Perturbation of the gut microbiome by prevotella spp. Enhances host susceptibility to mucosal inflammation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Diverse microbial signatures within the intestinal microbiota have been associated with intestinal and systemic inflammatory diseases, but whether these candidate microbes actively
modulate host phenotypes or passively expand within the altered microbial ecosystem is frequently not known. Here we demonstrate that colonization of mice with a member of the genus
_Prevotella_, which has been previously associated to colitis in mice, exacerbates intestinal inflammation. Our analysis revealed that _Prevotella intestinalis_ alters composition and
function of the ecosystem resulting in a reduction of short-chain fatty acids, specifically acetate, and consequently a decrease in intestinal IL-18 levels during steady state.
Supplementation of IL-18 to _Prevotella_-colonized mice was sufficient to reduce intestinal inflammation. Hence, we conclude that intestinal _Prevotella_ colonization results in metabolic
changes in the microbiota, which reduce IL-18 production and consequently exacerbate intestinal inflammation, and potential systemic autoimmunity. SIMILAR CONTENT BEING VIEWED BY OTHERS
LONG-DISTANCE RELATIONSHIPS - REGULATION OF SYSTEMIC HOST DEFENSE AGAINST INFECTIONS BY THE GUT MICROBIOTA Article 22 June 2022 STRAIN-SPECIFIC ALTERATIONS IN GUT MICROBIOME AND HOST IMMUNE
RESPONSES ELICITED BY TOLEROGENIC _BIFIDOBACTERIUM PSEUDOLONGUM_ Article Open access 19 January 2023 INTRODUCTION TO HOST MICROBIOME SYMBIOSIS IN HEALTH AND DISEASE Article 09 December 2020
INTRODUCTION Intestinal homeostasis is maintained by the dynamic interplay between the gut microbiota and the host immune system,1 in which multiple cell types including intestinal
epithelial cells (IECs) and goblet cells, serve not only as a passive barrier but also as a source of antimicrobial substances strengthening the barrier.2 Microbiota-derived metabolites
represent important signals that impact both the mucosal immune system and proper epithelial barrier function.3 Alterations in the composition and function of the microbiota have been
associated with a wide range of human disease including inflammatory bowel disease (IBD) and rheumatoid arthritis (RA). In IBD, it has been specifically hypothesized that immune-mediated
pathologies arise from dysregulated immune responses towards the intestinal microbiota,4,5 but different other non-exclusive concepts about how the microbiota promotes IBD and potentially
other autoimmune diseases are debated.6 For instance, an overall loss of microbial diversity, changes in the balance between beneficial commensals and potential pathobionts as well as
changes in microbial metabolites such as short-chain fatty acids (SCFAs) have been reported in diverse patient populations.7,8,9 Strikingly, altered SCFA production also modulates systemic
immune responses linking intestinal dysbiosis and extra-intestinal immunity.10 Still, the exact identity of intestinal bacteria and their metabolites that trigger aberrant host responses and
contribute to the development of IBD and other autoimmune diseases in humans are not exactly known, as the direct causal relationship between microbiota and complex diseases has been
difficult to prove outside animal models. For instance, several studies in humans described associations between IBD and increased abundance in Gammaproteobacteria and the presence of
Enterobacteriaceae, particularly adherent-invasive _E. coli_ (AIEC) strains.11 Notably, AIEC modulate colitis susceptibility in some mouse models12,13 and additional members of the
Enterobacteriaceae family, i.e., _Klebsiella pneumoniae_ and _Proteus mirabilis_ were also identified to promote colitis in mice.14,15 Moreover, several other members of the murine
microbiota were identified to directly exacerbate intestinal inflammation. This includes _Akkermansia muciniphila_16 as well as distinct _Bacteroides_17 and _Helicobacter_ species.18 Of
note, recent studies have also started to shed light on the role of non-bacterial members of the microbiome such as protozoa and phages in the development of IBD, i.e., the increased
intestinal inflammation in mice colonized with _Tritrichomonas muris_,19 and an enrichment of Caudovirales bacteriophages in IBD patients.20 Beyond these well-studied examples, microbiome
studies have identified many microbes that were found enriched in disease-promoting communities, but with unknown roles in host-microbiota crosstalk, i.e., members of the _Prevotella_
genus.21,22 In general, the role of members of the _Prevotella_ genus within the intestinal microbiota and their effects on the host is not completely understood and somewhat conflicting
interpretations have been reported. High prevalence and relative abundance of _Prevotella_ is found in non-Westerners who consume a plant-rich diet.23,24 Moreover, it has been shown that
_Prevotella_ spp. can improve glucose metabolism stimulated by the intake of prebiotics.25 Together, these studies suggest that _Prevotella_ spp. are beneficial microbes that have colonized
humans for extended periods of time. In contrast, other studies have associated _Prevotella_ spp. with autoimmune diseases, insulin resistance and diabetes, and gut inflammation.22,26,27
Specifically, an overabundance of _Prevotella copri_ was noted in new-onset rheumatoid arthritis (NORA) patients22 and also in patients with systemic autoimmunity associated with RA, but
without clinical symptoms yet.28 In mouse models, an altered gut microbiota dominated by a member of the genus _Prevotella_ was discovered in NLRP6-deficient mice and was associated with
higher susceptibility to chemically-induced colitis.21 Interestingly, _Prevotella_ spp. along with segmented filamentous bacteria (SFB) and _Helicobacter_ spp. are among the highest
immunoglobulin (Ig) A-coated bacteria in these mice, which has been interpreted to reflect their immunogenic features.29 These seemingly opposing effects by _Prevotella_ on the host’s
physiology may be caused by multiple factors including direct or community-mediated effects. Moreover, these effects may not be causally linked to the presence of _Prevotella_ and other
members of the _Prevotella_-dominated microbiome may have the propensity to promote inflammation and intestinal dysbiosis. However, the detailed investigation of the immunomodulatory
properties of _Prevotella_ spp. and their potential mechanisms is prohibited by the strain diversity, as well as the lacking availability of diverse intestinal _Prevotella_ isolates from
model organisms such as the mouse. As of now, culture collections include several _Prevotella_ species from the human intestine, while no species isolated from mice are available. In the
present study, we isolated a novel intestinal _Prevotella_ species (_Prevotella intestinalis_ nov. sp.) from the colitogenic microbiota of _Nlrp6__−/_− mice and then investigated the impact
of its colonization on the interplay between host and the microbiota during intestinal homeostasis and inflammation. We found that _P. intestinalis_ colonization of WT specific pathogen-free
(SPF) mice, devoid of any _Prevotella_ spp. in the intestine, reshapes the resident intestinal microbial community and significantly alters the metabolic profile in the intestine.
_Prevotella_-induced decrease in the levels of SCFA, in particular acetate, is associated with reduced colonic IL-18 expression and production during homeostasis. Notably, _P.
intestinalis_-induced decrease of IL-18 production modulates the exacerbation of colonic inflammation in immunocompetent mice. Strikingly, colonization of a distinct SPF mouse line with _P.
intestinalis_ phenocopied the alterations including the enhanced susceptibility to DSS-induced intestinal inflammation demonstrating the effect can be observed in diverse microbial
ecosystems. Colonization of SPF mice with another _Prevotella_ spp. or a member of the Muribaculaceae family, which are both able to establish high relative abundance in SPF mice thereby
disturbing the microbial ecosystem similarly reduce acetate production and affect intestinal inflammation. Together, our results firmly establish that a representative member of the genus
_Prevotella_, namely _Prevotella intestinalis_, can causally promote inflammation in the host, an effect it shares with other gut bacteria. Enhancement of disease severity is associated to
changes in the microbial ecosystem and microbially-produced metabolites. RESULTS A NOVEL _PREVOTELLA_ SPECIES_, PREVOTELLA INTESTINALIS_, COLONIZES SPF WT MICE IN HIGH ABUNDANCE AND RESHAPES
THE INTESTINAL MICROBIAL COMMUNITY STRUCTURE Alteration in the microbiota of some lines of _Nlrp6__−/−_ mice renders them more susceptible to chemically-induced intestinal inflammation30,31
and a high relative abundance of unknown members of the family Prevotellaceae was identified by 16S rRNA gene sequencing in mice with high disease susceptibility.21 In addition, we
identified that different species of the genus _Prevotella_ were also highly abundant in other colitogenic communities.32 In order to experimentally address whether _Prevotella_ spp.
modulate disease severity, we isolated novel _Prevotella_ species from the colon content of these mouse lines using a step-wise enrichment and isolation scheme under strictly anaerobic
conditions. This cultivation effort yielded three new species belonging to the genus _Prevotella_ based on the comparison of their 16S rRNA genes to other described _Prevotella_ species
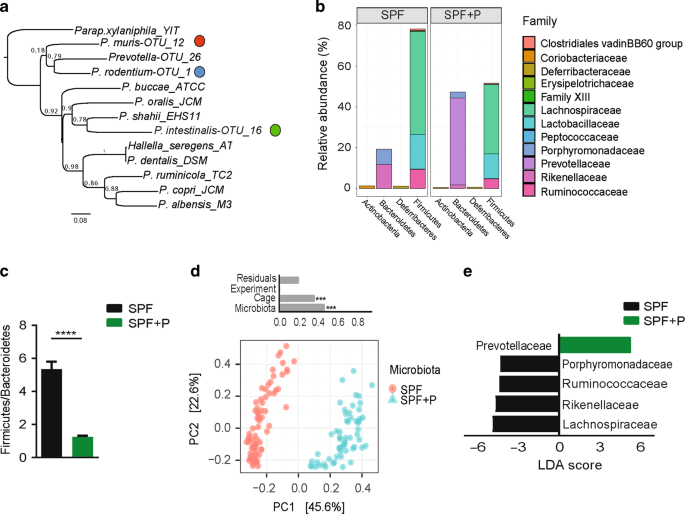
(Fig. 1a). Based on genotypic characterization we conclude that each of the isolates belongs to a novel bacterial taxon within the genus _Prevotella_, for OTU_16 isolated from _Nlrp6__−/−_
mice the name _Prevotella intestinalis_ is proposed (Table S1) (Gálvez, Iljazovic, manuscript in preparation). Of note, mining of metagenomic sequencing data from the murine gut using a
recently released resource, the integrated mouse gut metagenome catalog (iMGMC),33 revealed that _P. intestinalis_ can be found in both laboratory and wild mice demonstrating it is a
naturally occurring bacteria in the mouse gut (Table S2). To study the impact of _P. intestinalis_ on the intestinal ecosystem, we colonized specific pathogen-free (SPF) WT mice, devoid of
any _Prevotella_ species, by a single oral gavage (SPF + P). After 4–5 weeks, _P. intestinalis_ colonization was determined by analyzing fecal microbiota composition using 16S rRNA gene
sequencing. Strikingly, _P._ _intestinalis_ colonized SPF + P mice in high relative abundance (42.5% +/− 2.9, mean +/− SEM) (Fig. 1b), thereby significantly reshaping the microbial community
including a decreased Firmicutes to Bacteroidetes ratio (F/B) (Fig. 1c). In addition, analysis of β-diversity using principle coordinates analysis (PCoA) showed distinct clustering of SPF
and SPF + P communities (Fig. 1d). Based on permutational multivariant analysis of variance (ADONIS), over 45% of the differences were attributed to _Prevotella_ colonization (_R_2 = 0.46,
_p_ < 0.001). Although there was no difference in observed species richness (_p_ = 0.26), the complexity of the community structure, when accounting for species richness and evenness
(Shannon index), was significantly lower in SPF mice after _P. intestinalis_ colonization (_p_ < 0.0001) (Supplementary Fig. 1a). On a family level, comparison of SPF communities with and
without _P. intestinalis_ colonization by linear discriminant analysis (LDA) effect size (LEfSe) showed that _Prevotella_ colonization decreased relative abundance of resident families
within Bacteroidetes phyla, as well as the predominant Firmicutes, namely Lachnospiraceae and Ruminococcaceae (Fig. 1e). _Prevotella_ spp. have been found to predominantly colonize the lumen
of the lower gastrointestinal tract (GIT),30,34 but have been as well described as a part of the intestinal mucosal community.35,36,37 The combination of 16S rRNA gene sequencing with the
quantitation of microbial loads has recently described variation within the absolute abundances of intestinal bacteria and linked it to enterotypes in healthy humans.38 Hence, we quantified
bacterial loads using flow cytometry-based enumeration of bacterial concentrations in the luminal content (Supplementary Fig. 1b). This revealed no differences in the total bacterial cell
counts after _Prevotella_ colonization suggesting that _Prevotella_ is not simply increasing the total microbial density, but rather replaces other bacteria (Supplementary Fig. 1c). We
additionally analyzed the composition of the mucosa-associated microbiota in distal and proximal colon (DC and PC), locations with highest _P. intestinalis_ colonization. We found _P.
intestinalis_ to be present in both DC and PC mucosal sites, with higher abundance in the DC (23.9% +/− 2.6, mean +/− SEM), yet significantly lower than in the DC lumen (Supplementary Fig.
1d). No translocation into the colonic tissue, mesenteric lymph nodes or the liver were detected (Supplementary Fig. 1e). Altogether, this data demonstrate that _P. intestinalis_
colonization has a significant impact on SPF community structure, including the decrease in the microbial diversity and Firmicutes to Bacteroidetes ratio. In addition, _P. intestinalis_
predominantly colonizes the lumen of the colon, however, it is also found closely associated to the colonic mucosa, which is in line with previous findings regarding the niche of
_Prevotella_ spp. and where it may exert immunomodulatory effects on the host.39 _PREVOTELLA INTESTINALIS_ COLONIZATION IS SUFFICIENT TO EXACERBATE DSS-INDUCED COLITIS IN IMMUNOCOMPETENT
HOST We next investigated whether _P. intestinalis_ can exacerbate susceptibility to intestinal inflammation after induced damage to the intestinal barrier. Therefore, acute intestinal
inflammation was induced in littermate SPF and SPF + P mice by administering dextran sulfate sodium (DSS) in drinking water (2.1% w/v). While WT SPF mice used in this study have been
previously reported to be relatively resistant to induction of DSS colitis, displaying moderate colitis severity and mild weight loss,32 colonization of SPF + P mice resulted in a more
severe disease outcome such as significant increased body weight loss (Fig. 2a). Of note, SPF + P mice did not show an increase in mortality (Supplementary Fig. 2a). Colonoscopy on days 6
and 9 after induction of DSS colitis revealed increased tissue damage in _P._ _intestinalis_-colonized mice (Fig. 2b, c). Moreover, higher intestinal inflammation in SPF + P mice was
supported by pronounced colon shortening (Fig. 2d) and histological characterization of tissue damage during DSS colitis (Fig. 2e, f). Specifically, inflammation in SPF + P mice was highest
in the distal colon with pronounced tissue erosion, and higher hyperplasia, edema, and infiltration of inflammatory cells (Fig. 2g). Histological analysis of cecum and small intestine during
DSS colitis showed no significant differences between SPF and SPF + P mice (Supplementary Fig. 2b, c). Microbiota analysis demonstrated that the relative abundance _of P. intestinalis_
decreased during DSS colitis suggesting that it cannot benefit from the altered milieu during inflammation (Supplementary Fig. 2d). While damage of the intestinal barrier has been
demonstrated to result in the translocation of some groups of intestinal bacteria, no translocation of _P. intestinalis_ was detected using a in intestinal tissue, mLN nor the liver
(Supplementary Fig. 2e). Microbiota composition and functionality has the potential to influence the outcome of many types of animal models of diseases.40 Whether the enhancement of
intestinal inflammation by _P. intestinalis_ was only specific to the SPF mice raised in our facility, SPF mice devoid of _P. intestinalis_ were obtained from a commercial vendor (Taconic,
referred to as SPF2). SPF2 were colonized by a distinct _Prevotella_ species that was present in lower abundance (4.9% +/– 1.0, mean +/− SEM), but _P. intestinalis_ outcompeted this species
and further expanded within the SPF2 mice (SPF2 + P) (13.0% +/− 2.4) (Supplementary Fig. 2f, g). After induction of DSS colitis SPF2 + P displayed higher body weight loss, increased
reduction of colon length and higher mortality compared with SPF2 mice demonstrating that _P. intestinalis_-enhanced intestinal inflammation is not limited to a specific SPF mouse line
(Supplementary Fig. 2h-l). Together, these data establish that _P. intestinalis_ is able to alter susceptibility to DSS colitis in an immunocompetent host. ALTERED DSS SUSCEPTIBILITY BY
_PREVOTELLA INTESTINALIS_ COLONIZATION IS ASSOCIATED WITH ELEVATED PRO-INFLAMMATORY CYTOKINE RESPONSES AND IS INDEPENDENT OF ADAPTIVE IMMUNITY To characterize the differences in inflammation
between SPF and SPF + P mice, various cytokines and chemokines were quantified in the distal colon tissue in steady state and during inflammation. Levels of the pro-inflammatory cytokines
IL-6 and tumor necrosis factor alpha (TNF-α) were higher in mice harboring _P. intestinalis_ (Fig. 3a) on day 7 of DSS colitis. _P. intestinalis_ colonization also resulted in increased
levels of the anti-inflammatory cytokine IL-10 in the colon. Notably, contrary to the results observed in SPF mice colonized with the _Prevotella_-rich microbial community from _Nlrp6__−/−_
mice, from which _P. intestinalis_ was originally isolated, SPF + P mice did not display increased levels of interferon γ (IFN-γ), IL-17A, IL-1β, or CCL5 (Supplementary Fig. 3).32
Strikingly, during steady state there was no impact of _P. intestinalis_ colonization on the production of a range of tested cytokines such as IL-6, TNF-α, IL-10, IFN- γ, IL-17a, or IL-1β
(Supplementary Fig. 3), when compared to the SPF mice, except a 2.5-fold decrease of IL-18 levels in distal colons of _Prevotella_-colonized mice. Higher intestinal inflammation in SPF + P
mice during DSS colitis was also characterized by significant increases of multiple chemokines, including LIX and MCP-1, which have been involved in the recruitment and activation of
monocyte and neutrophils to the site of inflammation, as well as MIP-1α and MIP-1β (Fig. 3b and Supplementary Fig. 3). Inflammation in DSS colitis can be triggered by different effector
cells including innate and adaptive immune cells.32,41 To identify which subsets of immune cells are differently presented between the two groups, we analyzed the abundance and composition
of colonic lamina propria leukocytes (LPLs) before and 7 days after induction of DSS colitis by flow cytometry. _Prevotella_ colonization did not result in increased numbers of LPLs (CD45+
cells) in the steady state, but resulted in increased numbers of LPLs after the DSS induction (Fig. 4a, Supplementary Fig. 4b). The global analysis of immune cells subsets of the adaptive
immune system (Supplementary Fig. 4a) in colon tissue demonstrated no significant differences in cell numbers or frequencies, i.e., we observed no differences in the numbers and abundances
of total CD4+ and CD8+ T cells (Fig. 4b, c) as well as B220+ B cells (not shown). Notably, while the numbers and frequency of activated CD4+ T cells (CD62L−CD44+) were increased in colons of
SPF + P mice during DSS colitis (Fig. 4d, e), the numbers of different CD4+ T helper (Th) subsets including Th1 (CD4+IFN-γ+) and Th17 (CD4+IL-17A+) cells as well as in regulatory T cells
(CD4+Foxp3+) were not affected (Supplementary Fig. 4c). Analyzing the abundance of various subsets of innate immune cells (Supplementary Fig. 4d), we observed a significant increase in
frequency and numbers of neutrophils (Ly6C+Ly6G+ cells) in colons of mice colonized with _P. intestinalis_ during DSS colitis, but not in the steady state (Fig. 4f, g, Supplementary Fig.
4e). These findings are in line with increased levels of multiple neutrophil-attracting chemokines we measured in colons of SPF + P mice (Fig. 3b). While no significant increase of
infiltrating monocytes (Ly6c+Ly6g−MHCIIlow-mid+CCR2+) or dendritic cells (Ly6g−Ly6c+MHCIIhigh+CD11c+) was noted by numbers (Supplementary Fig. 4f) or frequencies (not shown), we noted
increased numbers of resident macrophages (Ly6g−Ly6c−CD11b+F4/80+MHCII+CX3CR1+) in SPF + P mice during DSS colitis (Supplementary Fig. 4f). These data suggested that exacerbation of DSS
colitis severity by _P. intestinalis_ is associated with differential recruitment and activation of innate and to a lesser degree of adaptive immune cells, respectively. We recently
demonstrated that the colitogenic community of _Nlrp6__−/−_ mice, which contains _P. intestinalis_, alters susceptibility to DSS colitis via modulation of adaptive immune cells, i.e.,
transfer of the community in _Rag2__−/−_ mice was unable to exacerbate disease severity.32 To test whether _P. intestinalis_ requires the presence of adaptive immune cells to alter colitis
susceptibility, we colonized WT and Rag2-deficient mice with _P. intestinalis_. Importantly, both WT and _Rag2__−/−_ mice harbored the same SPF microbiota before the _P. intestinalis_
colonization.30 Specifically, the comparison of their fecal microbiota composition before induction of DSS colitis showed that the mice clustered together in relation to their microbial
communities (SPF or SPF + P) (Fig. 4h). Multivariate analysis of variance using ADONIS showed microbiota contributed to the variability of the groups with 60% (_R_2 = 0.60, _p_ = 0.001),
while genotype contributing to the differences as little as 5% (_R_2 = 0.05, _p_ = 0.007). Strikingly, _P. intestinalis_ exacerbated DSS colitis severity both in WT and _Rag2__−/−_ mice, as
indicated by their weight loss (Fig. 4i) and colon shortening (Supplementary Fig. 4g). Taken together, we conclude that _P. intestinalis_ colonization promotes intestinal inflammation upon
damage to the intestinal barrier independent of adaptive immune cells. _P. INTESTINALIS_-INDUCED DECREASE OF IL-18 MODULATES THE EXACERBATION OF COLONIC INFLAMMATION Besides differences in
cytokine and chemokine production during DSS colitis, we also observed that _P. intestinalis_ colonization of SPF mice resulted in a decrease of IL-18 levels in colonic tissue before
induction of intestinal inflammation (Fig. 3a). The role of IL-18 during DSS colitis has been controversially discussed, either suggested to play a role in promoting intestinal epithelial
integrity and protection from acute experimental colitis,42,43,44 or to exacerbate intestinal inflammation due to impaired repair processes.45,46 This prompted us to investigate whether
lower levels of colonic IL-18 may be linked to the _Prevotella_-enhanced susceptibility to colonic inflammation during DSS-induced colitis. Since IL-18 has been previously shown to
ameliorate severity of DSS colitis,44,47,48 we aimed to determine whether IL-18 supplementation would be sufficient to reduce inflammation in _Prevotella_-colonized mice. SPF and SPF + P
mice were administered daily with recombinant IL-18 (rIL-18) or vehicle intraperitoneally (i.p.) starting 2 days prior and during the DSS colitis. Indeed, administration of rIL-18 attenuated
colitis severity in mice colonized with _P. intestinalis_, as assessed by reduced weight loss (Fig. 5a, b) and histological examination of colon sections performed on day 7 post DSS
induction (Fig. 5c, d). While both SPF mice groups, receiving PBS and rIL-18, showed similar mild crypt erosion, _Prevotella_-colonized mice administered with rIL-18, but not PBS, showed
diminished colitis severity. _Prevotella_-colonized mice injected with PBS displayed more severe epithelial hyperplasia and mucosal invasion of inflammatory cells in comparison to mice
supplemented with rIL-18 (SPF + P + rIL-18) (Fig. 5d). While administration of rIL-18 to SPF + P mice moderately reduced the levels of IL-6 and TNF-α (Fig. 5e), levels of chemoattractants
MCP-1, MIP-1a, MIP-1b, and LIX were significantly diminished in comparison to PBS treated mice (Fig. 5f). Notably, supplementation of rIL-18 did not alter the abundance of _P. intestinalis_
(Fig. 5g). Together, these results demonstrate that _Prevotella_-induced suppression of colonic IL-18 production alters susceptibility to intestinal inflammation upon tissue damage.
REDUCTION OF IL-18 IS ASSOCIATED WITH _PREVOTELLA_-INDUCED CHANGES IN THE MICROBIOTA AND MODULATION OF SCFAS PRODUCTION Distinct microbial metabolites, specifically taurine, histamine,
polyamines, and SCFAs modulate inflammasome signaling on the transcriptional and post-transcriptional level.47,48 Hence, we first addressed whether changes in IL-18 protein levels observed
in _Prevotella_-colonized mice were accompanied by changes on the transcriptional level. Indeed, _P. intestinalis_ colonization resulted in reduced _Il18_ expression, while _Casp1_
expression was not significantly affected (Supplementary Fig. 5a). While taurine has been demonstrated to enhance IL-18 processing via activation of the Nlrp6 inflammasome, histamine and
distinct polyamines have been shown to have an inhibitory effect.47 Therefore, we measured taurine, histamine, putrescine, spermine, spermidine, and cadaverine concentrations in cecal
content of SPF and SPF + P mice, however, we did not observe any correlation between the relative amounts of detected metabolites and levels of IL-18 (Fig. 6a). Conversely, mice with SPF
microbiota, which showed higher levels of colonic IL-18, displayed a twofold increase in luminal putrescine concentrations than the _Prevotella_-colonized mice (Fig. 6a). Also, antimicrobial
peptides previously identified to be modulated by IL-18 in the colon were not altered (Supplementary Fig. 5b).47 The role of SCFA in the maintenance of epithelial health has been
extensively investigated.48,49,50,51,52 Mackay and colleagues demonstrated that the SCFAs acetate and butyrate can act on GPR43 and GPR109a receptors on IECs, respectively, and stimulate the
expression of the _Il18_ gene in the intestine.48 Since we recently showed modulation of SCFA levels 8 weeks after _Prevotella_ spp. colonization resulting in alterations of osteoclast
metabolism in the bone,53 we hypothesized that _P. intestinalis_ also induced a decrease in SCFAs earlier after colonization, thereby resulting in distinct IL-18 production in SPF and SPF +
P mice. Measurements of the concentrations of SCFAs in the intestinal content and serum of SPF and SPF + P mice 4 weeks after _Prevotella_ colonization revealed that total SCFAs levels were
affected already at this time point (Fig. 6b). More specifically, we observed a decrease of SCFA concentration in the cecum and colon content by 30% and 43%, respectively, as well as in the
serum in SPF + P mice (23% decrease in SPF + P mice) (Fig. 6b). The decrease mainly derived from a significant reduction of acetate concentrations, the most abundant SCFA, in cecum (−50%),
colon (−35%), and serum (−25%) (Fig. 6c, Supplementary Fig. 5c, d). In contrast, propionate was slightly decreased only in the colon (Supplementary Fig. 5c), while butyrate showed minor
opposing site-specific concentrations changes (Fig. 6c, Supplementary Fig. 5c, d). A similar reduction of colonic SCFA and acetate as well as concomitantly colonic IL-18 concentrations was
observed in SPF2 + P mice (Supplementary Fig. 5e, f). These results show that _Prevotella_ colonization modulates SCFAs production and suggest that the lower concentration of IL-18 in
_Prevotella_-colonized mice is associated with changes in the concentration of acetate, but not other types of SCFA. Next, we investigated how _P. intestinalis_ colonization modulates the
concentration of acetate and considered different mechanisms, i.e., the conversion in other SCFAs54,55 or distinct SCFA production profiles between resident commensals and _Prevotella_.56
First, although frequently regarded as an end product of anaerobic fermentation, acetate is utilized by specific intestinal bacteria for butyrate production.55 As a significant decrease of
acetate and a concurrent increase of butyrate is observed after _P. intestinalis_ colonization in the cecum, we sought to investigate whether _P. intestinalis_ utilizes acetate for butyrate
production. Therefore, _P. intestinalis_ was cultured in the presence or absence of physiological concentrations of acetate. Notably, _P. intestinalis_ did not produce any butyrate
(Supplementary Fig. 5g) and acetate concentrations did not decrease when added exogenously. In the absence of added acetate, _P. intestinalis_ produced acetate, propionate and succinate in
similar concentrations as reported for _P. copri_.57 Next, we wanted to exclude that in vivo _P. intestinalis_ convert acetate itself or promotes its conversion by other bacteria reducing
its concentration. Therefore, SPF and SPF + P mice were given orally 50 mg of stable isotope (13C)-labeled acetate and SCFA were analyzed for 13C-enrichment after 4 h. The presence of
bacteria producing butyrate from acetate within the SPF microbiota was detected in general as evidenced by the presence of 13C-labeled butyrate, however, we did not observe higher
concentrations of 13C-labeled butyrate in SPF + P mice (Fig. 6d, e). Hence, we considered the presence of distinct fermentation profiles in _P. intestinalis_ as the basis for reduced acetate
concentration. Comparison of the production of metabolites after in vitro cultivation of _P. intestinalis_ to _Parabacteroides goldsteinii_, one of the Bacteroidales representatives who was
outcompeted by _P. intestinalis_ (Fig. 6f), identified as fermentation products formate and acetate, as well as succinate and to a certain degree also propionate. Notably, _P. intestinalis_
produced relatively more succinate and less acetate than _P. goldsteinii_ (Fig. 6g). Related changes in the concentrations in succinate were observed in vivo, i.e., higher in SPF + P mice
in the cecum (Fig. 6h). Of note, these changes were not observed in the colon (Fig. 6h). To investigate whether these alterations in SCFA production are specific to _P. intestinalis_, SPF
mice were colonized with another _Prevotella_ species (new isolate, proposed name: _Prevotella rodentium_) (Gálvez, Iljazovic, manuscript in preparation) or a member of the family
Muribaculaceae (phylum Bacteroidetes), i.e., _Duncaniella muris_,58 which is also absent in SPF mice. Microbiota analysis showed both strains were able to colonize SPF reaching abundances
similar to _P. intestinalis_ reducing the abundance of resident Bacteroidetes species (Supplementary Fig. 6a). Measurement of luminal SCFA concentrations revealed reductions in acetate
comparable to _P. intestinalis_ (Supplementary Fig. 6b, c). Concurrently, decrease of colonic IL-18 was measured upon _P. rodentium_ and _D. muris_ colonization (Supplementary Fig. 6d).
Finally, colonization with _D. muris_ enhanced susceptibility to DSS-induced intestinal inflammation in SPF mice, while a similar trend was observed for _P. rodentium_ (Supplementary Fig.
6d) suggesting that the decrease of acetate production as an indirect result of domination by specific groups of Bacteroidales is more generally linked to enhanced inflammation after
intestinal injury. DISCUSSION Numerous studies in humans and animal models have established associations between alteration in the microbiota composition and a wide range of inflammatory
diseases, e.g., IBD and RA.59,60 Specifically, the increased relative abundances of members of Prevotellaceae family within diverse microbial ecosystems have been associated with RA,22,61
periodontitis,62 and intestinal and vaginal dysbiosis,21,63,64,65 yet the direct functional relevance of increased _Prevotella_ colonization is largely unclear. Some of the known
_Prevotella_ species have been reported to be involved in opportunistic infections, while most of them are classically considered to be commensals colonizing different mucosal sites.39 In
the intestine, the presence of _Prevotella_ spp. has been proposed to be a biomarker of one of the three human gut enterotypes in developed countries.66 Besides its association to RA in
humans,22,61 some studies have observed changes in _Prevotella_ abundances in IBD patients,67,68 whereas other studies showed no association.9 Conversely, members of the genus _Prevotella_
have also been associated with beneficial effects on the health as well, such as improved glucose metabolism25 and correlation with plant-rich diet.23,24 Hence, whether the association of
different host responses to _Prevotella_ spp. colonization in human individuals is explained by high species diversity and different functional capabilities or by indirect community-mediated
effects is not known.69,70 Finally, _Prevotella_ may not be causatively involved and rather be a marker of a distinct state of the microbiome promoting inflammation. Altogether, these
observations highlight the interest to expand our understanding of the impact of _Prevotella_ spp. on the intestinal ecosystem and the host. The lack of intestinal _Prevotella_ isolates from
experimental models prevents addressing these highly relevant questions. Specifically, as the gut microbiota of conventionally raised mice is often not permissive to colonization by human
enteropathogens71,72 or commensal human-derived bacteria.73,74 Hence, commonly repetitive, daily gavage or antibiotics are used to overcome the colonization resistance, as in case of studies
with _P. copri_.22,25 Antibiotics use in such models can have profound effects on the host physiology directly or via antibiotic-induced dysbiosis, making these models ambiguous. Therefore,
we developed a targeted isolation strategy to recover novel _Prevotella_ species from the mouse intestine and characterized the outcome of _P. intestinalis_ colonization on a resident
community and intestinal health. _P._ _intestinalis_ colonized the mice in high relative abundance, similar to what has been reported for _P. copri_ in humans,70 occupying the niche of
resident bacteria in the colonic lumen and mucosa. The microbiota of recipient mice in this study was characterized by the high abundance of Firmicutes that have been in general associated
with beneficial effects on the host.8,59 In turn, microbiota composition of _Prevotella_-colonized mice was characterized by a decrease in α-diversity (Shannon) and members of the Firmicutes
phylum, both changes previously attributed to dysbiosis and microbiota of IBD, asthma, and RA patients.75,76,77 Colonization by _P. intestinalis_ did not lead to intestinal inflammation in
immunocompetent mice without inducing injury to the intestine (up to 5 weeks of colonization). Yet, upon induction of damage to the intestinal barrier and exposure to the luminal bacteria in
the DSS colitis model, _Prevotella_-colonized mice displayed signs of exacerbated inflammation in comparison to SPF mice. Importantly, exacerbation of inflammation was not restricted to the
mice with one specific gut microbiome composition used in this study (SPF), as comparable results were recapitulated in commercially obtained mice with a distinct microbiome composition
(SPF2). Specifically, the inflamed tissue of mice colonized with _P. intestinalis_ was characterized by increased levels of IL-6 and TNF-α, as well as higher levels of neutrophil-attracting
chemokines accompanied with neutrophils infiltration. Strikingly, while the intestinal community of Nlrp6-deficient mice, from which _P. intestinalis_ was isolated, exacerbates DSS colitis
in a T-cell dependent manner,32 _P. intestinalis_ did not require adaptive immune cells to exacerbate disease, i.e., Rag2-deficient mice showed _Prevotella_-exacerbated intestinal
inflammation. These results suggest that other members or combined effects of distinct microbes in the colitogenic community in _Nlrp6__−/−_ mice are responsible for induction of
pro-inflammatory adaptive immune cells. Subsequent analysis clearly demonstrated that _Prevotella_ colonization shapes host immunity already during the steady state, i.e., reduction of IL-18
production. Specifically, we observed that _P. intestinalis_ colonization of SPF microbiota results in significant decrease of colonic _Il18_ expression and IL-18 production (2.5-fold)
during steady state. Interestingly, previous studies demonstrated that decrease of IL-18 production by 1.3–1.5-fold was already sufficient to disturb the intestinal homeostasis.47,48 Hence,
we hypothesized that these changes affected the intestinal barrier during steady state, which in turn contributed to a more severe intestinal inflammation in _Prevotella_-colonized mice
during DSS colitis. Indeed, rIL-18 supplementation ameliorated the susceptibility to intestinal inflammation in _Prevotella_-colonized mice and alleviated colonic tissue damage. Notably,
even though IL-18 has been widely studied, no definitive role of IL-18 in intestinal homeostasis and inflammation has been conclusively established. While some studies suggested IL-18 has a
protective role, preventing dysbiosis21,47 and promoting epithelial barrier integrity and regeneration,44,48 others have linked IL-18 to increased colitis severity.45,46 Our data further add
to the complex role of IL-18 in the intestine during homeostasis and inflammation suggesting that perturbation of the levels of IL-18 levels may predispose to intestinal inflammation upon
tissue injury. Several microbiota-derived metabolites in the intestine have been shown to modulate IL-18 production, either by effecting _Il18_ expression49,78 or through modulation of
inflammasome activation,47,48 e.g., SCFAs, taurine and distinct polyamines such as spermidine. Targeted metabolomics revealed that colonization of the SPF community by _P. intestinalis_ is
accompanied by a significant decrease in SCFAs levels, in particular, acetate, but none of the other known Nlrp6 inflammasome modulators. As an end product of microbial fermentation,
intestinal production of SCFAs, specifically straight-chain SCFAs, are influenced by both diet and microbiota composition. While production of butyrate has been associated to Clostridia,
members of the Bacteroidetes have been reported to be the major contributor to propionate and acetate production.56,79 Surprisingly, _Prevotella_-induced microbiota changes in SPF mice
resulted in a decrease, rather than the expected increase, of acetate concentration in the intestine of SPF + P mice. We initially hypothesized _P. intestinalis_ may be directly utilizing
acetate for butyrate production as this has been reported for other gut commensals, however, our data showed that _P. intestinalis_ produced formate, acetate, propionate, and succinate,
while no butyrate production was detected in vitro. Experiments with stable isotope-labeled acetate showed that _Prevotella_ does not utilize acetate for butyrate production itself, or
stimulates the conversion in other bacteria in vivo as no significant increase of 13C-labeled butyrate in SPF + P mice was detected. As an alternative to an enhanced consumption of acetate,
we considered that _Prevotella_-induced changes in the microbiota reduce acetate production. Indeed, _P. goldsteinii_, which was strongly reduced by _Prevotella_ and is one of the two
members of the Bacteroidetes in SPF mice, produced relatively more acetate and formate, while _P. intestinalis_ fermentation resulted in higher levels of propionate. In turn, succinate, an
alternative fermentation product, was produced at higher levels by _P. intestinalis_ in vitro supporting the distinct metabolism of _P. intestinalis_ compared to other microbiota members. Of
note, succinate was not consistently changed in vivo being increased in the cecum but not colon of SPF + P in comparison to SPF mice. As succinate is a major fermentation product of _P.
copri_80 further research will be needed to evaluate the importance of changes in succinate and other metabolites produced by _Prevotella_ spp. of the mouse and human intestinal microbiota
to host biology. Specifically, we conclude that _P. intestinalis_ may be a valuable model organism for future studies of the association between _P. copri_ and diverse diseases including RA
enabling detailed longitudinal analysis of diverse physiological parameters and aspects of immune responses in vivo. It remains to be addressed which metabolic and associated changes in the
host are specific for _Prevotella_ spp. compared with other Bacteroidales groups. While colonization with a representative member of the family Muribaculaceae, which is highly prevalent in
mice, exacerbates disease similarly to _P. intestinalis_, notable additive effects were observed after colonization of SPF2 mice with _P. intestinalis_. Despite the presence of
Muribaculaceae and another _Prevotella_ spp. in SPF2 mice, colonization with _P. intestinalis_ further exacerbated disease severity likely reflecting its ability to efficiently colonize the
mouse gut Taken together, our data provide strong evidence for an immunomodulatory role of _Prevotella_ spp. in the intestine. The reduction of SCFA concentrations in SPF + P mice is linked
to a distinct fermentation profile in _P. intestinalis_ combined with a relative reduction of predominantly acetate-producing bacteria due _to P. intestinalis_ expansion. Down-modulation of
microbial acetate production after intestinal domination by specific groups of Bacteroidales may more general result in an enhancement of intestinal inflammation after chemical damage to the
intestinal barrier. MATERIALS AND METHODS MICE Wild-type (WT), _Rag2__−/−_, and IL-17AGFP IFN-γKatushka FoxP3RFP reporter mice used in the study were on the C57BL/6N background. They were
all bred and maintained at the animal facilities of the Helmholtz Centre for Infection Research (HZI) under enhanced specific pathogen-free conditions (SPF) (Table S3). Therefore, mice were
rederived into SPF microbiota by embryo transfer.30 C57BL/6N “SPF2” mice were obtained from a commercial vendor (Taconic) (Table S4). _Nlrp6__−/−_ mice were obtained from Yale University and
maintained under conventional housing conditions at the HZI without rederivation. All experiments were performed with 10- to 11-week-old age-matched and gender-matched animals. _P.
INTESTINALIS_ ISOLATION AND COLONIZATION OF MICE Fresh colonic content of conventionally housed donor _Nlrp6__−/−_ mice was collected in BBL thioglycollate media and fecal content homogenate
was further processed in an anaerobic chamber with following gas mixture: 70% nitrogen, 20% carbon dioxide, and 10% hydrogen. Bacteria were isolated by using the most probable number (MPN)
technique (Goodman et al.81) where homogenized content was diluted in a range in which maximal 30% of wells showed detectable growth. Specifically, 10-fold dilutions (10−6 and 10−7) of fecal
content homogenate were cultured in a sterile 96-well plate in Brain Hearth Infusion broth supplemented with 10% FBS and 0.5 g/L vitamin K3 (BHI-S medium) on 37 °C for 2 days.
_Prevotella_-positive wells were further enriched in BHI-S medium containing vancomycin (6 µg/ml) and selected on BHI-S blood agar (defibrinated sheep blood, 5% v/v) plates. Positive
colonies were passaged three times on agar plates before a pure culture was obtained and glycerol stocks were frozen in −80 °C. For every experiment, _P. intestinalis, P. rodentium_, or
_Duncaniella muris_ culture was grown anaerobically (70% N2, 20% CO2, and 10% H2) from a frozen glycerol stock in BHI-S medium on 37 °C for 2 days. All mice were colonized by oral gavage at
age of 5–6 weeks with each bacteria at a dose 3 × 108 CFU in 200 µl of BHI media. With exception, SPF2 mice were colonized with _P. intestinalis_ at the same dose by oral gavage, followed by
a 20–30 µl rectal administration. DSS-INDUCED COLITIS Acute colitis was induced by adding dextran sodium sulfate (DSS) in sterilized drinking water of 10–11-week-old WT and gene-deficient
mice. Mice with SPF and SPF2 microbiome composition were given 2.1% (w/v) and 1.6% (w/v) DSS in drinking water, respectively, for 7 days, followed by 5 days of access to regular drinking
water. During the course of DSS treatment fresh DSS solution was prepared and replaced on day 0 and day 4. Mice were monitored daily by measurement of body weight and clinical assessment,
including stool consistency and detection of blood in the stool. Animals which lost 20% or more of their initial body weight were euthanized. STATISTICAL ANALYSIS Statistical analysis was
performed using GraphPad Prism 7 program (GraphPad Software, Inc.) and R v3.3.0. Data are expressed as mean ± SEM (Standard error of mean). Differences were analyzed by Student’s _t_ test
and ANOVA. _P_ values indicated represent an unpaired nonparametric Mann–Whitney or two-way ANOVA by Tukey’s multiple comparison analysis. The permutational multivariate ANOVA analysis of
variance (ADONIS) was computed with 999 permutations. For ADONIS tests, an _R_2 > 0.1 (effect size, 10%) and _p_ value < 0.05 were considered as significant. _P_ values ≤ 0.05 were
considered as significant: *_p_ < 0.05, **_p_ < 0.01, ***_p_ < 0.001, ****_p_ < 0.0001. REFERENCES * Belkaid, Y. & Harrison, O. J. Homeostatic immunity and the microbiota.
_Immunity_ 46, 562–576 (2017). Article CAS PubMed PubMed Central Google Scholar * Peterson, L. W. & Artis, D. Intestinal epithelial cells: regulators of barrier function and immune
homeostasis. _Nat. Rev. Immunol._ 14, 141–153 (2014). Article CAS PubMed Google Scholar * Postler, T. S. & Ghosh, S. Understanding the holobiont: how microbial metabolites affect
human health and shape the immune system. _Cell Metab._ 26, 110–130 (2017). Article CAS PubMed PubMed Central Google Scholar * Swidsinski, A. et al. Mucosal flora in inflammatory bowel
disease. _Gastroenterology_ 122, 44–54 (2002). Article PubMed Google Scholar * Sartor, R. B. Mechanisms of Disease: pathogenesis of Crohn’s disease and ulcerative colitis. _Nat. Clin.
Pract. Gastroenterol. Hepatol._ 3, 390–407 (2006). Article CAS PubMed Google Scholar * Ni, J., Wu, G. D., Albenberg, L. & Tomov, V. T. Gut microbiota and IBD: causation or
correlation? _Nat. Rev. Gastroenterol. Hepatol._ 14, 573 (2017). Article PubMed PubMed Central Google Scholar * Tedelind, S., Westberg, F., Kjerrulf, M. & Vidal, A. Anti-inflammatory
properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. _World J. Gastroenterol._ 13, 2826–2832 (2007). Article CAS PubMed
PubMed Central Google Scholar * Manichanh, C. et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. _Gut_ 55, 205–211 (2006). Article CAS
PubMed PubMed Central Google Scholar * Morgan, X. C. et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. _Genome Biol._ 13, R79 (2012). Article
CAS PubMed PubMed Central Google Scholar * Makki, K., Deehan, E. C., Walter, J. & Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. _Cell Host
Microbe_ 23, 705–715 (2018). Article CAS PubMed Google Scholar * Darfeuille-Michaud, A. et al. High prevalence of adherent-invasive _Escherichia coli_ associated with ileal mucosa in
Crohn’s disease. _Gastroenterology_ 127, 412–421 (2004). Article PubMed Google Scholar * Carvalho, F. A. et al. Transient inability to manage proteobacteria promotes chronic gut
inflammation in TLR5-deficient mice. _Cell Host Microbe_ 12, 139–152 (2012). Article CAS PubMed PubMed Central Google Scholar * Carvalho, F. A. et al. Crohn’s disease adherent-invasive
_Escherichia coli_ colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. _J. Exp. Med._ 206, 2179–2189 (2009). Article CAS PubMed PubMed Central Google
Scholar * Garrett, W. S. et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. _Cell Host Microbe_ 8, 292–300 (2010).
Article CAS PubMed PubMed Central Google Scholar * Seo, S.-U. et al. Distinct commensals induce interleukin-1β via NLRP3 inflammasome in inflammatory monocytes to promote intestinal
inflammation in response to injury. _Immunity_ 42, 744–755 (2015). Article CAS PubMed PubMed Central Google Scholar * Seregin, S. S. et al. NLRP6 protects Il10−/− mice from colitis by
limiting colonization of _Akkermansia muciniphila_. _Cell Rep._ 19, 733–745 (2017). Article CAS PubMed PubMed Central Google Scholar * Bloom, S. M. et al. Commensal bacteroides species
induce colitis in host-genotype-specific fashion in a mouse model of inflammatory Bowel disease. _Cell Host Microbe_ 9, 390–403 (2011). Article CAS PubMed PubMed Central Google Scholar
* Kullberg, M. C. et al. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism.
_Infect. Immun._ 66, 5157–5166 (1998). Article CAS PubMed PubMed Central Google Scholar * Escalante, N. K. et al. The common mouse protozoa Tritrichomonas muris alters mucosal T cell
homeostasis and colitis susceptibility. _J. Exp. Med._ 213, 2841–2850 (2016). Article CAS PubMed PubMed Central Google Scholar * Fernandes, M. A. et al. Enteric virome and bacterial
microbiota in children with ulcerative colitis and Crohn disease. _J. Pediatr. Gastroenterol. Nutr._ 68, 30–36 (2019). Article PubMed PubMed Central Google Scholar * Elinav, E. et al.
NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. _Cell_ 145, 745–757 (2011). Article CAS PubMed PubMed Central Google Scholar * Scher, J. U. et al. Expansion
of intestinal _Prevotella copri_ correlates with enhanced susceptibility to arthritis. _Elife_ 2, e01202 (2013). * Clemente, J. C. et al. The microbiome of uncontacted Amerindians. _Sci.
Adv._ 1, e1500183–e1500183 (2015). Article PubMed PubMed Central CAS Google Scholar * Martínez, I. et al. The gut microbiota of rural papua new guineans: composition, diversity
patterns, and ecological processes. _Cell Rep._ 11, 527–538 (2015). Article PubMed CAS Google Scholar * Kovatcheva-Datchary, P. et al. Dietary fiber-induced improvement in glucose
metabolism is associated with increased abundance of prevotella. _Cell Metab._ 22, 971–982 (2015). Article CAS PubMed Google Scholar * Pedersen, H. K. et al. Human gut microbes impact
host serum metabolome and insulin sensitivity. _Nature_ 535, 376–381 (2016). Article CAS PubMed Google Scholar * Leite, A. Z. et al. Detection of increased plasma interleukin-6 levels
and prevalence of _Prevotella copri_ and _Bacteroides vulgatus_ in the feces of type 2 diabetes patients. _Front. Immunol._ 8, 1107 (2017). Article PubMed PubMed Central CAS Google
Scholar * Rodriguez, D. A. et al. _Prevotella copri_ in individuals at risk for rheumatoid arthritis. 1–4 https://doi.org/10.1136/annrheumdis-2018-214514 (2019). * Palm, N. W. et al.
Immunoglobulin A coating identifies colitogenic bacteria in inflammatory Bowel Disease. _Cell_ 158, 1000–1010 (2014). Article CAS PubMed PubMed Central Google Scholar * Gálvez, E. J.
C., Iljazovic, A., Gronow, A., Flavell, R. & Strowig, T. Shaping of intestinal microbiota in Nlrp6- and Rag2-deficient mice depends on community structure. _Cell Rep_. 21, 3914–3926
(2017). * Mamantopoulos, M. et al. Nlrp6- and ASC-dependent inflammasomes do not shape the commensal gut microbiota composition. _Immunity_ 47, 339–348.e4 (2017). Article CAS PubMed
Google Scholar * Roy, U. et al. Distinct microbial communities trigger colitis development upon intestinal barrier damage via innate or adaptive immune cells. _Cell Rep._ 21, 994–1008
(2017). Article CAS PubMed PubMed Central Google Scholar * Lesker, T. R. et al. An integrated metagenome catalog reveals new insights into the murine gut microbiome. _Cell Rep._ 30,
2909–2922.e6 (2020). Article CAS PubMed PubMed Central Google Scholar * Gu, S. et al. Bacterial community mapping of the mouse gastrointestinal tract. _PLoS ONE_ 8, e74957 (2013).
Article CAS PubMed PubMed Central Google Scholar * Yasuda, K. et al. Biogeography of the intestinal mucosal and lumenal microbiome in the rhesus macaque. _Cell Host Microbe_ 17, 385–391
(2015). Article CAS PubMed PubMed Central Google Scholar * Wang, A.-H. et al. Human colorectal mucosal microbiota correlates with its host niche physiology revealed by endomicroscopy.
_Sci. Rep._ 6, 21952 (2016). Article CAS PubMed PubMed Central Google Scholar * Rolhion, N. et al. A _Listeria monocytogenes_ bacteriocin can target the commensal _Prevotella copri_ and
modulate intestinal infection. _Cell Host Microbe_ 26, 691–701.e5 (2019). Article CAS PubMed PubMed Central Google Scholar * Vandeputte, D. et al. Quantitative microbiome profiling
links gut community variation to microbial load. _Nature_ 551, 507 (2017). Article CAS PubMed Google Scholar * Larsen, J. M. The immune response to Prevotella bacteria in chronic
inflammatory disease. _Immunology_ 151, 363–374 (2017). Article CAS PubMed PubMed Central Google Scholar * Macpherson, A. J. & McCoy, K. D. Standardised animal models of host
microbial mutualism. _Mucosal Immunol._ 8, 476–486 (2015). Article CAS PubMed Google Scholar * Chassaing, B., Aitken, J. D., Malleshappa, M. & Vijay-Kumar, M. in _Current Protocols
in Immunology_ Vol. 104, 15.25.1–15.25.14 (John Wiley & Sons, Inc., 2014). * Oficjalska, K. et al. Protective role for caspase-11 during acute experimental murine colitis. _J. Immunol._
194, 1252–1260 (2015). Article CAS PubMed Google Scholar * Salcedo, R. et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. _J.
Exp. Med._ 207, 1625–1636 (2010). Article CAS PubMed PubMed Central Google Scholar * Zaki, M. H. et al. The NLRP3 inflammasome protects against loss of epithelial integrity and
mortality during experimental colitis. _Immunity_ 32, 379–391 (2010). Article CAS PubMed PubMed Central Google Scholar * Błażejewski, A. J. et al. Microbiota normalization reveals that
canonical caspase-1 activation exacerbates chemically induced intestinal inflammation. _Cell Rep._ 19, 2319–2330 (2017). Article PubMed CAS Google Scholar * Nowarski, R. et al.
Epithelial IL-18 equilibrium controls barrier function in colitis. _Cell_ 163, 1444–1456 (2015). Article CAS PubMed PubMed Central Google Scholar * Levy, M. et al. Microbiota-modulated
metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. _Cell_ 163, 1428–1443 (2015). Article CAS PubMed PubMed Central Google Scholar * Macia, L.
et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. _Nat. Commun._ 6, 6734 (2015). Article CAS
PubMed Google Scholar * Singh, N. et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. _Immunity_
40, 128–139 (2014). Article CAS PubMed PubMed Central Google Scholar * Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis.
_Science_ 341, 569–573 (2013). Article CAS PubMed Google Scholar * Park, J. et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone
deacetylases and regulation of the mTOR–S6K pathway. _Mucosal Immunol._ 8, 80–93 (2015). Article CAS PubMed Google Scholar * Furusawa, Y. et al. Commensal microbe-derived butyrate
induces the differentiation of colonic regulatory T cells. _Nature_ 504, 446–450 (2013). Article CAS PubMed Google Scholar * Lucas, S. et al. Short-chain fatty acids regulate systemic
bone mass and protect from pathological bone loss. _Nat. Commun._ 9, 55 (2018). Article PubMed PubMed Central CAS Google Scholar * den Besten, G. et al. The short-chain fatty acid
uptake fluxes by mice on a guar gum supplemented diet associate with amelioration of major biomarkers of the metabolic syndrome. _PLoS ONE_ 9, e107392 (2014). Article CAS Google Scholar *
Duncan, S. H., Barcenilla, A., Stewart, C. S., Pryde, S. E. & Flint, H. J. Acetate utilization and butyryl coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from
the human large intestine. _Appl. Environ. Microbiol._ 68, 5186–5190 (2002). Article CAS PubMed PubMed Central Google Scholar * Flint, H. J., Duncan, S. H., Scott, K. P. & Louis, P.
Links between diet, gut microbiota composition and gut metabolism. _Proc. Nutr. Soc._ 74, 13–22 (2015). Article CAS PubMed Google Scholar * Franke, T. & Deppenmeier, U. Physiology
and central carbon metabolism of the gut bacterium _Prevotella copri_. _Mol. Microbiol._ 109, 528–540 (2018). Article CAS PubMed Google Scholar * Lagkouvardos, I. et al. Sequence and
cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. _Microbiome_ 7, 28 (2019). * Frank, D. N. et al.
Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. _Proc. Natl Acad. Sci. USA_ 104, 13780–13785 (2007). Article CAS PubMed
PubMed Central Google Scholar * Zhang, X. et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. _Nat. Med._ 21, 895–905 (2015).
Article CAS PubMed Google Scholar * de Aquino, S. G. et al. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response.
_J. Immunol._ 192, 4103–4111 (2014). Article PubMed CAS Google Scholar * Ji, S., Kim, Y., Min, B.-M., Han, S. H. & Choi, Y. Innate immune responses of gingival epithelial cells to
nonperiodontopathic and periodontopathic bacteria. _J. Periodontal Res._ 42, 503–510 (2007). Article CAS PubMed Google Scholar * Gosmann, C. et al. Lactobacillus-deficient cervicovaginal
bacterial communities are associated with increased HIV acquisition in young south african women. _Immunity_ 46, 29–37 (2017). Article CAS PubMed PubMed Central Google Scholar *
Onderdonk, A. B., Delaney, M. L. & Fichorova, R. N. The human microbiome during bacterial vaginosis. _Clin. Microbiol. Rev._ 29, 223–238 (2016). Article CAS PubMed PubMed Central
Google Scholar * Dillon, S. M. et al. Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T-cell activation in untreated HIV-1 infection. _Mucosal
Immunol._ 9, 24–37 (2016). Article CAS PubMed Google Scholar * Arumugam, M. et al. Enterotypes of the human gut microbiome. _Nature_ 473, 174–180 (2011). Article CAS PubMed PubMed
Central Google Scholar * Lucke, K. Prevalence of _Bacteroides_ and _Prevotella_ spp. in ulcerative colitis. _J. Med. Microbiol._ 55, 617–624 (2006). Article CAS PubMed Google Scholar *
Kleessen, B., Kroesen, A. J., Buhr, H. J. & Blaut, M. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. _Scand. J. Gastroenterol._ 37,
1034–1041 (2002). Article CAS PubMed Google Scholar * Kumar Gupta, V., Chaudhari, N. M., Iskepalli, S. & Dutta, C. Divergences in gene repertoire among the reference Prevotella
genomes derived from distinct body sites of human. https://doi.org/10.1186/s12864-015-1350-6 (2011). * De Filippis, F. et al. Distinct genetic and functional traits of human intestinal
_Prevotella copri_ strains are associated with different habitual diets. _Cell Host Microbe_ 25, 444–453.e3 (2019). Article PubMed CAS Google Scholar * Barthel, M. et al. Pretreatment of
mice with streptomycin provides a _Salmonella enterica_ serovar Typhimurium colitis model that allows analysis of both pathogen and host. _Infect. Immun._ 71, 2839–2858 (2003). Article CAS
PubMed PubMed Central Google Scholar * Ritchie, J. M. in _Enterohemorrhagic Escherichia coli and Other Shiga Toxin-Producing E. coli_ Vol. 2, 175–195 (American Society of Microbiology,
2014). * Chung, H. et al. Gut immune maturation depends on colonization with a host-specific microbiota. _Cell_ 149, 1578–1593 (2012). Article CAS PubMed PubMed Central Google Scholar *
Lee, S. M. et al. Bacterial colonization factors control specificity and stability of the gut microbiota. _Nature_ 501, 426–429 (2013). Article CAS PubMed PubMed Central Google Scholar
* Wright, E. K. et al. Recent advances in characterizing the gastrointestinal microbiome in Crohn’s disease: a systematic review. _Inflamm. Bowel Dis._ 21, 1219–1228 (2015). Article
PubMed Google Scholar * Abrahamsson, T. R. et al. Low gut microbiota diversity in early infancy precedes asthma at school age. _Clin. Exp. Allergy_ 44, 842–850 (2014). Article CAS PubMed
Google Scholar * Maeda, Y. et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. _Arthritis Rheumatol._ 68, 2646–2661 (2016).
Article CAS PubMed Google Scholar * Kalina, U. et al. Enhanced production of IL-18 in butyrate-treated intestinal epithelium by stimulation of the proximal promoter region. _Eur. J.
Immunol._ 32, 2635–2643 (2002). Article CAS PubMed Google Scholar * den Besten, G. et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host
energy metabolism. _J. Lipid Res._ 54, 2325–2340 (2013). Article CAS Google Scholar * De Vadder, F. et al. Microbiota-produced succinate improves glucose homeostasis via intestinal
gluconeogenesis. _Cell Metab._ 24, 151–157 (2016). Article PubMed CAS Google Scholar * Goodman, A. L. et al. Extensive personal human gut microbiota culture collections characterized and
manipulated in gnotobiotic mice. _Proc Natl Acad Sci U S A._ 108, 6252–6257 (2011). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank the
members of the Strowig laboratory for valuable discussions. We thank the staff of the animal unit and the genome analytics core facility at the Helmholtz Institute for Infection Research and
Sabine Kaltenhäuser and Gesa Martens for excellent technical support. We thank Dr. Sabine Gronow for valuable advice on anaerobic culturing. We are thankful to Dr. Lidia Bosurgi, Madeleine
Hamley, and Simon Delandre for the valuable input on flow cytometry data analysis. The project was kindly supported by the Helmholtz Association (#VH-NG-933 to T.S.) and by the Deutsche
Forschungsgemeinschaft (DFG, German Research Foundation, projects STR-1343/1 and STR-1343/2 as well as under Germany’s Excellence Strategy – EXC 2155 “RESIST” – project ID 39087428). Open
access funding provided by Projekt DEAL. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Microbial Immune Regulation, Helmholtz Center for Infection Research, Braunschweig,
Germany Aida Iljazovic, Urmi Roy, Eric J. C. Gálvez, Till R. Lesker, Bei Zhao, Achim Gronow, Lena Amend & Till Strowig * Hannover Medical School, Hannover, Germany Eric J. C. Gálvez
& Till Strowig * Bacterial Metabolomics, Leibniz institute DSMZ-German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany Sabine E. Will & Meina Neumann-Schaal *
Department of Bioinformatics and Biochemistry, BRICS, Technische Universität Braunschweig, Braunschweig, Germany Julia D. Hofmann & Kerstin Schmidt-Hohagen * Mouse Pathology, Helmholtz
Center for Infection Research, Braunschweig, Germany Marina C. Pils * Centre for Individualised Infection Medicine, Hannover, Germany Till Strowig Authors * Aida Iljazovic View author
publications You can also search for this author inPubMed Google Scholar * Urmi Roy View author publications You can also search for this author inPubMed Google Scholar * Eric J. C. Gálvez
View author publications You can also search for this author inPubMed Google Scholar * Till R. Lesker View author publications You can also search for this author inPubMed Google Scholar *
Bei Zhao View author publications You can also search for this author inPubMed Google Scholar * Achim Gronow View author publications You can also search for this author inPubMed Google
Scholar * Lena Amend View author publications You can also search for this author inPubMed Google Scholar * Sabine E. Will View author publications You can also search for this author
inPubMed Google Scholar * Julia D. Hofmann View author publications You can also search for this author inPubMed Google Scholar * Marina C. Pils View author publications You can also search
for this author inPubMed Google Scholar * Kerstin Schmidt-Hohagen View author publications You can also search for this author inPubMed Google Scholar * Meina Neumann-Schaal View author
publications You can also search for this author inPubMed Google Scholar * Till Strowig View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
A.I. and T.S. designed the experiments and wrote the paper with input from co-authors. A.I., U.R., B.Z., A.G., and L.A. performed experiments, and A.I. performed most of the analysis.
E.J.C.G. and T.R.L. supported the analysis of 16S rRNA sequencing. K.S.H., S.E.W., J.D.H., and M.N.S. performed metabolism-related experiments and analysis. M.C.P. performed histological
evaluation and analysis. T.S. supervised the study. CORRESPONDING AUTHOR Correspondence to Till Strowig. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIG. 1 SUPPLEMENTARY FIG. 2 SUPPLEMENTARY FIG. 3 SUPPLEMENTARY FIG. 4 SUPPLEMENTARY FIG. 5 SUPPLEMENTARY FIG. 6 SUPPLEMENTARY TABLE 1 SUPPLEMENTARY
TABLE 2 SUPPLEMENTARY TABLE 3 SUPPLEMENTARY TABLE 4 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Iljazovic, A., Roy, U., Gálvez, E.J.C. _et al._ Perturbation of the gut microbiome by _Prevotella spp_. enhances host susceptibility to
mucosal inflammation. _Mucosal Immunol_ 14, 113–124 (2021). https://doi.org/10.1038/s41385-020-0296-4 Download citation * Received: 17 September 2019 * Revised: 06 April 2020 * Accepted: 23
April 2020 * Published: 20 May 2020 * Issue Date: January 2021 * DOI: https://doi.org/10.1038/s41385-020-0296-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative