similar signatures of deterioration in urgent intubation and sepsis, although
their model performed best when predicting urgent intubations.106 MODELS USING CONTINUOUS PHYSIOLOGICAL VARIABLES AND NON-CONTINUOUS CLINICAL VARIABLES It is likely that a multidimensional
approach to modeling disease that includes continuous physiological variables, intermittently collected clinical variables (e.g., lab values, medication administrations), and patient factors
(e.g., age, comorbidities) will result in better performing models through a more complete picture of an individual patient. However, we found only seven studies that integrated
intermittently collected clinical variables or patient-level characteristics into continuous cardiorespiratory physiology-based models. Only one of those studies incorporated all three types
of data, demonstrating that two machine learning models of sepsis prediction in a large PICU achieved good performance.8 Integrated laboratory results with the HRV-based HeRO score
demonstrated an improved performance predicting sepsis in neonates when compared to HeRO score alone.10 A comparison of three machine learning models integrating continuous cardiorespiratory
variables with respiratory support and medication data automatically generated asthma scores that were noninferior to expert-derived manual scores.108 A comparison of models to predict at
least four mechanical ventilation days in PICU patients found that a model containing both patient-level factors and continuous data performed better than those containing continuous or
patient-level factors alone.109 Three machine learning models with continuous versus non-continuous data in a small CICU population found that the best strategy for non-continuous,
continuous, combination models had an equivalent performance.110 Finally, two studies investigated the commercially available oxygen delivery index (IDO2; Etiometry, Boston, MA), an index
containing both continuous and non-continuous clinical variables (but not demographic data), finding IDO2 to be associated with failed vasoactive wean in CICU patients111 and improved
specificity of clinical concern for cardiac arrest in PICU patients.112 DISCUSSION In this comprehensive scoping review we have summarized the existing evidence on novel approaches to the
capture and use of continuous cardiorespiratory physiological data in hospitalized children. We identified 58 articles describing novel devices or measurement approaches and these were found
to be primarily small, single-center, observational trials. Only three of these novel devices or measurement approaches were further explored in prediction or inference models in
hospitalized children. We also identified 47 articles of prediction or inference modeling using continuous cardiorespiratory variables and these varied greatly in sample size. Most
multivariable models did not incorporate multidimensional data (i.e., intermittently collected clinical variables or patient-level factors). Only two studies were randomized control trials
in which a cardiorespiratory physiological variable-based model was implemented and tested in hospitalized children (in both cases neonates).67,86 Finally, no study in either section took
place entirely outside of an ICU. Based on these findings, we identified three key areas for future development: (1) further external validation of promising novel devices and approaches to
measure continuous cardiorespiratory physiological variables; (2) more studies of models integrating multidimensional data; and (3) further dissemination, implementation, and prospective
validation of prediction models using continuous cardiorespiratory physiological data. Though novel cardiorespiratory monitoring devices and approaches have been an important pediatric
research focus for the last two decades, the majority of studies are small and single-center. It is difficult to assess the value of these novel devices without further study and external
validation. Furthermore, we found only three devices (PiCCO, NICOM, PRAM) that were used in prediction or inference models in hospitalized children.47,89,90 All three systems showed clinical
utility but all require proprietary devices and specialized training, and only NICOM is noninvasive. As such, despite availability for over 15 years, none have become routinely incorporated
into standard of care. Most of the other novel devices described in this review were developed for neonates where a major motivator is to avoid wire-based sensors, which can be obtrusive
and potentially harmful to delicate skin.1,2 It is unclear what the utility of these types of devices will have outside of the NICU, but they may gain more prominence as there is a movement
toward higher patient mobility and fewer wires.2 Machine learning-based prediction models that use continuous cardiorespiratory physiological data are steadily emerging in the literature,
with the majority of studies published after 2018. In general, we found that the integration of continuous cardiorespiratory physiological data with non-continuous clinical variables is
associated with improved performance in various clinical deterioration models. Of the seven prediction models that performed this integration, all but one found that continuous physiological
data improved model performance8,10,108,109,111,112 (and the outlier showed non-inferiority110). These types of integrated, dynamic physiological-clinical models will likely serve as a
foundation for personalized medicine in the acute care setting.113 Machine learning will be an important tool in this endeavor, as more traditional statistical methods typically require data
reduction steps that may discard useful information. However, for these models to be successful, we first need to: (1) identify which patients will benefit most from cardiorespiratory
monitoring and the prediction models based on physiological data; (2) share data, resources, and methods to develop more accurate models; and (3) develop strategies to disseminate,
implement, and prospectively validate these models. Identifying the patients who will most benefit from continuous cardiorespiratory monitoring will be an essential step in the successful
development and implementation of integrated, dynamic physiological-clinical models. All children with an acute illness may not need continuous cardiorespiratory monitoring. A recent
multicenter RCT comparing intermittent versus continuous pulse oximetry in the first episode of bronchiolitis in stable children found no difference in clinical outcomes.114 Clinicians may
not, however, be as good at predicting clinical deterioration as we would like–a recent study of emergent PICU transfers due to clinical deterioration found only 35% of patients were on
continuous cardiorespiratory monitoring prior to the event requiring transfer.115 Perhaps we should first focus on assessing the pre-test probability of a patient benefitting from continuous
cardiorespiratory monitoring to identify the “best” populations of hospitalized children to either escalate to continuous monitoring (with or without associated prediction models) or
de-escalate to intermittent monitoring. These strategies may eventually apply to patients who would benefit from remote patient monitoring after discharge from the hospital13 or those who
may avoid an admission altogether by being discharged from the emergency department with a wearable device.2 Advances in this field will hinge on our ability to share data, models,
resources, and methods. Clinical data science has advanced considerably in the last few years thanks to EHR data-sharing initiatives that leverage common data models, standard terminologies,
and data exchange resources.116,117 Indeed, automated sepsis alerts, clinical decision support tools, and real-time continuous vital sign visualization tools have already impacted the way
we provide care. However, physiological data from monitoring devices have not seen this same level of sharing and collaboration, with few exceptions (e.g., the MIMIC dataset).118 This lack
of collaboration is in large part due to three technical reasons: (1) physiological monitoring data is rarely stored by hospital systems beyond a few days; (2) there is a lack of standards
to make physiological data more interoperable across institutions; and (3) the complexity and volume of physiological data makes sharing these data more complicated than other types of
clinical data.119 The first problem is the most critical and urgent: we owe it to our patients to store any physiological data we collect to learn how to improve the care of future patients.
The other two problems are more easily surmountable through the development of new standards and data collaboration approaches (e.g., federated learning).120 The final hurdle that we must
overcome to take full advantage of continuous physiological data and machine learning models is the so-called last mile of artificial intelligence: dissemination, implementation, and
prospective validation.121 We identified very few examples of successful implementation and prospective validation of models based on cardiorespiratory physiological data in children,
including only two RCTs.67,86 A recent systematic review of real-time clinical analytics implementations found only 14 studies and identified 37 implementation challenges across people,
processes, information, and technology.122 A few key steps may improve implementation success: (1) making the models informative, actionable, timely, and interpretable; (2) tailoring each
intervention to the particular hospital environment in which the model will be implemented, both with buy-in and education; (3) integrating into routine clinical care without significantly
impacting workflows; and (4) providing support and ongoing monitoring of performance after implementation.123,124,125 CONCLUSION In this scoping review we have summarized the existing
evidence on novel approaches to the capture and use of continuous cardiorespiratory physiological data in hospitalized children. We identified three key areas for future development: (1)
further validation of promising novel devices; (2) more studies of models integrating multidimensional data with continuous cardiorespiratory data; and (3) further dissemination,
implementation, and validation of prediction models using continuous cardiorespiratory data. DATA AVAILABILITY All data generated or analyzed during this study are included in this published
article and its Supplementary information files. REFERENCES * Atallah, L. et al. Unobtrusive ECG monitoring in the NICU using a capacitive sensing array. _Physiol. Meas._ 35, 895–913
(2014). Article CAS PubMed Google Scholar * Xu, S., Jayaraman, A. & Rogers, J. A. Skin sensors are the future of health care. _Nature_ 571, 319–321 (2019). Article CAS PubMed
Google Scholar * Sanchez-Pinto, L. N., Luo, Y. & Churpek, M. M. Big data and data science in critical care. _Chest_ 154, 1239–1248 (2018). Article PubMed PubMed Central Google
Scholar * Amiri, P. et al. Potential prognostic markers in the heart rate variability features for early diagnosis of sepsis in the pediatric intensive care unit using convolutional neural
network classifiers. _Conf. Proc. IEEE Eng. Med. Biol. Soc._ 2020, 5627–5630 (2020). Google Scholar * Cabrera-Quiros, L. et al. Prediction of late-onset sepsis in preterm infants using
monitoring signals and machine learning. _Crit. Care Explor._ 3, e0302 (2021). Article PubMed PubMed Central Google Scholar * Kamaleswaran, R. et al. Applying artificial intelligence to
identify physiomarkers predicting severe sepsis in the PICU. _Pediatr. Crit. Care Med._ 19, e495–e503 (2018). Article PubMed Google Scholar * Leon, C., Carrault, G., Pladys, P. &
Beuchee, A. Early detection of late onset sepsis in premature infants using visibility graph analysis of heart rate variability. _IEEE J. Biomed. Health Inf._ 25, 1006–1017 (2021). Article
Google Scholar * Spaeder, M. C. et al. Predictive analytics in the pediatric intensive care unit for early identification of sepsis: capturing the context of age. _Pediatr. Res._ 86,
655–661 (2019). Article PubMed Google Scholar * Stanculescu, I., Williams, C. K. I. & Freer, Y. Autoregressive hidden Markov models for the early detection of neonatal sepsis. _IEEE
J. Biomed. Health Inf._ 18, 1560–1570 (2014). Article Google Scholar * Xiao, Y., Griffin, M. P., Lake, D. E. & Moorman, J. R. Nearest-neighbor and logistic regression analyses of
clinical and heart rate characteristics in the early diagnosis of neonatal sepsis. _Med. Decis. Mak._ 30, 258–266 (2010). Article Google Scholar * Rusin, C. G. et al. Prediction of
imminent, severe deterioration of children with parallel circulations using real-time processing of physiologic data. _J. Thorac. Cardiovasc. Surg._ 152, 171–177 (2016). Article PubMed
PubMed Central Google Scholar * Bose, S. N. et al. Early identification of impending cardiac arrest in neonates and infants in the cardiovascular ICU: a statistical modelling approach
using physiologic monitoring data. _Cardiol. Young._ 29, 1340–1348 (2019). Article PubMed Google Scholar * Badke, C. M., Swigart, L., Carroll, M. S., Weese-Mayer, D. E. &
Sanchez-Pinto, L. N. Autonomic nervous system dysfunction is associated with re-hospitalization in pediatric septic shock survivors. _Front. Pediatr._ 9, 745844 (2021). Article PubMed
Google Scholar * Yiallourou, S. R., Walker, A. M. & Horne, R. S. C. Validation of a new noninvasive method to measure blood pressure and assess baroreflex sensitivity in preterm infants
during sleep. _Sleep_ 29, 1083–1088 (2006). Article PubMed Google Scholar * Lemson, J. et al. The reliability of continuous noninvasive finger blood pressure measurement in critically
ill children. _Anesth. Analg._ 108, 814–821 (2009). Article PubMed Google Scholar * Andriessen, P. et al. Feasibility of noninvasive continuous finger arterial blood pressure measurements
in very young children, aged 0-4 years. _Pediatr. Res._ 63, 691–696 (2008). Article PubMed Google Scholar * Kapur, G. et al. Noninvasive determination of blood pressure by heart sound
analysis compared with intra-arterial monitoring in critically ill children–a pilot study of a novel approach. _Pediatr. Crit. Care Med._ 20, 809–816 (2019). Article PubMed Google Scholar
* Liu, C. et al. Wireless, skin-interfaced devices for pediatric critical care: application to continuous, noninvasive blood pressure monitoring. _Adv. Healthc. Mater._ 10, e2100383
(2021). Article PubMed Google Scholar * Chandler, J. R. et al. Pulse oximeter plethysmograph variation and its relationship to the arterial waveform in mechanically ventilated children.
_J. Clin. Monit. Comput._ 26, 145–151 (2012). Article CAS PubMed Google Scholar * Hay, W. W. Jr et al. Reliability of conventional and new pulse oximetry in neonatal patients. _J.
Perinatol._ 22, 360–366 (2002). Article PubMed Google Scholar * Chaichulee, S. et al. Cardio-respiratory signal extraction from video camera data for continuous non-contact vital sign
monitoring using deep learning. _Physiol. Meas._ 40, 115001 (2019). Article PubMed PubMed Central Google Scholar * Chen, Q. et al. Non-contact heart rate monitoring in neonatal intensive
care unit using RGB camera. _Conf. Proc. IEEE Eng. Med. Biol. Soc._ 2020, 5822–5825 (2020). Google Scholar * Cobos-Torres, J.-C., Abderrahim, M. & Martínez-Orgado, J. Non-contact,
simple neonatal monitoring by photoplethysmography. _Sensors_ 18, 4362 (2018). * Mestha, L. K., Kyal, S., Xu, B., Lewis, L. E. & Kumar, V. Towards continuous monitoring of pulse rate in
neonatal intensive care unit with a webcam. _Conf. Proc. IEEE Eng. Med. Biol. Soc._ 2014, 3817–3820 (2014). Google Scholar * Villarroel, M. et al. Non-contact physiological monitoring of
preterm infants in the neonatal intensive care unit. _NPJ Digit. Med._ 2, 128 (2019). * Lee, W. H. et al. Feasibility of non-contact cardiorespiratory monitoring using impulse-radio
ultra-wideband radar in the neonatal intensive care unit. _PLoS One_ 15, e0243939 (2020). Article CAS PubMed PubMed Central Google Scholar * Marchionni, P., Scalise, L., Ercoli, I.
& Tomasini, E. P. An optical measurement method for the simultaneous assessment of respiration and heart rates in preterm infants. _Rev. Sci. Instrum._ 84, 121705 (2013). Article CAS
PubMed Google Scholar * Sato, S. et al. Assessment of a new piezoelectric transducer sensor for noninvasive cardiorespiratory monitoring of newborn infants in the NICU. _Neonatology_ 98,
179–190 (2010). Article PubMed Google Scholar * Grubb, M. R. et al. Forehead reflectance photoplethysmography to monitor heart rate: preliminary results from neonatal patients. _Physiol.
Meas._ 35, 881–893 (2014). Article CAS PubMed Google Scholar * Simmen, P. et al. Multichannel esophageal heart rate monitoring of preterm infants. _IEEE Trans. Biomed. Eng._ 68,
1903–1912 (2021). Article PubMed Google Scholar * Kraaijenga, J. V., Hutten, G. J., de Jongh, F. H. & van Kaam, A. H. Transcutaneous electromyography of the diaphragm: a
cardio-respiratory monitor for preterm infants. _Pediatr. Pulmonol._ 50, 889–895 (2015). Article PubMed Google Scholar * Cabrera-Quiros, L. et al. Estimation of heart rate directly from
ECG spectrogram in neonate intensive care units. _Conf. Proc. IEEE Eng. Med. Biol. Soc._ 2020, 320–323 (2020). Google Scholar * Abdel-Rahman, Y., Jeremic, A. & Tan, K. Neonatal heart
rate prediction. _2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society_. https://doi.org/10.1109/iembs.2009.5334205 (2009). * Badke, C. M., Marsillio,
L. E., Weese-Mayer, D. E. & Sanchez-Pinto, L. N. Autonomic nervous system dysfunction in pediatric sepsis. _Front. Pediatr._ 6, 280 (2018). Article PubMed PubMed Central Google
Scholar * Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society
of Pacing and Electrophysiology. _Eur. Heart J._ 17, 354–381 (1996).. * Mayampurath, A., Volchenboum, S. L. & Sanchez-Pinto, L. N. Using photoplethysmography data to estimate heart rate
variability and its association with organ dysfunction in pediatric oncology patients. _NPJ Digit. Med._ 1, 29 (2018). Article PubMed PubMed Central Google Scholar * Litton, E. &
Morgan, M. The PiCCO monitor: a review. _Anaesth. Intensive Care_ 40, 393–408 (2012). Article CAS PubMed Google Scholar * Egan, J. R. et al. Clinical assessment of cardiac performance in
infants and children following cardiac surgery. _Intensive Care Med._ 31, 568–573 (2005). Article PubMed Google Scholar * Fakler, U. et al. Cardiac index monitoring by pulse contour
analysis and thermodilution after pediatric cardiac surgery. _J. Thorac. Cardiovasc. Surg._ 133, 224–228 (2007). Article CAS PubMed Google Scholar * Aslan, N. et al. Comparison of
cardiac output and cardiac index values measured by critical care echocardiography with the values measured by pulse index continuous cardiac output (PiCCO) in the pediatric intensive care
unit: a preliminary study. _Ital. J. Pediatr._ 46, 47 (2020). Article PubMed PubMed Central Google Scholar * Gergely, M. et al. Assessment of global tissue perfusion and oxygenation in
neonates and infants after open-heart surgery. _Interact. Cardiovasc. Thorac. Surg._ 18, 426–431 (2014). Article PubMed PubMed Central Google Scholar * Calamandrei, M. et al. Assessment
of cardiac output in children: a comparison between the pressure recording analytical method and Doppler echocardiography. _Pediatr. Crit. Care Med._ 9, 310–312 (2008). Article PubMed
Google Scholar * Saxena, R., Durward, A., Puppala, N. K., Murdoch, I. A. & Tibby, S. M. Pressure recording analytical method for measuring cardiac output in critically ill children: a
validation study. _Br. J. Anaesth._ 110, 425–431 (2013). Article CAS PubMed Google Scholar * Gatelli, I. F., Vitelli, O., Chiesa, G., De Rienzo, F. & Martinelli, S. Noninvasive
cardiac output monitoring in newborn with hypoplastic left heart syndrome. _Am. J. Perinatol._ 37, S54–S56 (2020). Article PubMed Google Scholar * Blohm, M. E. et al. Impedance
cardiography (electrical velocimetry) and transthoracic echocardiography for non-invasive cardiac output monitoring in pediatric intensive care patients: a prospective single-center
observational study. _Crit. Care_ 18, 603 (2014). Article PubMed PubMed Central Google Scholar * Schubert, S. et al. Continuous, non-invasive techniques to determine cardiac output in
children after cardiac surgery: evaluation of transesophageal Doppler and electric velocimetry. _J. Clin. Monit. Comput._ 22, 299–307 (2008). Article PubMed Google Scholar * Lee, J. Y. et
al. The ability of stroke volume variation measured by a noninvasive cardiac output monitor to predict fluid responsiveness in mechanically ventilated children. _Pediatr. Cardiol._ 35,
289–294 (2014). Article PubMed Google Scholar * Botte, A. et al. Evaluation of a noninvasive cardiac output monitor in mechanically ventilated children. _Pediatr. Crit. Care Med._ 7,
231–236 (2006). Article PubMed Google Scholar * Gil-Anton, J. et al. Cardiac index monitoring by femoral arterial thermodilution after cardiac surgery in children. _J. Crit. Care_ 29,
1132.e1–4 (2014). Article PubMed Google Scholar * Bay-Hansen, R., Elfving, B. & Greisen, G. Use of near infrared spectroscopy for estimation of peripheral venous saturation in
newborns: comparison with co-oximetry of central venous blood. _Biol. Neonate_ 82, 1–8 (2002). Article PubMed Google Scholar * Massa-Buck, B., Amendola, V., McCloskey, R. &
Rais-Bahrami, K. Significant correlation between regional tissue oxygen saturation and vital signs of critically ill infants. _Front. Pediatr._ 5, 276 (2017). * Dabal, R. J. et al. Inferior
vena cava oxygen saturation monitoring after the Norwood procedure. _Ann. Thorac. Surg._ 95, 2114–2120 (2013). discussion 2120–2121. Article PubMed Google Scholar * Gillam-Krakauer, M. et
al. Correlation of abdominal rSO2 with superior mesenteric artery velocities in preterm infants. _J. Perinatol._ 33, 609–612 (2013). Article CAS PubMed PubMed Central Google Scholar *
Piasek, C. Z., Van Bel, F. & Sola, A. Perfusion index in newborn infants: a noninvasive tool for neonatal monitoring. _Acta Paediatr._ 103, 468–473 (2014). Article PubMed Google
Scholar * Coutrot, M. et al. Perfusion index: physical principles, physiological meanings and clinical implications in anaesthesia and critical care. _Anaesth. Crit. Care Pain. Med._ 40,
100964 (2021). Article PubMed Google Scholar * Roback, K., Nelson, N., Johansson, A., Hass, U. & Strömberg, T. A new fiberoptical respiratory rate monitor for the neonatal intensive
care unit. _Pediatr. Pulmonol._ 39, 120–126 (2005). Article PubMed Google Scholar * Janssen, R., Wang, W., Moço, A. & de Haan, G. Video-based respiration monitoring with automatic
region of interest detection. _Physiol. Meas._ 37, 100–114 (2016). Article PubMed Google Scholar * Abbas, A. K., Heimann, K., Jergus, K., Orlikowsky, T. & Leonhardt, S. Neonatal
non-contact respiratory monitoring based on real-time infrared thermography. _Biomed. Eng. Online_ 10, 93 (2011). Article PubMed PubMed Central Google Scholar * Sauthier, M., Tuli, G.,
Jouvet, P. A., Brownstein, J. S. & Randolph, A. G. Estimated Pao2: a continuous and noninvasive method to estimate Pao2 and oxygenation index. _Crit. Care Explor._ 3, e0546 (2021).
Article PubMed PubMed Central Google Scholar * Thandaveshwara, D., Chandrashekar Reddy, A. H., Gopalakrishna, M. V. & Doreswamy, S. M. Saturation oxygenation pressure index: a
non-invasive bedside measure for severity of respiratory disease in neonates on CPAP. _Eur. J. Pediatr._ 180, 1287–1292 (2021). Article CAS PubMed Google Scholar * Isenberg, S. J.,
Neumann, D., Fink, S. & Rich, R. Continuous oxygen monitoring of the conjunctiva in neonates. _J. Perinatol._ 22, 46–49 (2002). Article PubMed Google Scholar * Pediatric Acute Lung
Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. _Pediatr. Crit. Care
Med._ 16, 428–439 (2015). Article Google Scholar * Duyu, M. et al. Comparing the novel microstream and the traditional mainstream method of end-tidal CO2 monitoring with respect to PaCO2
as gold standard in intubated critically ill children. _Sci. Rep._ 10, 22042 (2020). * Wu, C.-H. et al. Good estimation of arterial carbon dioxide by end-tidal carbon dioxide monitoring in
the neonatal intensive care unit. _Pediatr. Pulmonol._ 35, 292–295 (2003). Article PubMed Google Scholar * Hejlesen, O. K., Cichosz, S. L., Vangsgaard, S., Andresen, M. F. & Madsen,
L. P. Clinical implications of a quality assessment of transcutaneous CO2 monitoring in preterm infants in neonatal intensive care. _Stud. Health Technol. Inform._ 150, 490–494 (2009).
PubMed Google Scholar * Williams, E., Dassios, T., O’Reilly, N., Walsh, A. & Greenough, A. End-tidal capnography monitoring in infants ventilated on the neonatal intensive care unit.
_J. Perinatol._ 41, 1718–1724 (2021). Article CAS PubMed PubMed Central Google Scholar * Kugelman, A. et al. Impact of continuous capnography in ventilated neonates: a randomized,
multicenter study. _J. Pediatr._ 168, 56–61.e2 (2016). Article PubMed Google Scholar * Belal, S. Y., Taktak, A. F. G., Nevill, A. & Spencer, A. An intelligent ventilation and
oxygenation management system in neonatal intensive care using fuzzy trend template fitting. _Physiol. Meas._ 26, 555–570 (2005). Article PubMed Google Scholar * McSwain, S. D. et al.
End-tidal and arterial carbon dioxide measurements correlate across all levels of physiologic dead space. _Respir. Care_ 55, 288–293 (2010). PubMed Google Scholar * Riou, Y. et al.
Reproducibility of the respiratory dead space measurements in mechanically ventilated children using the CO2SMO monitor. _Intensive Care Med._ 30, 1461–1467 (2004). Article CAS PubMed
Google Scholar * Bhalla, A. K. et al. Monitoring dead space in mechanically ventilated children: volumetric capnography versus time-based capnography. _Respir. Care_ 60, 1548–1555 (2015).
Article PubMed Google Scholar * Agus, M. S. D., Alexander, J. L. & Mantell, P. A. Continuous non-invasive end-tidal CO2 monitoring in pediatric inpatients with diabetic ketoacidosis.
_Pediatr. Diabetes_ 7, 196–200 (2006). Article PubMed Google Scholar * Erdoğan, S., Oto, A. & Boşnak, M. Reliability of cerebral oximeter in non-invasive diagnosis and follow-up of
hypercapnia. _Turk. J. Pediatr._ 58, 389–394 (2016). Article PubMed Google Scholar * Sochet, A. A. et al. Transcutaneous carbon dioxide monitoring during apnea testing for determination
of neurologic death in children: a retrospective case series. _Pediatr. Crit. Care Med._ 21, 437–442 (2020). Article PubMed Google Scholar * Frerichs, I., Schiffmann, H., Hahn, G. &
Hellige, G. Non-invasive radiation-free monitoring of regional lung ventilation in critically ill infants. _Intensive Care Med._ 27, 1385–1394 (2001). Article CAS PubMed Google Scholar *
Davies, P., Yasin, S., Gates, S., Bird, D. & Silvestre, C. Clinical scenarios of the application of electrical impedance tomography in paediatric intensive care. _Sci. Rep._ 9, 5362
(2019). Article PubMed PubMed Central Google Scholar * Krause, U. et al. Monitoring of regional lung ventilation using electrical impedance tomography after cardiac surgery in infants
and children. _Pediatr. Cardiol._ 35, 990–997 (2014). Article PubMed Google Scholar * Griffin, M. P. et al. Heart rate characteristics: novel physiomarkers to predict neonatal infection
and death. _Pediatrics_ 116, 1070–1074 (2005). Article PubMed Google Scholar * Griffin, M. P., Lake, D. E., O’Shea, T. M. & Moorman, J. R. Heart rate characteristics and clinical
signs in neonatal sepsis. _Pediatr. Res._ 61, 222–227 (2007). Article PubMed Google Scholar * Griffin, M. P. & Moorman, J. R. Toward the early diagnosis of neonatal sepsis and
sepsis-like illness using novel heart rate analysis. _Pediatrics_ 107, 97–104 (2001). Article CAS PubMed Google Scholar * Ellenby, M. S. et al. Uncoupling and recoupling of autonomic
regulation of the heart beat in pediatric septic shock. _Shock_ 16, 274–277 (2001). Article CAS PubMed Google Scholar * Gee, A. H., Barbieri, R., Paydarfar, D. & Indic, P. Predicting
bradycardia in preterm infants using point process analysis of heart rate. _IEEE Trans. Biomed. Eng._ 64, 2300–2308 (2017). Article PubMed Google Scholar * Jost, K., Datta, A. N., Frey,
U. P., Suki, B. & Schulzke, S. M. Heart rate fluctuation after birth predicts subsequent cardiorespiratory stability in preterm infants. _Pediatr. Res._ 86, 348–354 (2019). Article
PubMed Google Scholar * Perez–Zabalza, M. et al. Analysis of heart rate variability in children during high flow nasal cannula therapy. _Biomed. Phys. Eng. Express_ 5, 045028 (2019).
Article Google Scholar * Massaro, A. N. et al. Heart rate variability in encephalopathic newborns during and after therapeutic hypothermia. _J. Perinatol._ 34, 836–841 (2014). Article CAS
PubMed PubMed Central Google Scholar * Moorman, J. R. et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. _J.
Pediatr._ 159, 900–906.e1 (2011). Article PubMed PubMed Central Google Scholar * Fairchild, K. D. et al. Septicemia mortality reduction in neonates in a heart rate characteristics
monitoring trial. _Pediatr. Res._ 74, 570–575 (2013). Article PubMed PubMed Central Google Scholar * Coggins, S. A. et al. Heart rate characteristic index monitoring for bloodstream
infection in an NICU: a 3-year experience. _Arch. Dis. Child. Fetal Neonatal Ed._ 101, F329–F332 (2016). Article PubMed Google Scholar * Favia, I. et al. Cardiac index assessment by the
pressure recording analytical method in infants after paediatric cardiac surgery: a pilot retrospective study. _Interact. Cardiovasc. Thorac. Surg._ 23, 919–923 (2016). Article PubMed
Google Scholar * Wang, F. et al. The fluid management and hemodynamic characteristics of PiCCO employed on young children with severe hand, foot, and mouth disease—a retrospective study.
_BMC Infect. Dis._ 21, 208 (2021). * Ehrmann, D. E. et al. Lessons learned from the first pilot study of the compensatory reserve index after congenital heart surgery requiring
cardiopulmonary bypass. _World J. Pediatr. Congenit. Heart Surg._ 12, 176–184 (2021). Article PubMed Google Scholar * Berg, R. A. et al. End-tidal carbon dioxide during pediatric
in-hospital cardiopulmonary resuscitation. _Resuscitation_ 133, 173–179 (2018). Article PubMed PubMed Central Google Scholar * Baserga, M., Reich, B. & Braski, K. Abnormal splanchnic
regional saturations in a preterm infant that developed necrotizing enterocolitis following a red blood cell transfusion. _Adv. Neonatal Care_ 20, 401–405 (2020). Article PubMed Google
Scholar * Maher, K. O., Phelps, H. M. & Kirshbom, P. M. Near infrared spectroscopy changes with pericardial tamponade. _Pediatr. Crit. Care Med._ 10, e13–e15 (2009). Article PubMed
Google Scholar * Chock, V. Y., Rose, L. A., Mante, J. V. & Punn, R. Near-infrared spectroscopy for detection of a significant patent ductus arteriosus. _Pediatr. Res._ 80, 675–680
(2016). Article PubMed Google Scholar * Hansen, M. L. et al. Cerebral near-infrared spectroscopy monitoring (NIRS) in children and adults: a systematic review with meta-analysis.
_Pediatr. Res_. (2022) https://doi.org/10.1038/s41390-022-01995-z. * Sivaprasath, P., Mookka Gounder, R. & Mythili, B. Prediction of shock by peripheral perfusion index. _Indian J.
Pediatr._ 86, 903–908 (2019). Article CAS PubMed Google Scholar * Bhalla, A. K., Dong, J., Klein, M. J., Khemani, R. G. & Newth, C. J. L. The association between ventilatory ratio
and mortality in children and young adults. _Respir. Care_ 66, 205–212 (2021). Article PubMed Google Scholar * Rasera, C. C., Gewehr, P. M. & Domingues, A. M. T. PET(CO2) measurement
and feature extraction of capnogram signals for extubation outcomes from mechanical ventilation. _Physiol. Meas._ 36, 231–242 (2015). Article PubMed Google Scholar * Matam, B. R.,
Rajeswari Matam, B., Duncan, H. & Lowe, D. Automated prediction of deterioration of infants in paediatric intensive care using SpO2. _Int. J. Biomed. Eng. Technol._ 13, 341 (2013).
Article Google Scholar * Nagori, A., Dhingra, L. S., Bhatnagar, A., Lodha, R. & Sethi, T. Predicting hemodynamic shock from thermal images using machine learning. _Sci. Rep._ 9, 91
(2019). Article PubMed PubMed Central Google Scholar * Rusin, C. G. et al. Automated prediction of cardiorespiratory deterioration in patients with single ventricle. _J. Am. Coll.
Cardiol._ 77, 3184–3192 (2021). Article PubMed Google Scholar * Erez, E., Mazwi, M. L., Marquez, A. M., Moga, M.-A. & Eytan, D. Hemodynamic patterns before inhospital cardiac arrest
in critically ill children: an exploratory study. _Crit. Care Explor_ 3, e0443 (2021). Article PubMed PubMed Central Google Scholar * Matam, B. R., Duncan, H. & Lowe, D. Machine
learning based framework to predict cardiac arrests in a paediatric intensive care unit. _J. Clin. Monit. Comput._ 33, 713–724 (2019). Article CAS PubMed Google Scholar * Duncan, H. P.,
Fule, B., Rice, I., Sitch, A. J. & Lowe, D. Wireless monitoring and real-time adaptive predictive indicator of deterioration. _Sci. Rep._ 10, 11366 (2020). Article PubMed PubMed
Central Google Scholar * Moss, T. J. et al. Signatures of subacute potentially catastrophic illness in the ICU: model development and validation. _Crit. Care Med._ 44, 1639–1648 (2016).
Article PubMed PubMed Central Google Scholar * Clark, M. T. et al. Predictive monitoring for respiratory decompensation leading to urgent unplanned intubation in the neonatal intensive
care unit. _Pediatr. Res._ 73, 104–110 (2013). Article PubMed Google Scholar * Messinger, A. I. et al. Novel pediatric-automated respiratory score using physiologic data and machine
learning in asthma. _Pediatr. Pulmonol._ 54, 1149–1155 (2019). Article PubMed PubMed Central Google Scholar * Castiñeira, D. et al. Adding continuous vital sign information to static
clinical data improves the prediction of length of stay after intubation: a data-driven machine learning approach. _Respir. Care_ 65, 1367–1377 (2020). Article PubMed PubMed Central
Google Scholar * Rooney, S. R. et al. Prediction of extubation failure in the paediatric cardiac ICU using machine learning and high-frequency physiologic data. _Cardiol. Young_ 1–8 (2021).
* Goldsmith, M. P. et al. Use of a risk analytic algorithm to inform weaning from vasoactive medication in patients following pediatric cardiac surgery. _Crit. Care Explor._ 3, e0563
(2021). Article PubMed PubMed Central Google Scholar * Dewan, M., Cooper, D. S. & Tegtmeyer, K. Low inadequate oxygen delivery index is associated with decreased cardiac arrest risk
in high-risk pediatric ICU patients. _Crit. Care Explor._ 4, e0600 (2022). Article PubMed PubMed Central Google Scholar * Holder, A. L. & Clermont, G. Using what you get: dynamic
physiologic signatures of critical illness. _Crit. Care Clin._ 31, 133–164 (2015). Article PubMed PubMed Central Google Scholar * Mahant, S. et al. Intermittent vs continuous pulse
oximetry in hospitalized infants with stabilized bronchiolitis: a randomized clinical trial. _JAMA Pediatr._ 175, 466–474 (2021). Article PubMed Google Scholar * Kowalski, R. L., Lee, L.,
Spaeder, M. C., Moorman, J. R. & Keim-Malpass, J. Accuracy and monitoring of Pediatric Early Warning Score (PEWS) scores prior to emergent pediatric intensive care unit (ICU) transfer:
retrospective analysis. _JMIR Pediatr. Parent_ 4, e25991 (2021). Article PubMed PubMed Central Google Scholar * Forrest, C. B., Margolis, P., Seid, M. & Colletti, R. B. PEDSnet: how
a prototype pediatric learning health system is being expanded into a national network. _Health Aff._ 33, 1171–1177 (2014). Article Google Scholar * Brant, E. B. et al. Developing a shared
sepsis data infrastructure: a systematic review and concept map to FHIR. _NPJ Digit. Med._ 5, 44 (2022). Article PubMed PubMed Central Google Scholar * Johnson, A. E. W. et al.
MIMIC-III, a freely accessible critical care database. _Sci. Data_ 3, 160035 (2016). Article CAS PubMed PubMed Central Google Scholar * Maslove, D. M., Elbers, P. W. G. & Clermont,
G. Artificial intelligence in telemetry: what clinicians should know. _Intensive Care Med._ 47, 150–153 (2021). Article PubMed PubMed Central Google Scholar * Crowson, M. G. et al. A
systematic review of federated learning applications for biomedical data. _PLOS Digital Health_ 1, e0000033 (2022). Article Google Scholar * Coiera, E. The last mile: where artificial
intelligence meets reality. _J. Med. Internet Res._ 21, e16323 (2019). Article PubMed PubMed Central Google Scholar * Lim, H. C. et al. Toward a learning health care system: a systematic
review and evidence-based conceptual framework for implementation of clinical analytics in a digital hospital. _Appl. Clin. Inform._ 13, 339–354 (2022). Article PubMed PubMed Central
Google Scholar * Moorman, L. P. Principles for real-world implementation of bedside predictive analytics monitoring. _Appl. Clin. Inform._ 12, 888–896 (2021). Article PubMed PubMed
Central Google Scholar * Futoma, J., Simons, M., Panch, T., Doshi-Velez, F. & Celi, L. A. The myth of generalisability in clinical research and machine learning in health care. _Lancet
Digit Health_ 2, e489–e492 (2020). Article PubMed PubMed Central Google Scholar * Sanchez-Pinto, L. N. & Bennett, T. D. Evaluation of machine learning models for clinical prediction
problems. _Pediatr. Crit. Care Med._ 23, 405–408 (2022). Article PubMed Google Scholar * McQuillen, P. S. et al. Regional and central venous oxygen saturation monitoring following
pediatric cardiac surgery: concordance and association with clinical variables. _Pediatr. Crit. Care Med._ 8, 154–160 (2007). Article PubMed Google Scholar * Ong, T. et al. Higher
pulmonary dead space may predict prolonged mechanical ventilation after cardiac surgery. _Pediatr. Pulmonol._ 44, 457–463 (2009). Article PubMed PubMed Central Google Scholar * Heskamp,
L., Lansdorp, B., Hopman, J., Lemson, J. & de Boode, W.-P. Ventilator-induced pulse pressure variation in neonates. _Physiol. Rep._ 4, e12716 (2016). * Joshi, R. et al. Cardiorespiratory
coupling in preterm infants. _J. Appl. Physiol._ 126, 202–213 (2019). Article PubMed Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, IL, USA Sarah B. Walker, Colleen M. Badke, Michael S. Carroll, Kyle S. Honegger, Andrea Fawcett, Debra E.
Weese-Mayer & L. Nelson Sanchez-Pinto * Stanley Manne Children’s Research Institute, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, USA Sarah B. Walker, Colleen
M. Badke, Michael S. Carroll, Kyle S. Honegger, Andrea Fawcett, Debra E. Weese-Mayer & L. Nelson Sanchez-Pinto Authors * Sarah B. Walker View author publications You can also search for
this author inPubMed Google Scholar * Colleen M. Badke View author publications You can also search for this author inPubMed Google Scholar * Michael S. Carroll View author publications You
can also search for this author inPubMed Google Scholar * Kyle S. Honegger View author publications You can also search for this author inPubMed Google Scholar * Andrea Fawcett View author
publications You can also search for this author inPubMed Google Scholar * Debra E. Weese-Mayer View author publications You can also search for this author inPubMed Google Scholar * L.
Nelson Sanchez-Pinto View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors made substantial contributions to the conception and
design of the study. S.B.W., C.M.B., K.S.H., M.S.C., D.E.W.-M., and L.N.S.-P. contributed to the acquisition, analysis, and interpretation of data. S.B.W., C.M.B., K.S.H., M.S.C., D.E.W.-M.,
and L.N.S.-P. helped draft the article, revise the manuscript critically for important intellectual content, and gave final approval of the version to be submitted for peer review.
CORRESPONDING AUTHOR Correspondence to Sarah B. Walker. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION MONITOR REVIEW_SUPPLEMENTARY TABLES_REVISION MONITOR
REVIEW_SUPPLEMENTARY TABLES_REVISION RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing
agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement
and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Walker, S.B., Badke, C.M., Carroll, M.S. _et al._ Novel approaches to capturing and using continuous
cardiorespiratory physiological data in hospitalized children. _Pediatr Res_ 93, 396–404 (2023). https://doi.org/10.1038/s41390-022-02359-3 Download citation * Received: 02 June 2022 *
Revised: 16 August 2022 * Accepted: 11 October 2022 * Published: 03 November 2022 * Issue Date: January 2023 * DOI: https://doi.org/10.1038/s41390-022-02359-3 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative