The effect of methylphenidate on social cognition and oxytocin in children with attention deficit hyperactivity disorder
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The current study aimed to explore the possible effect of stimulants on oxytocin (OT), a neuropeptide which regulates social behavior, as a mediator of the pro-social effect of
methylphenidate (MPH) in children with attention deficit hyperactivity disorder (ADHD) compared to healthy controls (HCs). Utilizing a double-blind placebo-controlled design, we compared the
performance of 50 children with ADHD and 40 HCs in “theory of mind” (ToM) tasks and examined the effect of a single dose of MPH/placebo on ToM and salivary OT levels in children with ADHD
at baseline and following an interpersonal interaction. Children with ADHD displayed significantly poorer ToM performance; however, following MPH administration, their performance normalized
and differences between children with ADHD and HC were no longer found. Salivary OT levels at baseline did not differ between children with ADHD and HCs. However, after a parent–child
interaction, OT levels were significantly higher in the HC group compared to children with ADHD. Administration of MPH attenuated this difference such that after parent–child interaction
differences in OT levels between children with ADHD and HC were no longer found. In the ADHD group, OT levels decreased from administration of placebo to the parent–child interaction.
However, the administration of MPH to children with ADHD was associated with an increase in OT levels after the parent–child interaction. We conclude that OT might play a role as a mediator
of social deficits in children with ADHD and that the reactivity of the OT system to social interaction in children with ADHD might be impaired. Stimulants may improve ToM and social
functions in children with ADHD via its impact on the OT system. PRS: OT and Social Cognition in Children with ADHD: Impact of MPH. You have full access to this article via your institution.
Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS MEASURING POSITIVE MENTAL HEALTH IN CHILDREN WITH ATTENTION-DEFICIT/HYPERACTIVE DISORDER Article Open access 29 April 2025 SLEEP AND
INTERNALIZING PROBLEMS IN PRIMARY SCHOOL CHILDREN WITH ATTENTION-DEFICIT HYPERACTIVITY DISORDER Article 18 April 2024 ADDITIVE EFFECTS OF EEG NEUROFEEDBACK ON MEDICATIONS FOR ADHD: A
SYSTEMATIC REVIEW AND META-ANALYSIS Article Open access 27 November 2022 INTRODUCTION Attention deficit hyperactivity disorder (ADHD), a neurodevelopmental disorder affecting ~7% of children
and adolescents [1], is associated with considerable impairment in social functioning [2, 3]. Compared to healthy children, children with ADHD suffer more social rejection and difficulty
forming reciprocal relationships [4]. Deficits in interpersonal functioning in children with ADHD have been attributed in part to impairments in performance of tasks related to theory of
mind (ToM). ToM refers to the ability to attribute mental states, beliefs, and intentions to self and others [5, 6]. For example, some studies have shown children with ADHD having deficits
in the ability to recognize facial expressions [7, 8], whereas other studies have found impairments in first- and second-order ToM tests [9, 10]. Evidence also points to impairments in
empathic functions in children with ADHD [11, 12]. However, these studies have some notable limitations, including small sample sizes and high percentages of comorbid disruptive disorders.
An individual’s ToM abilities depend, at least partly, on the integrity of the dopaminergic and serotonergic systems as well as on their interaction with other neurotransmitters and
neurohormones [5]. Oxytocin (OT) is a neuropeptide that underpins the formation and quality of social relationships, the expression of closeness, and the recognition of affect in the facial
expressions of others [13]. Oxytocin has been hypothesized to increase the salience of social signals by modulating attention-orienting responses to external contextual social cues [13]. Its
secretion increases in response to interpersonal interactions [13, 14]. Research has shown associations between peripheral levels of OT, as measured in blood or saliva, and the quality of
social relationships and affiliative behaviors in healthy individuals as well as in patients suffering from a range of mental disorders [14]. Oxytocin reciprocally interacts with
dopaminergic neurons in the mesolimbic tract [15] and such links serve as the basis for the formation of social attachments via neurons that express both OT and DA [16]. Anatomical and
immunocytochemical studies have found that neuronal fibers and receptor binding-sites of OT and dopamine are located in the same areas in the central nervous system (CNS), often in very
close proximity to one another [15]. Oxytocin-secreting cells in the hypothalamus carry dopamine receptors. Indeed, patients with mental disorders associated with the dysregulation of
dopamine (e.g., autistic spectrum disorders, schizophrenia, depression) exhibit changes in CNS and peripheral OT levels [15]. Given that injury in dopaminergic transporters and receptors is
a central component in the etiology of ADHD [17], it is possible that the OT system also plays a role as a mediator of social deficits, particularly ToM impairment, in children with ADHD. To
date, only a few studies have shown decreased OT levels in children with ADHD compared to healthy controls. These studies found a negative correlation between serum OT levels and total ADHD
total scores, and especially aggression scores, and a positive correlation between serum OT levels and empathy scores in patients with ADHD [18, 19]. However, in both studies, only baseline
OT levels were assessed, while changes in OT levels following an interpersonal interaction were not measured. This is an important omission, as social abilities are dynamic and
interaction-related, and the reactivity of the OT system to interpersonal interaction may be highly relevant to understanding the social difficulties in ADHD. Stimulants reduce negative
social interactions and improve social functioning in children with ADHD [20, 21] and also improve empathy scores [22]. In a previous study, we showed that a single dose of methylphenidate
(MPH) improved the performance of children with ADHD on ToM tests [23]. To date, no study examined the potential effect of stimulants on OT levels in children with ADHD. This is a pivotal
issue in understanding the neurobiological underpinnings of improvement in social cognition among children with ADHD who are treated with stimulants, given that OT might have a role in
mediating this improvement. In the current study we utilized a double-blind placebo-controlled design to test the effects of stimulants on socio-cognitive abilities in children with ADHD and
the role of the OT system in this effect. We hypothesized that dysfunction in the OT system may account for the social difficulties of children suffering from ADHD and that the dynamics in
the OT system may explain the pro-social effect of stimulants on these children. Thus, the objectives of the current study were (1) to compare ToM abilities and salivary OT levels between
children with ADHD and healthy controls (HCs), and (2) to examine the effect of a single dose of MPH on ToM and salivary OT levels in children with ADHD following an interpersonal
interaction. METHODS SUBJECTS We recruited fifty children (28 males, 22 females; 88% children of married couples) aged 6–12 diagnosed with ADHD and 40 HCs (22 males, 18 females; 85% children
of married couples) were recruited. Patients were recruited from the ADHD clinic of the Shalvata Mental Health Center and from Tel-Aviv University School of Medicine. The HC subjects were
recruited from the community via the internet and social media. ADHD was diagnosed by child and adolescent psychiatrists using the Diagnostic and Statistical Manual of Mental Disorders,
fourth or fifth edition (DSM-IV-TR and DSM-5) [24, 25]. We excluded children with past or current affective disorders, psychosis, substance abuse disorder, conduct disorder or any medical or
neurological condition or medication use that might affect the child’s participation in the study, including all psychotropic medications. We also excluded children who had a first-degree
relative with a major psychiatric diagnosis. Inclusion criteria for the control group were the same as those of the ADHD group but with no diagnosis of ADHD or history of a first-degree
relative with ADHD. Participants were reimbursed for their expanses and received a small gift for participation. The IRB of the Shalvata Mental Health Center approved the study. Both parents
of all participants signed a consent form and the children gave their consent verbally. PROCEDURE Apart from the initial clinical assessment at the clinic, all assessments were performed in
the children’s homes. Children with ADHD participated in two sessions: one session an hour after taking a short-acting MPH (in an adjusted dosage of 0.3–0.5 mg/kg) and one session an hour
after taking a placebo (PLC). Children routinely prescribed with MPH treatment were asked not to take the medicine 48 h before the examination, since the clinical effect of the long-acting
MPH is no longer than 12 h [26]. The study was randomized-controlled, such that children were assigned to the sessions randomly in a double-blind manner. Each session lasted about 60–90 min.
In order to reduce a possible learning effect of the computerized tasks (to be elaborated upon below), the sessions were performed at least two weeks apart. Control subjects participated in
only one session and did not take any medication. Parents completed questionnaires regarding demographics and general information about the child’s academic and social functioning. In
addition, parents completed the Swanson, Nolan and Pelham Questionnaire-IV (SNAP-IV) [27]. This instrument contains subscales for inattention, hyperactive/impulsive behavior, and
oppositional behavior. Parents also filled out the Strengths and Difficulties Questionnaire (SDQ) [28], a screening inventory composed of five distinct dimensions: conduct problems,
emotional symptoms, hyperactivity, peer problems, and pro-social behavior. Intelligence was measured using the similarities subtest from the Wechsler Intelligence Scale for Children
(WISC-IV) [29]. Self-reported anxiety was measured using the State-Trait Anxiety Inventory (STAI) [30], a 40-item questionnaire scored by a Likert scale. State anxiety represents a transient
emotional status that results from situational stress; trait anxiety represents a predisposition to react with anxiety in stressful situations. We measured ToM performance using the ToM
test [31], which consists of vignettes, stories, and drawings about which the child has to answer a number of questions. Results are given on three subscales: ToM1—precursors of ToM (i.e.,
recognition of emotions); ToM2—first manifestations of a real ToM (first-order belief, understanding of false belief); and ToM3—more advanced aspects of ToM (second-order belief,
understanding of humor). A second ToM task was the Faux Pas Recognition task (FPR), designed by Baron-Cohen et al. [32]. This task is designated to evaluate the ability of participants to
recognize social “faux pas”—social situations in which a speaker says something without understanding that there might be a difference between his/her state of knowledge and that of the
listener (“cognitive” ToM), and should recognize the potential emotional impact of a statement on the listener (“affective” ToM) [33]. At each session, participants were given 10 short
stories, five of which contained faux pas situations to be identified. After hearing every story, participants were asked ToM questions. The score consisted of the total number of correct
identifications of faux pas situations. The Hebrew version of the FPR was employed after validation by a group of normative subjects [34]. Executive functions (EF) and attention were tested
via the cognition module in the NIH Toolbox for the Assessment of Neurological and Behavioral Function (NIH-TB) [35]. We used the Dimensional Change Card Sort Test (DCCS) and the Flanker
Inhibitory Control and Attention Test, which measure cognitive flexibility and inhibitory control, respectively [36]. In order to ensure maximal effect of the drug, cognitive tests were
performed at least 60 min after the administration of MPH/PLC. Salivary OT levels were measured at three time points. The first time point was at the beginning of each session (“T1”). The
second was 40 min after the administration of MPH/PLC (“T2”; only for the ADHD group), in order to assess the effect of MPH/PLC on OT levels, regardless of social interaction. Since studies
show that changes in central salivary OT levels are best reflected after 15 min when measured by peripheral OT [14], the third OT measurement (“T3”) was set to 15 min after a “positive
social interaction” in which the child and the parent were asked to plan a “fun day” that would include both of them, and to talk about it for 5 min. The parent–child interaction occurred
after the cognitive tasks (i.e., around 75 min after the administration of MPH/PLC). Interactions were performed with participants’ mothers in 88 and 85% of the encounters of ADHD patients
and HC, respectively. Other interactions were with the children’s fathers. Participants were asked to avoid drinking and eating an hour before the test and to avoid caffeine 3 h before the
test. Saliva samples were collected by passive drool into designated tube at each of the three time points. In order to precipitate the mucus, samples underwent four freeze-thaw cycles:
freeze at −70 °C and thaw at 4 °C. After the forth cycle the tubes were centrifuged twice at 4000 rpm for 30 min. Supernatants were collected and stored at −20 °C until assayed.
Determination of OT from saliva samples was performed using a commercial OT ELISA kit (ENZO, NY, USA). Measurements were performed in duplicate according to the manufacturer’s instructions.
The concentrations of samples were calculated using MatLab-7 according to relevant standard curves. The intra-assay and inter-assay coefficients of samples were 14.7 and 22.7 percent,
respectively. The intra-assay and inter-assay coefficients of controls were 4.9 and 13.2 percent, respectively. STATISTICAL ANALYSIS Demographic and clinical comparisons between groups
(ADHD, HCs) were performed using independent t-tests with group as a between-subjects factor, and _χ_2 tests or Fisher’s exact tests, as appropriate. Pearson correlations were used to assess
correlations between OT and SNAP scores or performance in FPR. All tests were two-sided with a significance level set at 0.05. The ideal study design to test our hypothesis would have been
2 (PLC, MPH) X 2 (ADHD, HC). However, the effects of psychostimulants in control subjects could not be tested due to ethical limitations. Therefore, we took a between-subjects approach, to
examine the differences between: (1) HCs and children with ADHD who took PLC and (2) HCs and patients with ADHD who took MPH. We also took a within-subjects approach to examine the effect of
MPH on the same variables. THE EFFECT OF MPH ON SALIVARY OT LEVELS Given that the design of the study was mixed, consisting of a between-groups comparison and a within-group comparison, we
applied two strategies for data analysis. First, we used independent sample _t_-tests (with the Bonferroni correction) to compare OT levels between the ADHD group and the HCs. Then, we
carried out two-way repeated measures ANOVA with time (T1, T2, T3) and drug (MPH/PLC) as within-subjects’ factors to examine drug effects on OT levels in the ADHD group. To assess the effect
of order of drug administration (MPH/PLC first), we carried out the same analysis with order as a covariant (while the effect of order was kept fixed). Appropriate follow-up analyses using
paired t-tests were used to examine differences in the mean score of each of the three measurements of OT. It should be noted that for the ADHD group, three saliva samples were collected at
each session, with a total of six samples for each participant. Only twelve participants produced enough saliva for analyses of all six samples, and the repeated measures ANOVA included only
these subjects. There were no between-group differences in cognitive performance or ToM tests between children that produced enough saliva for analyses of all six samples and children for
whom we could only use part of the samples. Given the small sample size, we repeated the follow-up t-tests comparing each of the two time-points with all subjects who produced enough saliva
for these two time-points (e.g., 28 children with ADHD who produced enough saliva for measurements of T2 and T3). For each of the HCs, saliva samples were collected twice (T1 and T3);
therefore, we carried out paired t-tests to examine drug effects on OT levels of control subjects. THE EFFECT OF MPH ON TOM To examine between-group differences in ToM and FPR, we performed
the same set of analyses as described above (for OT level). To examine within-group effects for the ToM task, we conducted two-way repeated measures ANOVA with drug (MPH/PLC) and with ToM
type (ToM1, ToM2, ToM3) as within-subject factors. To examine within-group effects for the FPR, we conducted the same analysis but with story type (control, faux-pas) as a between-subject
factor. To assess the effect of order of drug administration, we repeated the same analysis with order as a covariant (while the effect of order was kept fixed). RESULTS Table 1 presents
between-group comparisons of demographic and clinical parameters. As shown, parents of HCs had significantly higher incomes. Participants with ADHD had fewer friends and showed significantly
lower achievements on academic and behavioral measures. Children with ADHD showed significantly higher scores on SDQ scales of overall stress, emotional distress, behavioral difficulties,
and hyperactivity, and lower scores on pro-social behavior. Children with ADHD had slightly lower scores on the similarities subtest of WISC (mean score of 9.40 ± 2.84 compared to 10.62 ±
1.68 in the control group, _P_-value = 0.02). There were no between-group differences in the cognitive tests between children with ADHD taking PLC and HC. However, following the
administration of MPH, children with ADHD significantly outperformed the HCs in the Flanker task. TOM As shown in Table 2, there was a significant difference in the ToM1 and ToM2 subscales
of the ToM test between children with ADHD and HCs (all _p_-values ≤ 0.001). These differences remained significant after controlling for between-group IQs and parental income differences.
Differences in ToM disappeared after the administration of MPH to children with ADHD. There was also a significant difference in the control stories of the FPR between children with ADHD and
HCs (mean scores of 3.11 and 3.97, _p_-value > 0.01), which also disappeared following the administration of MPH. We did not correct the FPR analysis for between-group differences in
WISC as a covariant, given that IQ was not significantly correlated with FPR measurements (as opposed to the ToM test). Within the group of patients with ADHD, the two-way repeated measures
ANOVAs revealed significant main effects for drug in both tasks (_F_(1,41) = 4.37, _p_ = 0.043 for the ToM task and _F_(1,41) = 6.29, _p_ = 0.016 for the FPR), and non-significant ToM type
by drug interaction, suggesting that the improvement in ToM following the administration of MPH was not a function of ToM type (in the ToM task) or story type (in the FPR). We then repeated
the analysis with order as a covariant which yielded the same pattern of results. OT There were no between-group (ADHD/HCs) differences in baseline salivary OT level concentrations. However,
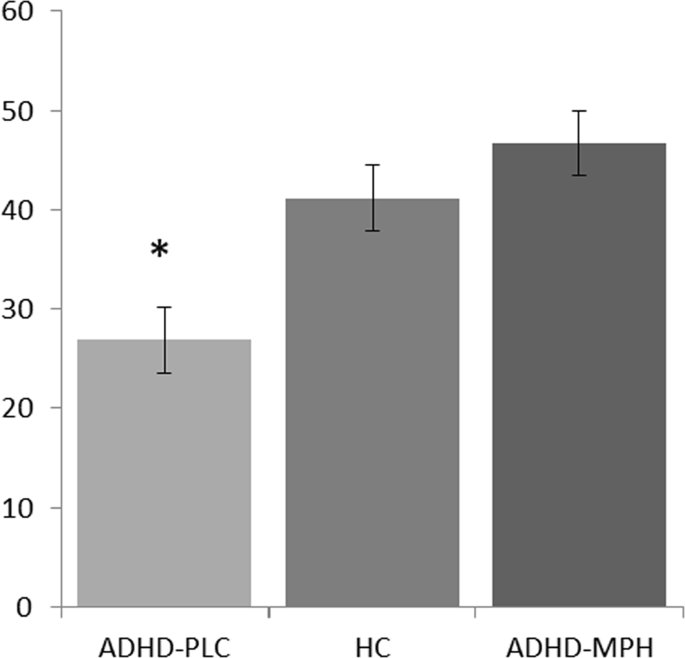
at the third time point, 15 min after the parent–child interaction, OT levels were significantly higher in HCs compared to children with ADHD who took PLC (41.24 pg/mL vs. 26.86 pg/mL,
_t_(60) = −2.359, _p_-value = 0.022). Interestingly, following the administration of MPH there was no difference in salivary OT levels between HCs and children with ADHD 15 min after the
parent–child interaction, _t_(63) = 0.776, _p_ = 0.441, (see Fig. 1). These findings gained support from the within-subjects analysis: the two-way repeated measures ANOVA revealed no
significant main effects for OT or time. Importantly, a significant drugXtime interaction was found (which remained significant after controlling for the effect of order, _F_(2,20) = 4.70,
_p_ = 0.021, _η__p_2 = 0.320). To examine the source of the interaction, we carried out follow-up paired samples _t_-tests which revealed that within the group of children with ADHD,
salivary OT levels were not significantly changed at T2 following the administration of either MPH or PLC. However, within the group of children with ADHD, OT levels decreased between T2 to
the T3 following the administration of PLC (_t_(11) = 2.266, _p_ = 0.045, _η__p_2 = 0.318), but not following the administration of MPH (see Fig. 2). As noted above, we repeated the
follow-up analyses using a larger sample size, and the same pattern of results was revealed (significant decrease in OT levels between T2 and T3 following the administration of PLC (_t_(26)
= 2.444, _p_ = 0.022, _η__p_2 = 0.187), but not MPH). These findings may suggest that although children with ADHD have social interactions generally associated with a decrease in OT levels,
MPH may act to maintain the reactivity of the OT system levels following a social interaction. It is important to note that in the HC group there were no differences in OT levels at baseline
and 15 min after a “positive social interaction.” DISCUSSION The goals of our study were to compare ToM abilities between children with ADHD and HCs and to examine, for the first time, the
effect of a single dose of MPH on ToM performance and salivary OT levels in children with ADHD at baseline and following an interpersonal interaction. The key novel finding of our study is
the possible impairment in OT reactivity to social interactions in children with ADHD and the action of MPH in attenuating this OT impairment. Our findings, therefore, point to the
involvement of the OT system in the socio-cognitive deficits in ADHD and point to a potential mechanism by which stimulants improve social abilities in children with ADHD. In real life,
children with ADHD are more prone to suffering from social difficulties. As expected, the children with ADHD in our study had fewer friends compared with HCs. Indeed, several previous
studies showed that children with ADHD are considered to be more peer-rejected and less popular as compared to healthy children [4, 37, 38]. Also, as expected, the children with ADHD showed
significantly lower achievements on academic and behavioral measures, and significantly higher scores on SDQ scales of overall stress, emotional distress, behavioral difficulties, and
hyperactivity, and lower scores on pro-social behavior. These findings are also in line with previous studies [39, 40]. A main finding of the current study was that children with ADHD had
lower scores on the ToM test and FPR compared with HCs. However, these differences disappeared after the MPH administration. This finding corresponds with clinical reports indicating that
administration of stimulants improves social functioning in children with ADHD [2, 41, 42]. In previous studies [23, 43], we also showed that ToM performance of children with ADHD was
impaired when compared with that of HCs, and was significantly improved following the administration of MPH. One of the goals of the current study was to explore whether improvement in ToM
tasks following the administration of MPH is derived from a direct action of MPH in brain regions associated with reasoning about mental states of others, or whether the improvement is
secondary to enhancement of other cognitive functions required in these tasks, like the participants’ ability to stay concentrated during a continuous task, better attention to specific
details, or a decrease in impulse responding. Surprisingly, in the current study, we found no improvement in EF, as measured by DCCS and the Flanker Inhibitory Control and Attention Test,
following the use of MPH. Possibly, these tests were experienced as very easy for most children and there was a ceiling effect in the performance on both tests. Improvement in ToM might also
have resulted from a direct effect of stimulants on dopaminergic circuits, as ToM abilities have been shown to be supported by dopaminergic pathways and other catecholaminergic systems in
the prefrontal cortex and the striatum [5, 22, 44]. Improvement in FPR might have resulted from improvement in EF [23, 43], and might also derive from a direct action of MPH on brain regions
associated with understanding the mental states of others, such as the bilateral temporal-parietal junction and cortical midline structures (medial prefrontal cortex, adjacent rostral
anterior cingulate cortex, and medial posterior parietal cortices) [45]. More comprehensive studies are warranted to address the yet open question of the effect of stimulants on social
cognition with respect to changes in other cognitive functions (e.g. EF such as working memory and inhibition). Among the key novel findings of our study is the between-group differences in
patterns of baseline salivary OT and reactivity levels. We found no differences between groups in baseline OT. However, consistent with our hypothesis, differences emerged in the dynamics of
the OT response, and OT levels increased following mother–child interaction in the control groups, but OT levels did not increase in the children with ADHD in the placebo condition; those
children showed no elevation in OT in response to mother–child interaction. Across mammalian species, mother–child interaction had been shown to result in central OT release and, in humans,
salivary OT increase was found after parent–child interaction. Notably, administration of a single dose of MPH was able to reverse this disruption and following administration, children’s OT
levels increased in response to interaction with their mothers. This suggests that stimulants may improve the flexibility of the OT system in children with ADHD. To date, only a few studies
have shown decreased OT levels in children with ADHD when compared to HCs, but those studies did not test changes in OT levels following social interactions [18, 19]. Our study shows for
the first time that, in children with ADHD, the reactivity of the OT system to a social interaction might be impaired and that, paradoxically, in this group, levels of OT decreased following
social interactions. Although we did not find any reference to such findings in the literature regarding the population at the heart of the current study, studies of other high-risk
populations show disruptions in OT reactivity to social and affiliative experiences. In one study, a trust-related interaction did not increase OT levels in patients with schizophrenia but
did increase such levels in the control group [46]. In another study, OT levels in children with early life neglect did not increase following physical contact with their mothers, as
compared to OT increase in non-neglected children [47]. Another important finding in the current study was that salivary OT levels decreased between T2 and T3 in children with ADHD after
placebo, but not after MPH, and after MPH administration there was no difference in OT levels between children with ADHD and HCs following a social interaction. These findings suggest that,
while children with ADHD have social interactions generally associated with a decrease in OT levels, MPH may act to maintain the OT levels during social interactions. This finding could be
explained by weak dopaminergic activity in children with ADHD, as studies have shown that both dopamine and OT, and the interaction between them, are needed for a successful social
interaction [15, 48]. As OT reciprocally interacts with dopaminergic pathways, particularly in the formation of the mother–child attachment [16] it is possible that the administration of a
dopaminergic compound has an indirect effect on the central OT system. MPH is known to block dopamine transporters in the striatum. In addition, studies show that it significantly enhances
dopamine activation in bilateral inferior frontal cortex/insula, especially the right inferior frontal cortex [49]. OT receptors are also expressed in the prefrontal cortex and the nucleus
accumbens, which may serve as potential integrative sites for dopamine and OT pathways underlying natural reward circuits and social attachment behaviors [15, 16]. Hence, our findings
provide further evidence for the possibility that OT plays a role as a mediator of social deficits in children with ADHD. Our study is consistent with research in animal models. In a study
conducted on rats, the administration of dopamine alone did not directly induce OT release. However, when the dopamine was administered during the rats’ suckling, there was an acceleration
in the firing rate of oxytocinergic cells, and milk-ejection was facilitated, indicating that the dopamine-controlled OT release during suckling by acting as a neuromodulator rather than as
a neurotransmitter [50]. This finding is in accord with the findings of our study and may explain why OT levels were raised in the MPH group only following the social interaction (T3), but
not directly after MPH administration (T2). Our findings suggest a possible biological explanation for the real-life social difficulties experienced by children with ADHD and for the
mechanism by which MPH improves social functioning in children with ADHD. _Limitation_ The findings of the current study must be viewed considering several limitations. First, with the aim
of achieving high ecological validity, we chose to perform this study in the children’s homes. Hence, results might have been affected by “non-sterile” conditions, such as interruptions of
other family members who were in the home at the time of the examination. Also, OT levels might have been affected by food or drink consumed prior to the examination. However, we did ask
participants to refrain from eating and drinking an hour prior to the examination. Second, only twelve participants produced enough saliva for analyses of all six samples, suggesting that
the findings may be limited by a relatively small sample size. However, the data presented by follow-up tests with the small sample size and by follow-up tests with all subjects who produced
enough saliva for each of the specific two time-points, revealed the same pattern of results. Furthermore, these follow-up analyses showed a large effect size [51]. Third, HC participants
did not take any drug, and the OT levels were measured only twice in this group (T1 and T3). These factors may have masked a potential impact of time or the PLC itself on OT levels. Last, OT
levels were measured in the saliva and not in the CNS. Previous studies assessing the extent to which salivary and central (i.e., CNS) OT concentrations are correlated show mixed results
[52, 53]. In light of this debate, it is important to note that even though OT concentrations in the saliva and blood are highly correlated [14], the former were found to better correlate
with OC concentrations in the cerebrospinal fluid [54]. In conclusion, the current study suggests that the positive effect of MPH on social cognition, as measured by the ToM tests and FPR,
might be mediated, at least in part, by an effect of MPH on the reactivity of the OT system, probably via dopaminergic circuits. This effect might provide a wider understanding of the
neuronal underpinning of impaired social cognition in children with ADHD and the improvement in social cognition associated with the use of stimulants. Future studies are needed to replicate
our findings and to examine whether the effect of a single dose of MPH is similar to continuous use of MPH. The results of this study may be used as the basis for future randomized
controlled trials (RCT) studying the effect of MPH compared to a PLC, and the effect of OT and other neurohormones on ToM functions in larger groups of children. FUNDING AND DISCLOSURE The
study was funded by a grant from the brain and behavior research foundation (NARSAD young investigator grant number 21165). The authors declare no competing interests. CHANGE HISTORY * _ 07
OCTOBER 2019 The original HTML and PDF versions of this Article were updated shortly after publication to correct an author name. _ * _ 09 OCTOBER 2019 A Correction to this paper has been
published: https://doi.org/10.1038/s41386-019-0533-2 _ REFERENCES * Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic
review and meta-analysis. Pediatrics. 2015;135:e994–1001. Article PubMed Google Scholar * Greene RW, Biederman J, Faraone SV, Sienna M, Garcia-Jetton J. Adolescent outcome of boys with
attention-deficit/hyperactivity disorder and social disability: results from a 4-year longitudinal follow-up study. J Consult Clin Psychol. 1997;65:758–67. Article PubMed Google Scholar *
Mash EJ, Barkley RA. Child Psychopathology. 2nd ed. New York: Gilford Press; 2003. * McQuade JD, Hoza B. Peer problems in attention deficit hyperactivity disorder: current status and future
directions. Dev Disabil Res Rev. 2008;14:320–4. Article PubMed Google Scholar * Abu-Akel A, Shamay-Tsoory S. Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia.
2011;49:2971–84. Article PubMed Google Scholar * Uekermann J, Kraemer M, Abdel-Hamid M, Schimmelmann BG, Hebebrand J, Daum I, et al. Social cognition in attention-deficit hyperactivity
disorder (ADHD). Neurosci Biobehav Rev. 2010;34:734–43. Article CAS PubMed Google Scholar * Buhler E, Bachmann C, Goyert H, Heinzel-Gutenbrunner M, Kamp-Becker I. Differential diagnosis
of autism spectrum disorder and attention deficit hyperactivity disorder by means of inhibitory control and “theory of mind. J Autism Dev Disord. 2011;41:1718–26. Article PubMed Google
Scholar * Da Fonseca D, Seguier V, Santos A, Poinso F, Deruelle C. Emotion understanding in children with ADHD. Child Psychiatry Hum Dev. 2009;40:111–21. Article PubMed Google Scholar *
Buitelaar JK, van der Wees M, Swaab-Barneveld H, van der Gaag RJ. Theory of mind and emotion-recognition functioning in autistic spectrum disorders and in psychiatric control and normal
children. Dev Psychopathol. 1999;11:39–58. Article CAS PubMed Google Scholar * Sodian B, Hulsken C, Thoermer C. The self and action in theory of mind research. Conscious Cogn.
2003;12:777–82. Article PubMed Google Scholar * Braaten EB, Rosen LA. Self-regulation of affect in attention deficit-hyperactivity disorder (ADHD) and non-ADHD boys: differences in
empathic responding. J Consult Clin Psychol. 2000;68:313–21. Article PubMed Google Scholar * Marton I, Wiener J, Rogers M, Moore C, Tannock R. Empathy and social perspective taking in
children with attention-deficit/hyperactivity disorder. J Abnorm Child Psychol. 2009;37:107–18. Article PubMed Google Scholar * Shamay-Tsoory SG, Abu-Akel A. The social salience
hypothesis of oxytocin. Biol Psychiatry. 2016;79:194–202. Article CAS PubMed Google Scholar * Feldman R. Oxytocin and social affiliation in humans. Horm Behav. 2012;61:380–91. Article
CAS PubMed Google Scholar * Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther.
2010;16:e92–123. Article CAS PubMed PubMed Central Google Scholar * Feldman R. The neurobiology of human attachments. Trends Cogn Sci. 2017;21:80–99. Article PubMed Google Scholar *
Wu J, Xiao H, Sun H, Zou L, Zhu LQ. Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol. 2012;45:605–20. Article CAS PubMed Google Scholar * Sasaki T, Hashimoto
K, Oda Y, Ishima T, Kurata T, Takahashi J, et al. Decreased levels of serum oxytocin in pediatric patients with attention deficit/hyperactivity disorder. Psychiatry Res. 2015;228:746–51.
Article CAS PubMed Google Scholar * Demirci E, Ozmen S, Kilic E, Oztop DB. The relationship between aggression, empathy skills and serum oxytocin levels in male children and adolescents
with attention deficit and hyperactivity disorder. Behav Pharm. 2016;27:681–8. Article CAS Google Scholar * Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The
MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484–500. Article PubMed PubMed Central
Google Scholar * Shaw M, Hodgkins P, Caci H, Young S, Kahle J, Woods AG, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects
of treatment and non-treatment. BMC Med. 2012;10:99. Article PubMed PubMed Central Google Scholar * Golubchik P, Weizman A. The possible effect of methylphenidate treatment on empathy in
children diagnosed with attention-deficit/hyperactivity disorder, both with and without comorbid oppositional defiant disorder. J Child Adolesc Psychopharmacol. 2017.
https://doi.org/10.1089/cap.2016.0111. * Maoz H, Tsviban L, Gvirts HZ, Shamay-Tsoory SG, Levkovitz Y, Watemberg N, et al. Stimulants improve theory of mind in children with attention
deficit/hyperactivity disorder. J Psychopharmacol 2014;28. https://doi.org/10.1177/0269881113492030. * American Psychiatric Association. Diagnostic and Statistical Manual of Mental
Disorders: DSM-IV-TR. 4th ed. Washington, DC: American Psychiatric Association; 2000. * American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th
ed. Washington, DC: American Psychiatric Association; 2013. * Coghill D, Banaschewski T, Zuddas A, Pelaz A, Gagliano A, Doepfner M. Long-acting methylphenidate formulations in the treatment
of attention-deficit/hyperactivity disorder: a systematic review of head-to-head studies. BMC Psychiatry. 2013;13:237. Article PubMed PubMed Central Google Scholar * Swanson JM, Kraemer
HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, et al. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of
treatment. J Am Acad Child Adolesc Psychiatry. 2001;40:168–79. Article CAS PubMed Google Scholar * Goodman R. The strengths and difficulties questionnaire: a research note. J Child
Psychol Psychiatry Allied Discip. 1997;38:581–6. Article CAS Google Scholar * Prifitera A, Saklofske DH, Weiss LG. WISC-IV Clinical Assessment and Intervention. 2nd ed. Amsterdam; Boston:
Elsevier: Academic; 2008. * Spielberger CD, Gorsuch RL, Lushene RE Manual for the State-Trait Anxiety Inventory. Palo Alto: Consult Psychol Press; 1970. * Muris P, Steerneman P, Meesters C,
Merckelbach H, Horselenberg R, van den Hogen T, et al. The TOM test: a new instrument for assessing theory of mind in normal children and children with pervasive developmental disorders. J
Autism Dev Disord. 1999;29:67–80. Article CAS PubMed Google Scholar * Baron-Cohen S, O’Riordan M, Stone V, Jones R, Plaisted K. Recognition of faux pas by normally developing children
and children with Asperger syndrome or high-functioning autism. J Autism Dev Disord. 1999;29:407–18. Article CAS PubMed Google Scholar * Shamay-Tsoory SG, Tomer R, Berger BD,
Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. J Cogn Neurosci. 2003;15:324–37. Article CAS
PubMed Google Scholar * Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cogn
Behav Neurol. 2005;18:55–67. Article CAS PubMed Google Scholar * Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and
behavioral function. Neurology. 2013;80:S2–6. Article PubMed PubMed Central Google Scholar * Zelazo PD. The Dimensional Change Card Sort (DCCS): a method of assessing executive function
in children. Nat Protoc. 2006;1:297–301. Article PubMed Google Scholar * Hoza B, Mrug S, Gerdes AC, Hinshaw SP, Bukowski WM, Gold JA, et al. What aspects of peer relationships are
impaired in children with attention-deficit/hyperactivity disorder? J Consult Clin Psychol. 2005;73:411–23. Article PubMed Google Scholar * Blachman DR, Hinshaw SP. Patterns of friendship
among girls with and without attention-deficit/hyperactivity disorder. J Abnorm Child Psychol. 2002;30:625–40. Article PubMed Google Scholar * Biederman J, Faraone S, Milberger S, Guite
J, Mick E, Chen L, et al. A prospective 4-year follow-up study of attention-deficit hyperactivity and related disorders. Arch Gen Psychiatry. 1996;53:437–46. Article CAS PubMed Google
Scholar * Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. J Pediatr Psychol. 2007;32:643–54. Article PubMed Google Scholar * Biederman J, Spencer TJ.
Psychopharmacological interventions. Child Adolesc Psychiatr Clin N Am. 2008;17:439–58, xi. Article PubMed Google Scholar * Swanson J, Baler RD, Volkow ND. Understanding the effects of
stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology. 2011;36:207–26. Article CAS PubMed Google
Scholar * Maoz H, Gvirts HZ, Sheffer M, Bloch Y Theory of Mind and Empathy in Children With ADHD. J Atten Disord. 2017. https://doi.org/10.1177/1087054717710766. * Lackner CL, Bowman LC,
Sabbagh MA. Dopaminergic functioning and preschoolers’ theory of mind. Neuropsychologia. 2010;48:1767–74. Article PubMed Google Scholar * Mahy CEV, Moses LJ, Pfeifer JH. How and where:
theory-of-mind in the brain. Dev Cogn Neurosci. 2014;9:68–81. Article PubMed PubMed Central Google Scholar * Kei S, Kiss I, Kelemen O. Sharing secrets: Oxytocin and trust in
schizophrenia. Soc Neurosci. 2009;4:287–93. https://doi.org/10.1080/17470910802319710. * Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is
associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci USA. 2005;102:17237–40. * Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions
in the social brain. Trends Cogn Sci. 2009;13:27–35. Article CAS PubMed Google Scholar * Rubia K, Alegria A, Cubillo A, Smith A, Brammer M, Radua J. Effects of stimulants on brain
function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol Psychiatry. 2014;76:616–28. Article CAS PubMed PubMed Central Google Scholar * Moos F,
Richard P. Excitatory effect of dopamine on oxytocin and vasopressin reflex releases in the rat. Brain Res. 1982;241:249–60. Article CAS PubMed Google Scholar * Maher JM1, Markey JC,
Ebert-May D. The other half of the story: effect size analysis in quantitative research. CBE Life Sci Educ. 2013;12:345–51. Article PubMed PubMed Central Google Scholar * Carter CS,
Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, et al. Oxytocin: behavioral associations and potential as a salivary biomarker. Ann N Y Acad Sci. 2007;1098:312–22.
Article CAS PubMed Google Scholar * Valstad M, Alvares GA, Egknud M, Matziorinis AM, Andreassen OA, Westlye LT, et al. The correlation between central and peripheral oxytocin
concentration: a systemic review and meta-analysis. Neurosci Biobehav Rev. 2017;78:117–24. Article CAS PubMed Google Scholar * Martin J, Kagerbauer SM, Gempt J, Podtschaske A,
Hapfelmeier A, Schneider G. Oxytocin levels in saliva correlate better than plasma levels with concentrations in the cerebrospinal fluid of patients in neurocritical care. J Neuroendocrinol.
2018. https://doi.org/10.1111/jne.12596. Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel Orit
Levi-Shachar, Yuval Bloch & Hagai Maoz * Shalvata Mental Health Center, Hod-Hasharon, Israel Orit Levi-Shachar, Yiftach Goldwin, Yuval Bloch & Hagai Maoz * Department of Behavioral
Sciences and Psychology, Ariel University, Ariel, Israel Hila Z. Gvirts * Department of Psychology, Haifa University, Haifa, Israel Simone Shamay-Tsoory * Baruch Ivcher School of Psychology,
Interdisciplinary Center, Herzlia, Israel Orna Zagoory-Sharon & Ruth Feldman Authors * Orit Levi-Shachar View author publications You can also search for this author inPubMed Google
Scholar * Hila Z. Gvirts View author publications You can also search for this author inPubMed Google Scholar * Yiftach Goldwin View author publications You can also search for this author
inPubMed Google Scholar * Yuval Bloch View author publications You can also search for this author inPubMed Google Scholar * Simone Shamay-Tsoory View author publications You can also search
for this author inPubMed Google Scholar * Orna Zagoory-Sharon View author publications You can also search for this author inPubMed Google Scholar * Ruth Feldman View author publications
You can also search for this author inPubMed Google Scholar * Hagai Maoz View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR
Correspondence to Hagai Maoz. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTAL FILE RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Levi-Shachar, O., Gvirts, H.Z., Goldwin, Y. _et al._ The
effect of methylphenidate on social cognition and oxytocin in children with attention deficit hyperactivity disorder. _Neuropsychopharmacol._ 45, 367–373 (2020).
https://doi.org/10.1038/s41386-019-0522-5 Download citation * Received: 18 April 2019 * Revised: 26 August 2019 * Accepted: 03 September 2019 * Published: 12 September 2019 * Issue Date: 02
January 2020 * DOI: https://doi.org/10.1038/s41386-019-0522-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative