Regulating colonic dendritic cells by commensal glycosylated large surface layer protein A to sustain gut homeostasis against pathogenic inflammation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Microbial interaction with the host through sensing receptors, including SIGNR1, sustains intestinal homeostasis against pathogenic inflammation. The newly discovered commensal
Propionibacterium strain, P. UF1, regulates the intestinal immunity against pathogen challenge. However, the molecular events driving intestinal phagocytic cell response, including colonic
dendritic cells (DCs), by this bacterium are still elusive. Here, we demonstrate that the glycosylation of bacterial large surface layer protein A (LspA) by protein O-mannosyltransferase 1
(Pmt1) regulates the interaction with SIGNR1, resulting in the control of DC transcriptomic and metabolomic machineries. Programmed DCs promote protective T cell response to intestinal
Listeria infection and resist chemically induced colitis in mice. Thus, our findings may highlight a novel molecular mechanism by which commensal surface glycosylation interacting with
SIGNR1 directs the intestinal homeostasis to potentially protect the host against proinflammatory signals inducing colonic tissue damage.
Commensal bacteria, via their surface layer (S-layer) gene products, and the gastrointestinal phagocytic cells expressing sensing receptors (e.g., SIGNR1) synergistically interact to
fine-tune the T cell signaling that is critical for protecting the host against pathogenic inflammation exerted by intestinal infections.1,2 In this process, bacterial S-layer macromolecules
along with induced metabolites transduce critical signals via cognate receptors into these cells that profoundly control the host homeostasis to protect against tissue damage.3,4 Although
the bacterial S-layer proteins display a similar architecture composed of a peptidoglycan layer decorated with proteins and polysaccharides, various modifications, particularly
glycosylation, exhibit strain-specific properties that differentially modify the host immune physiology.5 Disruption of mutualistic interactions of the commensal’s S-layer with the host
triggers deleterious signals that may manifest in pathogenic inflammation potentially impairing the intestinal barrier function.6 Thus, understanding how host intestinal immunity is
regulated through the recognition of these well-structured bacterial gene products by their cognate receptors7 to coordinate protective immune responses is currently of particular
therapeutic significance and requires further mechanistic investigations.8
Propionibacterium strain, P. UF1, is a newly discovered commensal bacterium isolated from the gut microbiota of premature infants fed human breast milk.9 This bacterium increases the
frequency of colonic Th17 and Treg cells9 involved in mucosal barrier repair and regulation of the intestinal inflammation.10 Induced bacteria-specific Th17 cell differentiation requires the
bacterial dihydrolipoamide acetyltransferase (DlaT), an enzymatic component of the pyruvate dehydrogenase complex.9 Chromosomal deletion of dlaT gene impairs the regulation of protective
Th17 cell response to intestinal and systemic Listeria monocytogenes (L. m) infection.9,11 Furthermore, P. UF1 regulates the neonatal T cells against necrotizing enterocolitis (NEC)-like
injury in mice9 and enhances the neonatal protective T cells against intestinal pathogen infection over time.12 However, the bacterial effector mechanisms potentially instructing the
function of colonic DCs to possibly control protective T cell immunity remain largely unknown. Here, we demonstrate that the glycosylation of bacterial LspA interacting with SIGNR1 is a
pivotal factor, which transcriptionally and metabolically programs colonic DCs, leading to protective T cell activation in steady state and during intestinal infection. Further, glycosylated
LspA-SIGNR1 interaction critically protects mice against colitis-induced intestinal barrier injury. Errors in the bacterial glycosylation significantly disrupt the intestinal homeostasis,
manifesting in an inflammatory condition resulting in pathogen persistence and colonic tissue damage. Thus, this finding highlights the critical relevance of the glycosylated LspA in
programming DC immunophysiology to mitigate pathogenic inflammation and the induced colitogenic potential in mice.
Knowing the significance of bacterial S-layer complexes in communicating with host cells,13 we sought to investigate the functional relevance of P. UF1 S-layer proteins potentially involved
in the regulation of colonic DC function. One of the S-layer proteins of P. UF1 is LspA, which contains six N-terminal LGFP repeats [L-G-X-P-X(7-8)-D/N-G] involved in cell membrane anchoring
and a C-terminal N-acetylglucosaminidase-like domain, potentially implicated in bacterial cell wall metabolism (Supplementary Fig. 1a). Phylogenetic analysis demonstrated that LspA was
highly conserved in P. UF1 and closely related Propionibacterium strains. Moreover, LspA homologs were also found in evolutionarily distantly related bacterial species, including
Bifidobacterium and Geodermatophilus (Supplementary Fig. 1b). Thus, to elucidate the functional significance of LspA within P. UF1 molecular machinery, the lspA gene was deleted from the
bacterial chromosome, resulting in ΔlspA P. UF1 (Fig. 1a, b). ΔlspA P. UF1 demonstrated enhanced bacterial clusters and autoagglutination (Fig. 1c), suggesting the critical involvement of
this protein in bacterial S-layer structures. Further, deletion of LspA significantly affected the bacterial transcriptomic and metabolomic signaling, including differential metabolic
pathways involved in peptidoglycan biosynthesis, amino and nucleotide sugar metabolism, fructose and mannose metabolism (Supplementary Fig. 2a). The analyzed metabolites involved in protein
glycosylation (e.g., GDP-mannose and mannose 1-phosphate), along with those important for cell wall metabolism (e.g., GlcNAc-6-phosphate and UDP-GlcNAc), were significantly deregulated
within ΔlspA P. UF1 compared to P. UF1 (Supplementary Fig. 2b). RNA-Seq analysis further documented differentially expressed genes implicated in bacterial mannosylation and nucleotide sugar
metabolism, including phosphatidylinositol mannosyltransferase pimA14 and GDP-mannose-dependent alpha-mannosyltransferase mgtA15 (Supplementary Fig. 2c). Thus, these data emphasize the
importance of LspA in the regulation of glycan metabolism that may fundamentally impact the bacterial S-layer glycosylation.
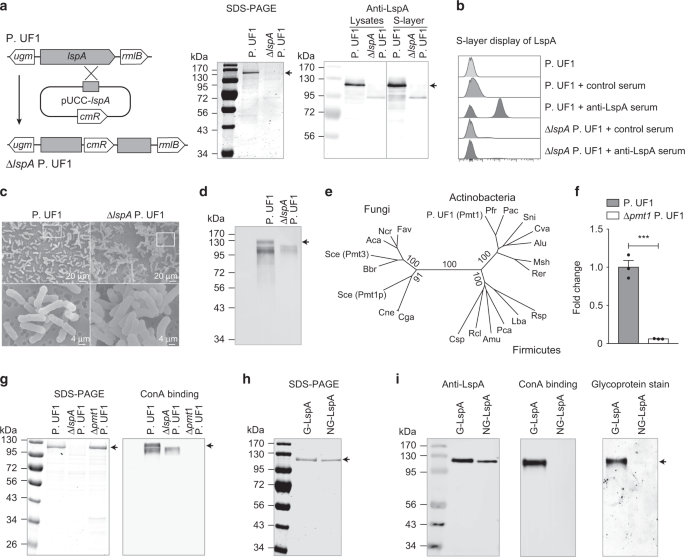
Glycosylation of LspA by Pmt1. a Identification of ΔlspA P. UF1 strain. Genetic scheme for disruption of lspA gene by chromosomal insertion of plasmid pUCC-lspA (left). SDS-PAGE (middle) and
Western blot (right) showing LspA protein was completely absent in ΔlspA P. UF1. CmR, chloramphenicol resistant gene. b Flow cytometric analysis of S-layer expression of LspA in P. UF1 and
ΔlspA P. UF1 using anti-LspA serum antibodies. Control serum was derived from unimmunized mice. c Scanning electron microscopy (SEM) images of P. UF1 and ΔlspA P. UF1. SEM images in the
bottom panel are magnified from the indicated zoom in the top panel. d ConA binding assay for S-layer proteins isolated from P. UF1 and ΔlspA P. UF1. e Neighbor-joining phylogenetic tree
showing the relationship of Pmt proteins from Actinobacteria, Firmicutes, and Fungi. f qRT-PCR analysis of pmt1 expression in P. UF1 and Δpmt1 P. UF1. g SDS-PAGE analysis and ConA binding
assay of S-layer proteins isolated from P. UF1, ΔlspA P. UF1, and Δpmt1 P. UF1. h SDS-PAGE analysis of purified glycosylated LspA (G-LspA) and non-glycosylated LspA (NG-LspA). i Equal
amounts of purified G-LspA and NG-LspA proteins were separated by SDS-PAGE and analyzed by Western blot using anti-LspA antibodies, ConA binding assay, and ProQ Emerald 300 glycoprotein
staining. Arrows indicate the LspA protein
The bacterial S-layer proteins are generally glycosylated for their noncovalent anchoring to the cell surface and interactions with environmental factors and host immune cells.5 Data
demonstrated that the S-layer of P. UF1 reacted with concanavalin A (ConA), a mannose/glucose-binding lectin, while LspA deficiency resulted in the loss of ConA binding (Fig. 1d), suggesting
that LspA may be glycosylated. Therefore, we investigated the glycosyltransferases responsible for adding glycol moieties to the bacterial S-layer using genome-wide bioinformatic analysis.
Pmt1, a potential member of protein O-mannosyltransferase family responsible for mannose transfer to serine and threonine residues of proteins in yeast16, was identified in P. UF1 genome.
Further analysis demonstrated that Pmt1 homologs fell into separate and loosely related groups of bacteria, including Actinobacteria (Fig. 1e). The pmt1 gene was then deleted in P. UF1 to
assess the status of LspA glycosylation (Fig. 1f). Although Δpmt1 P. UF1 showed similar S-layer protein patterns when compared to P. UF1, no binding to ConA was observed for S-layer proteins
isolated from Δpmt1 P. UF1 (Fig. 1g). To underscore the role of Pmt1 in the glycosylation of LspA, this protein was overproduced by ΔlspA P. UF1 and Δpmt1 P. UF1 strains to isolate and
purify the glycosylated LspA (G-LspA) and non-glycosylated LspA (NG-LspA), respectively (Fig. 1h). While both G-LspA and NG-LspA were recognized by anti-LspA serum antibodies, only purified
G-LspA bound to ConA and illuminated staining for glycoprotein (Fig. 1i). Thus, Pmt1 is critically required for the glycosylation of LspA.
To elaborate on the nature of LspA glycosylation, the purified G-LspA and NG-LspA proteins were treated with PNGase F to release any N-glycans, permethylated and analyzed by MALDI-MS. Here,
no N-linked glycans were detected in either of the LspA proteins (Supplementary Fig. 1c). The O-linked glycans were released by β-elimination procedure and permethylated prior to MALDI-MS
analysis. Signals corresponding to Hex1-Hex6 were observed in the G-LspA protein (Fig. 2a), but not in the NG-LspA (Supplementary Fig. 1d). Furthermore, glycan compositional analysis
demonstrated that mannose (Man) was the major monosaccharide of G-LspA, with a retention time of 10.9 min (Fig. 2b). In contrast, NG-LspA showed no traces of Man (Supplementary Fig. 1e).
Note that a minor peak of glucose was also detected in both samples. However, glucose, as a very common contaminant, could be derived from reagents and detected as a free and minor glucose
peak in HPAEC analysis. Moreover, glycomic analysis of released oligosaccharides demonstrated that Man3 was the major glycan in the G-LspA, comprising 77% of the total glycans. While Man2
and Man4 oligosaccharides were minor glycans, only traces of Man1, Man5 and Man6 were detected in the G-LspA (Fig. 2c).
Recognition of glycosylated LspA by SIGNR1. a O-linked glycan analysis of β-eliminated and permethylated G-LspA protein. Asterisk (*) indicates the contamination peak derived from reagents.
b Glycosyl composition analysis of monosaccharides in the G-LspA sample. Trace levels of glucose were detected as a common contaminant derived from reagents. c Summary table showing the
relative percentage of O-linked glycans from G-LspA. d LspA glycopeptides identified by glycoproteomics. The glycan composition and potential glycosylation sites (bolded) are shown. e
Glycosyl linkage analysis of the O-glycans. Asterisks (*) indicate non-carbohydrate peaks. RT, retention time. f Binding of G-LspA to SIGNR1-hFc. Equal amounts of G-LspA and NG-LspA proteins
were separated by SDS-PAGE and the specific interactions with SIGNR1-hFc were demonstrated, as no binding was detected in the presence of EDTA. g ELISA binding assays demonstrating G-LspA
binding specificity with SIGNR1-hFc. The binding was abolished in the presence of EDTA, competitive zymosan, or blocking antibody to SIGNR1. h ELISA showing G-LspA did not bind to
Dectin-1-hFc, as a control fusion protein. Zymosan served as a positive control. i Binding kinetics between G-LspA and SIGNR1-hFc. Various amounts of LspA proteins were coated on ELISA
plates and incubated with SIGNR1-hFc (0.5 μg/ml). Binding was detected using HRP-conjugated anti-human IgG antibody. Kd, LspA concentration required to achieve a half-maximum binding with
SIGNR1-hFc
The purified G-LspA protein was then digested with trypsin and elastase, resulting in peptides with >75% coverage (Supplementary Fig. 1a). LC-MS/MS analysis of the enriched peptides revealed
seven O-glycopeptides at the N-terminus of LspA (Fig. 2d). In addition to the 41 threonine/serine residues involved in Man attachment, adjacent proline and alanine residues that may
facilitate local conformational changes for protein O-glycosylation17 were also found in all the glycopeptides (Fig. 2d). Further, GC-MS analysis was performed to investigate the glycosyl
linkages and positions of released O-glycans. Data demonstrated that Man oligosaccharides of LspA were short linear chains interconnected via (1 → 6)-linkage and (1 → 2)-linkage (Fig. 2e).
Man(1 → 6)Man(1 → 2)Man was the major trisaccharide component in the G-LspA, while disaccharide Man(1 → 6)Man and tetrasaccharide Man(1 → 6)Man(1 → 2)Man(1 → 2)Man comprised a small
percentage (Fig. 2e). These data indicate that LspA is a mannosylated S-layer glycoprotein with linear short-chain O-glycans.
SIGNR1 expressed by myeloid DCs recognizes characteristic molecular patterns with complex mannose and fucose structures in bacteria and fungi.18 Recently, we observed the binding of P. UF1
to SIGNR1, but not SIGNR3.9 To precisely delineate the role of G-LspA binding to SIGNR1 in regulating DCs to subsequently initiate T cell commitment, the interaction of G-LspA with SIGNR1
was biochemically investigated. Here, G-LspA and NG-LspA proteins were first separated by SDS-PAGE, transferred to PVDF membrane, and then incubated with SIGNR1-hFc fusion protein. This
protein binding complex was analyzed by subsequent incubation with anti-human Fc secondary antibody. Data demonstrated that the purified G-LspA bound specifically to SIGNR1-hFc, and this
binding was abolished in the presence of EDTA (Fig. 2f). Further, SIGNR1 interaction with G-LspA was assessed by ELISA showing G-LspA binding to SIGNR1-hFc (Fig. 2g), but not to Dectin-1-hFc
used as a control fusion protein (Fig. 2h). This binding was completely blocked by pre-incubation of SIGNR1-hFc with anti-SIGNR1 antibody, or with the competitive ligand zymosan that is
composed of β-glucan, α-mannan and mannosyl proteins (Fig. 2g). In contrast, no binding was observed for NG-LspA using similar assays (Fig. 2g). Furthermore, the G-LspA exhibited a
dose-dependent binding with SIGNR1-hFc using protein concentrations ranging from 0.08 μg/ml to 10 μg/ml with a Kd value of 2.617 μg/ml G-LspA (Fig. 2i). In contrast, NG-LspA did not react
with SIGNR1-hFc, even with higher protein concentrations up to 20 μg/ml (Fig. 2i), highlighting the specificity of SIGNR1 binding to glycosylated LspA.
SIGNR1 was majorly expressed by colonic CD11chi MHCIIhi DCs (Supplementary 3a, b). To shed light on the relevance of LspA glycosylation interacting with colonic DCs, CD11chi MHCIIhi CD11b+
F4/80− DCs were FACS sorted (Supplementary Fig. 4a, b) from mice gavaged with P. UF1 or ΔlspA P. UF1 to analyze their transcriptome by RNA-Seq. Data demonstrated the modulation of
costimulatory molecules (Cd40, Cd80, Cd86, and Tnfsf4) in colonic DCs by P. UF1 (Fig. 3a). NF-κB signaling (Casp4, Traf1, Tnfrsf1b, Mapk6, Nfkbie, and Nfkbiz), cytokine/chemokine transcripts
(e.g., Il1b, Il12b, Cxcl1, and Cxcl2), and antigen presentation-related genes (e.g., Serpinb9, Rab8b) were also significantly augmented in DCs derived from mice gavaged with P. UF1 compared
to ΔlspA P. UF1. In contrast, DCs derived from ΔlspA P. UF1-gavaged mice showed activation of Sod3, Rhoh and Klf2, which may instruct functional suppression in these cells. Migrating DCs
constitutively express genes with regulatory functions.19 Accordingly, DCs derived from mice gavaged with P. UF1 had elevated expression of genes, such as Cd274, Spred1, Etv3, Tnfnip2,
Stat3, Stat4, and Stat5a. These cells were also enriched with genes implicated in DC development (Edn1, Cish), migration (Nrp2, Ccr10, Eps8), and differentiation (Pdk1, Hilpda), while DCs of
ΔlspA P. UF1-gavaged mice exhibited increased quantities of genes suppressing cellular regulatory functions (e.g., Cyr61, Sdc1). In addition, transcription factor Irf4 controlling Th17 cell
cytokine machinery,20 cell cycle inhibitor Cdkn1a involved in Treg cell formation,21 and T cell-attracting chemokines Ccl17 and Ccl22, were all significantly activated in DCs of
P.UF1-gavaged mice. In contrast, ΔlspA P. UF1 enhanced the DC expression of Cd55 and Gilz genes associated with suppression of T cell function.22,23
Modulation of DC activation by glycosylated LspA in steady state. a–b C57BL/6 (Signr1+/+) mice were gavaged with P. UF1 or ΔlspA P.UF1, and colonic CD11chi MHCIIhi CD11b+ F4/80− DCs were
isolated for RNA-Seq analysis. Heatmap showing a selection of top differentially expressed genes (FDR P