The HSF1/miR-135b-5p axis induces protective autophagy to promote oxaliplatin resistance through the MUL1/ULK1 pathway in colorectal cancer
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Oxaliplatin (oxa) is widely used in the treatment of colorectal cancer (CRC), but the development of oxaliplatin resistance is a major obstacle to the therapeutic efficacy in patients.
MicroRNAs (miRNAs), endogenous noncoding RNAs measuring between 22 and 24 nucleotides, have been shown to be involved in the development of CRC drug resistance. However, the mechanism by
which differentially expressed miRNAs induce chemotherapy resistance in CRC has not been fully elucidated to date. Here, we showed the differentially expressed miRNAs in
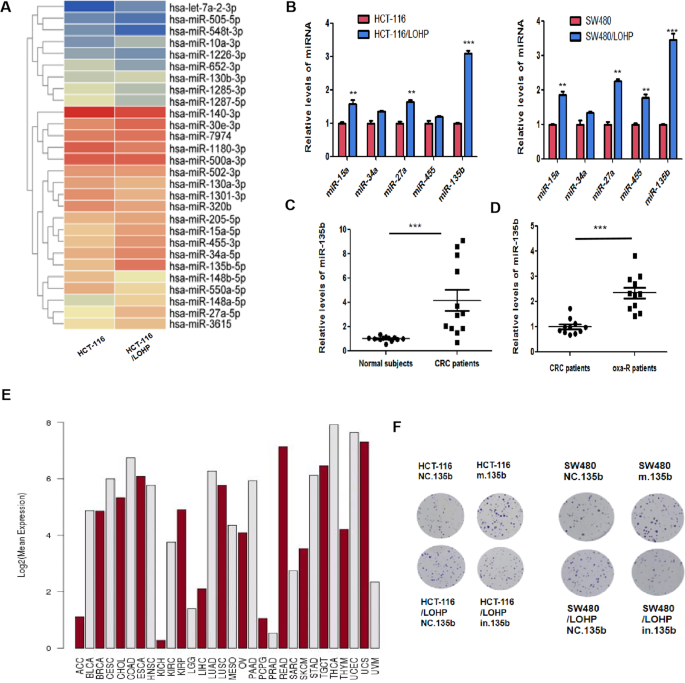
oxaliplatin-sensitive and oxaliplatin-resistant CRC cells through miRNA microarray technology and found that miR-135b-5p was significantly increased in oxaliplatin-resistant cells. And
miR-135b-5p was increased in the serum of colorectal cancer patients. More importantly, the miR-135b-5p level in the serum of oxaliplatin-resistant patients was further increased compared to
that of oxaliplatin-sensitive patients. Recent studies have shown that protective autophagy is an important mechanism that promotes drug resistance in tumors. The potential role of
miR-135b-5p in inducing protective autophagy and promoting oxaliplatin resistance was evaluated in two stable oxaliplatin-resistant CRC cell lines and their parental cells. We further
identified MUL1 as a direct downstream target of miR-135b-5p and showed that MUL1 could degrade the key molecule of autophagy, ULK1, through ubiquitination. Mouse xenograft models were
adopted to evaluate the correlation between miR-135b-5p and oxaliplatin-induced autophagy in vivo. Furthermore, we also investigated the regulatory factors for the upregulation of
miR-135b-5p in CRC cells under oxaliplatin chemotoxicity. These results indicated that miR-135b-5p upregulation in colorectal cancer could induce protective autophagy through the MUL1/ULK1
signaling pathway and promote oxaliplatin resistance. Targeting miR-135b-5p may provide a new treatment strategy for reversing oxaliplatin resistance in CRC.
This work was supported by grants from the National Natural Science Foundation of China (Nos. 82072664, 81772629, 81802363, 81702431, 81772843, 81974374) and the Demonstrative Research
Platform of Clinical Evaluation Technology for New Anticancer Drugs (No. 2018ZX09201015). This work was also supported by the Tianjin Science Foundation (Nos. 18JCQNJC81900, 18JCYBJC92000,
18JCYBJC25400, 18JCYBJC92900, 20JCYBJC00100) and the Science & Technology Development Fund of the Tianjin Education Commission for Higher Education (2018KJ046, 2017KJ227). The funders had no
role in the study design, the data collection and analysis, the interpretation of the data, the writing of the report, and the decision to submit this article for publication.
These authors contributed equally: Huiya Wang, Xia Wang Haiyang Zhang.
Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin’s Clinical Research
Center for Cancer, Tianjin, China
Huiya Wang, Xia Wang, Haiyang Zhang, Ting Deng, Rui Liu, Ying Liu, Hongli Li, Ming Bai, Tao Ning, Junyi Wang, Shaohua Ge & Yi Ba
HW, XW and HZ performed most of the experiments, analyzed the data, and wrote the manuscript. TD, RL, YL and HL reviewed and edited the manuscript. MB, TN, JW, GS performed some of the
experiments. YB designed the experiments and is the guarantor of this work, had full access to all of the data in the study, takes responsibility for the integrity of the data and the
accuracy of the data analysis.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anyone you share the following link with will be able to read this content: