Muc16 depletion diminishes kras-induced tumorigenesis and metastasis by altering tumor microenvironment factors in pancreatic ductal adenocarcinoma
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
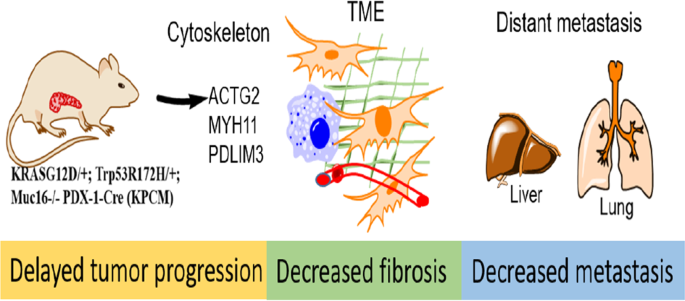
ABSTRACT MUC16, membrane-bound mucin, plays an oncogenic role in pancreatic ductal adenocarcinoma (PDAC). However, the pathological role of MUC16 in the PDAC progression, tumor
microenvironment, and metastasis in cooperation with KrasG12D and Trp53R172H mutations remains unknown. Deletion of Muc16 with activating mutations KrasG12D/+ and Trp53R172H/+ in mice
significantly decreased progression and prolonged overall survival in KrasG12D/+; Trp53R172H/+; Pdx-1-Cre; Muc16−/− (KPCM) and KrasG12D/+; Pdx-1-Cre; Muc16−/− (KCM), as compared to
KrasG12D/+; Trp53R172H/+; Pdx-1-Cre (KPC) and KrasG12D/+; Pdx-1-Cre (KC) mice, respectively. Muc16 knockout pancreatic tumor (KPCM) displays decreased tumor microenvironment factors and
significantly reduced incidence of liver and lung metastasis compared to KPC. Furthermore, in silico data analysis showed a positive correlation of MUC16 with activated stroma and
metastasis-associated genes. KPCM mouse syngeneic cells had significantly lower metastatic and endothelial cell binding abilities than KPC cells. Similarly, KPCM organoids significantly
decreased the growth rate compared to KPC organoids. Interestingly, RNA-seq data revealed that the cytoskeletal proteins Actg2, Myh11, and Pdlim3 were downregulated in KPCM tumors. Further
knockdown of these genes showed reduced metastatic potential. Overall, our results demonstrate that Muc16 alters the tumor microenvironment factors during pancreatic cancer progression and
metastasis by changing the expression of Actg2, Myh11, and Pdlim3 genes. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 50 print issues and online access $259.00 per year only $5.18 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS LOSS OF THE WILD-TYPE _KRAS_ ALLELE PROMOTES PANCREATIC CANCER
PROGRESSION THROUGH FUNCTIONAL ACTIVATION OF YAP1 Article 18 October 2021 ROFLUMILAST INHIBITS TUMOR GROWTH AND MIGRATION IN STK11/LKB1 DEFICIENT PANCREATIC CANCER Article Open access 09
March 2024 MUC4 LOSS MITIGATES EPIDERMAL GROWTH FACTOR RECEPTOR ACTIVITY ESSENTIAL FOR PDAC TUMORIGENESIS Article 09 January 2023 DATA AVAILABILITY The accession number for RNA sequencing
data of Muc16 knockout pancreatic tumor tissues is GSE212777, and materials associated with the current study are available from the corresponding author upon reasonable request. REFERENCES
* Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: Cancer J Clin. 2021;71:7–33. PubMed Google Scholar * Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: an
update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol. 2020;17:487–505. * Encarnación-Rosado J, Kimmelman AC. Harnessing metabolic dependencies in pancreatic cancers. Nat
Rev Gastroenterol Hepatol. 2021;18:482–92. * Buscail L, Bournet B, Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol
Hepatol. 2020;17:153–68. Article CAS PubMed Google Scholar * Benjamin JR, Ralph HH, Andrew JA, Richard AM, Jen JY, Chip S, et al. Integrated Genomic Characterization of Pancreatic Ductal
Adenocarcinoma. Cancer Cell. 2017;32:185–203.e13. * Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic
cancer. Nature 2015;518:495–501. Article CAS PubMed PubMed Central Google Scholar * Tian C, Huang Y, Clauser KR, Rickelt S, Lau AN, Carr SA, et al. Suppression of pancreatic ductal
adenocarcinoma growth and metastasis by fibrillar collagens produced selectively by tumor cells. Nat Commun. 2021;12:2328. Article CAS PubMed PubMed Central Google Scholar * Tian C,
Clauser KR, Öhlund D, Rickelt S, Huang Y, Gupta M, et al. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal
cells. Proc Natl Acad Sci USA. 2019;116:19609–18. Article CAS PubMed PubMed Central Google Scholar * Bafna S, Kaur S, Momi N, Batra SK. Pancreatic cancer cells resistance to
gemcitabine: the role of MUC4 mucin. Br J Cancer. 2009;101:1155–61. Article CAS PubMed PubMed Central Google Scholar * Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. Mucins in pancreatic
cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10:607–20. Article CAS PubMed PubMed Central Google Scholar * Caffrey T, Sagar S, Thomas D, Lewallen ME,
Hollingsworth MA, Radhakrishnan P. The glycoprotein mucin-1 negatively regulates GalNAc transferase 5 expression in pancreatic cancer. FEBS Lett. 2019;593:2751–61. Article CAS PubMed
PubMed Central Google Scholar * Chen SH, Dallas MR, Balzer EM, Konstantopoulos K. Mucin 16 is a functional selectin ligand on pancreatic cancer cells. FASEB J: Off Publ Fed Am Soc Exp
Biol. 2012;26:1349–59. Article CAS Google Scholar * Chen SH, Hung WC, Wang P, Paul C, Konstantopoulos K. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and
invasion via MMP-7 activation. Sci Rep. 2013;3:1870. Article PubMed PubMed Central Google Scholar * Das S, Rachagani S, Torres-Gonzalez MP, Lakshmanan I, Majhi PD, Smith LM, et al.
Carboxyl-terminal domain of MUC16 imparts tumorigenic and metastatic functions through nuclear translocation of JAK2 to pancreatic cancer cells. Oncotarget 2015;6:5772–87. Article PubMed
PubMed Central Google Scholar * Felder M, Kapur A, Gonzalez-Bosquet J, Horibata S, Heintz J, Albrecht R, et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol
Cancer. 2014;13:129. Article PubMed PubMed Central Google Scholar * Haridas D, Chakraborty S, Ponnusamy MP, Lakshmanan I, Rachagani S, Cruz E, et al. Pathobiological implications of
MUC16 expression in pancreatic cancer. PLoS One. 2011;6:e26839. Article CAS PubMed PubMed Central Google Scholar * Lakshmanan I, Ponnusamy MP, Das S, Chakraborty S, Haridas D,
Mukhopadhyay P, et al. MUC16 induced rapid G2/M transition via interactions with JAK2 for increased proliferation and anti-apoptosis in breast cancer cells. Oncogene 2012;31:805–17. Article
CAS PubMed Google Scholar * Lakshmanan I, Salfity S, Seshacharyulu P, Rachagani S, Thomas A, Das S, et al. MUC16 regulates TSPYL5 for lung cancer cell growth and chemoresistance by
suppressing p53. Clin Cancer Res. 2017;23:3906–17. Article CAS PubMed PubMed Central Google Scholar * Das S, Batra SK. Understanding the unique attributes of MUC16 (CA125): Potential
implications in targeted therapy. Cancer Res. 2015;75:4669–74. Article CAS PubMed PubMed Central Google Scholar * Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault M, Ho M, et al.
Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50. Article PubMed PubMed Central
Google Scholar * Akita K, Tanaka M, Tanida S, Mori Y, Toda M, Nakada H. CA125/MUC16 interacts with Src family kinases, and over-expression of its C-terminal fragment in human epithelial
cancer cells reduces cell-cell adhesion. Eur J Cell Biol. 2013;92:257–63. Article CAS PubMed Google Scholar * Muniyan S, Haridas D, Chugh S, Rachagani S, Lakshmanan I, Gupta S, et al.
MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism. Genes cancer. 2016;7:110–24. Article CAS PubMed PubMed Central
Google Scholar * Belisle JA, Horibata S, Jennifer GA, Petrie S, Kapur A, Andre S, et al. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes.
Mol Cancer. 2010;9:118. Article PubMed PubMed Central Google Scholar * Lee JW, Komar CA, Bengsch F, Graham K, Beatty GL. Genetically engineered mouse models of pancreatic cancer: The KPC
Model (LSL-Kras(G12D/+);LSL-Trp53(R172H/+);Pdx-1-Cre), its variants, and their application in immuno-oncology drug discovery. Curr Protoc Pharm. 2016;73:14.39.1–14.39.20. Article Google
Scholar * Nimmakayala RK, Leon F, Rachagani S, Rauth S, Nallasamy P, Marimuthu S, et al. Metabolic programming of distinct cancer stem cells promotes metastasis of pancreatic ductal
adenocarcinoma. Oncogene 2021;40:215–31. Article CAS PubMed Google Scholar * Chugh S, Barkeer S, Rachagani S, Nimmakayala RK, Perumal N, Pothuraju R, et al. Disruption of C1galt1 gene
promotes development and metastasis of pancreatic adenocarcinomas in mice. Gastroenterology 2018;155:1608–24. Article CAS PubMed Google Scholar * Hingorani SR, Wang L, Multani AS, Combs
C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell.
2005;7:469–83. Article CAS PubMed Google Scholar * Lakshmanan I, Seshacharyulu P, Haridas D, Rachagani S, Gupta S, Joshi S, et al. Novel HER3/MUC4 oncogenic signaling aggravates the
tumorigenic phenotypes of pancreatic cancer cells. Oncotarget 2015;6:21085–99. Article PubMed PubMed Central Google Scholar * Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, et
al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–38. Article CAS PubMed Google Scholar * Mallya K, Haridas D, Seshacharyulu P, Pothuraju R, Junker WM,
Krishn SR, et al. Acinar transformed ductal cells exhibit differential mucin expression in a tamoxifen-induced pancreatic ductal adenocarcinoma mouse model. Biol Open. 2020;9:bio052878. *
Cheon DJ, Wang Y, Deng JM, Lu Z, Xiao L, Chen CM, et al. CA125/MUC16 is dispensable for mouse development and reproduction. PLoS One. 2009;4:e4675. Article PubMed PubMed Central Google
Scholar * Kaushik G, Seshacharyulu P, Rauth S, Nallasamy P, Rachagani S, Nimmakayala RK, et al. Selective inhibition of stemness through EGFR/FOXA2/SOX9 axis reduces pancreatic cancer
metastasis. Oncogene 2021;40:848–62. Article CAS PubMed Google Scholar * Rauth S, Karmakar S, Batra SK, Ponnusamy MP. Recent advances in organoid development and applications in disease
modeling. Biochim Biophys Acta Rev Cancer. 2021;1875:188527. Article CAS PubMed PubMed Central Google Scholar * Lakshmanan I, Rachagani S, Hauke R, Krishn SR, Paknikar S, Seshacharyulu
P, et al. MUC5AC interactions with integrin β4 enhances the migration of lung cancer cells through FAK signaling. Oncogene 2016;35:4112–21. Article CAS PubMed PubMed Central Google
Scholar * Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp
Med. 2017;214:579–96. Article PubMed PubMed Central Google Scholar * Li G, Kim YJ, Mantel C, Broxmeyer HE. P-selectin enhances generation of CD14+CD16+ dendritic-like cells and inhibits
macrophage maturation from human peripheral blood monocytes. J Immunol. 2003;171:669–77. Article CAS PubMed Google Scholar * Lennon S, Oweida A, Milner D, Phan AV, Bhatia S, Van Court B,
et al. Pancreatic tumor microenvironment modulation by EphB4-ephrinB2 inhibition and radiation combination. Clin Cancer Res. 2019;25:3352–65. Article CAS PubMed PubMed Central Google
Scholar * Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet 2020;395:2008–20. Article CAS PubMed Google Scholar * Das S, Majhi PD, Al-Mugotir MH, Rachagani S, Sorgen
P, Batra SK. Membrane proximal ectodomain cleavage of MUC16 occurs in the acidifying Golgi/post-Golgi compartments. Sci Rep. 2015;5:9759. Article CAS PubMed PubMed Central Google Scholar
* Marimuthu S, Lakshmanan I, Muniyan S, Gautam SK, Nimmakayala RK, Rauth S, et al. MUC16 promotes liver metastasis of pancreatic ductal adenocarcinoma by upregulating NRP2-associated cell
adhesion. Mol Cancer Res. 2022;20:1208–21. Article CAS PubMed PubMed Central Google Scholar * Nallasamy P, Nimmakayala RK, Karmakar S, Leon F, Seshacharyulu P, Lakshmanan I, et al.
Pancreatic tumor microenvironment factor promotes cancer stemness via SPP1-CD44 axis. Gastroenterology 2021;161:1998–2013.e7. Article CAS PubMed Google Scholar * Sperb N, Tsesmelis M,
Wirth T. Crosstalk between tumor and stromal cells in pancreatic ductal adenocarcinoma. Int J Mol Sci. 2020;21:5486. * Connor AA, Denroche RE, Jang GH, Lemire M, Zhang A, Chan-Seng-Yue M, et
al. Integration of genomic and transcriptional features in pancreatic cancer reveals increased cell cycle progression in metastases. Cancer Cell. 2019;35:267–82.e7. Article CAS PubMed
PubMed Central Google Scholar * Alistar A, Morris BB, Desnoyer R, Klepin HD, Hosseinzadeh K, Clark C, et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with
modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017;18:770–8. Article CAS PubMed PubMed
Central Google Scholar * Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, et al. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine versus
Nab-Paclitaxel/Gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2018;36:359–66. Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS We thank Dr. Richard R. Behringer, Department of Genetics, Division of Basic Science Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA, for
providing the Muc16 whole-body knockout (C57BL/6J) mice. We thank Corinn Grabow for all the technical support. We thank the University of Nebraska Medical Center core facilities such as
Advanced Microscopy Core Facility, Genomics Core Facility, and Tissue Science Facility. This work was, in parts, supported by the support from the National Institutes of Health P01 CA217798,
R01 CA263575, R01 CA256973, R01 CA273349, R01 CA247471, R01 CA254036, R01 CA206444, R01 CA210637, R01 CA228524, F99 CA234962, U01 CA200466, and U01 CA210240, and the Nebraska Department of
Health and Human Services LB595, US Department of Veterans Affairs (I01 BX004676). AUTHOR INFORMATION Author notes * These authors contributed equally: Imayavaramban Lakshmanan,
Saravanakumar Marimuthu. AUTHORS AND AFFILIATIONS * Department of Biochemistry and Molecular Biology, University of Nebraska Medical Center, Omaha, NE, 68198-5870, USA Imayavaramban
Lakshmanan, Saravanakumar Marimuthu, Sanjib Chaudhary, Parthasarathy Seshacharyulu, Satyanarayana Rachagani, Sakthivel Muniyan, Ramakanth Chirravuri-Venkata, Pranita Atri, Sanchita Rauth,
Rama Krishna Nimmakayala, Jawed Akhtar Siddiqui, Shailendra K. Gautam, Ashu Shah, Gopalakrishnan Natarajan, Seema Parte, Namita Bhyravbhatla, Kavita Mallya, Dhanya Haridas, Sushil Kumar,
Maneesh Jain, Moorthy P. Ponnusamy & Surinder K. Batra * Department of Pathology and Microbiology, University of Nebraska Medical Center, Omaha, NE, 68198-5900, USA Geoffrey A. Talmon *
Department of Biostatistics, College of Public Health, University of Nebraska Medical Center, Omaha, NE, 68198-4375, USA Lynette M. Smith * Fred & Pamela Buffett Cancer Center,
University of Nebraska Medical Center, Omaha, NE, 68198-5870, USA Apar Kishor Ganti, Maneesh Jain, Moorthy P. Ponnusamy & Surinder K. Batra * Division of Oncology-Hematology, Department
of Internal Medicine, VA Nebraska Western Iowa Health Care System, and University of Nebraska Medical Center, Omaha, NE, 68105-1850, USA Apar Kishor Ganti * Eppley Institute for Research in
Cancer and Allied Diseases, University of Nebraska Medical Center, Omaha, NE, 68198-5870, USA Surinder K. Batra Authors * Imayavaramban Lakshmanan View author publications You can also
search for this author inPubMed Google Scholar * Saravanakumar Marimuthu View author publications You can also search for this author inPubMed Google Scholar * Sanjib Chaudhary View author
publications You can also search for this author inPubMed Google Scholar * Parthasarathy Seshacharyulu View author publications You can also search for this author inPubMed Google Scholar *
Satyanarayana Rachagani View author publications You can also search for this author inPubMed Google Scholar * Sakthivel Muniyan View author publications You can also search for this author
inPubMed Google Scholar * Ramakanth Chirravuri-Venkata View author publications You can also search for this author inPubMed Google Scholar * Pranita Atri View author publications You can
also search for this author inPubMed Google Scholar * Sanchita Rauth View author publications You can also search for this author inPubMed Google Scholar * Rama Krishna Nimmakayala View
author publications You can also search for this author inPubMed Google Scholar * Jawed Akhtar Siddiqui View author publications You can also search for this author inPubMed Google Scholar *
Shailendra K. Gautam View author publications You can also search for this author inPubMed Google Scholar * Ashu Shah View author publications You can also search for this author inPubMed
Google Scholar * Gopalakrishnan Natarajan View author publications You can also search for this author inPubMed Google Scholar * Seema Parte View author publications You can also search for
this author inPubMed Google Scholar * Namita Bhyravbhatla View author publications You can also search for this author inPubMed Google Scholar * Kavita Mallya View author publications You
can also search for this author inPubMed Google Scholar * Dhanya Haridas View author publications You can also search for this author inPubMed Google Scholar * Geoffrey A. Talmon View author
publications You can also search for this author inPubMed Google Scholar * Lynette M. Smith View author publications You can also search for this author inPubMed Google Scholar * Sushil
Kumar View author publications You can also search for this author inPubMed Google Scholar * Apar Kishor Ganti View author publications You can also search for this author inPubMed Google
Scholar * Maneesh Jain View author publications You can also search for this author inPubMed Google Scholar * Moorthy P. Ponnusamy View author publications You can also search for this
author inPubMed Google Scholar * Surinder K. Batra View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS IL, MPP, and SKB conceived and designed
the experiments. IL and SM performed the experiments. IL, SM, PS, and SC assisted with in vivo experiments. Data were collected and analyzed by IL, SM, SC, PS, SR, SM, RCV, PA, SR, RKN, JAS,
SKG, AS, GN, SP, NB, KM, DH, GAT, LMS, SK, AKG, MJ, MPP. The manuscript was written by IL and SM with input from MPP, MJ, and SKB and reviewed by all authors. Statistical analysis and IHC
scoring were done by LMS and GAT, respectively. All authors have read and approved the final manuscript. CORRESPONDING AUTHORS Correspondence to Moorthy P. Ponnusamy or Surinder K. Batra.
ETHICS DECLARATIONS COMPETING INTERESTS SKB is one of the co-founders of Sanguine. Diagnostics and Therapeutics, Inc. The other authors declare no potential conflicts of interest. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION SUPPLIMENTORY FIGURES 1 SUPPLIMENTORY FIGURES 2 SUPPLIMENTORY FIGURES 3 SUPPLIMENTORY FIGURES 4 SUPPLEMENTARY TABLE 1 RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g.
a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript
version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lakshmanan, I.,
Marimuthu, S., Chaudhary, S. _et al._ Muc16 depletion diminishes KRAS-induced tumorigenesis and metastasis by altering tumor microenvironment factors in pancreatic ductal adenocarcinoma.
_Oncogene_ 41, 5147–5159 (2022). https://doi.org/10.1038/s41388-022-02493-6 Download citation * Received: 28 April 2022 * Revised: 23 September 2022 * Accepted: 28 September 2022 *
Published: 21 October 2022 * Issue Date: 25 November 2022 * DOI: https://doi.org/10.1038/s41388-022-02493-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative