Strain sensor on a chip for quantifying the magnitudes of tensile stress on cells
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT During cardiac development, mechanotransduction from the in vivo microenvironment modulates cardiomyocyte growth in terms of the number, area, and arrangement heterogeneity.
However, the response of cells to different degrees of mechanical stimuli is unclear. Organ-on-a-chip, as a platform for investigating mechanical stress stimuli in cellular mimicry of the in
vivo microenvironment, is limited by the lack of ability to accurately quantify externally induced stimuli. However, previous technology lacks the integration of external stimuli and
feedback sensors in microfluidic platforms to obtain and apply precise amounts of external stimuli. Here, we designed a cell stretching platform with an in-situ sensor. The in-situ liquid
metal sensors can accurately measure the mechanical stimulation caused by the deformation of the vacuum cavity exerted on cells. The platform was applied to human cardiomyocytes (AC16) under
cyclic strain (5%, 10%, 15%, 20 and 25%), and we found that cyclic strain promoted cell growth induced the arrangement of cells on the membrane to gradually unify, and stabilized the cells
at 15% amplitude, which was even more effective after 3 days of culture. The platform’s precise control and measurement of mechanical forces can be used to establish more accurate in vitro
microenvironmental models for disease modeling and therapeutic research. SIMILAR CONTENT BEING VIEWED BY OTHERS ON-CHIP INTEGRATED OPTICAL STRETCHING AND ELECTROROTATION ENABLING SINGLE-CELL
BIOPHYSICAL ANALYSIS Article Open access 15 June 2020 MICROENGINEERED PLATFORMS FOR CHARACTERIZING THE CONTRACTILE FUNCTION OF IN VITRO CARDIAC MODELS Article Open access 28 February 2022 A
SOFT AND ULTRASENSITIVE FORCE SENSING DIAPHRAGM FOR PROBING CARDIAC ORGANOIDS INSTANTANEOUSLY AND WIRELESSLY Article Open access 25 November 2022 INTRODUCTION Multidimensional mechanical
stresses are produced in human organs by the in vivo microenvironment1,2,3. The human cardiomyocyte cell line AC16 serves as a resource for mimicking heart dynamics4, and cardiomyocytes can
be subjected to continuous mechanical stimulation to mimic in vivo dynamics. By adding external stimuli to cells cultivated in vitro, scientists have created several platforms and techniques
to examine changes in cell dynamics at the cellular level. These techniques include electrical stimulation5,6,7,8, direct stretching9,10,11, multiple spindle stretching12,13,14, and a range
of additional approaches. Clerc et al.15 demonstrated that transient mechanical stimulation causes cardiomyocytes to develop hypertrophic lesions. In contrast, Saucerman et al.16 discovered
that the lack of mechanical loading of the stimulus in vivo hampered the growth of cardiomyocytes, which in turn reduced cardiac contractility. This highlights the challenge in research on
using mechanical methods to prompt cells to develop their biological characteristics: accurately measuring the mechanical force applied to cells within an in vitro culture system designed
for stimulation. Microfluidic systems have been utilized in cell culture more frequently in recent years17,18,19,20, particularly in dynamic cell culture systems that imitate the intricate
movements of bodily organs21,22,23,24. Microfluidic devices allow human manipulation to mimic intricate movements and possess precise dimensions that simplify the process of structural
design. The elastic film deforms when the pressure in the vacuum chamber is changed by an external air valve, which is a simple way to provide tension stimulation inside the chip. However,
the necessity for exact quantification versus externally induced stimuli cannot be satisfied by microfluidic chips using vacuum chambers25,26 and cell drums27. To address this need,
microfluidic chips with in-situ sensors need to be designed. Sensors28,29 are incorporated into chips, and accurate strain can be obtained by piezoresistive30, piezoelectric31,32,33, and
crack sensing34 methods. However, there are few examples of in-situ sensors on chips that include mechanical stimulation. These techniques are either not suitable for continuous monitoring
or require complicated observation equipment. In our work, we introduced a cell stretching platform that features an in-situ sensor. This platform continuously applies mechanical stimulation
to cells attached to a flexible film. The stimulation is achieved by stretching and contracting the film, which is driven by the expansion and compression of a vacuum cavity inside a
microfluidic chip. The in-situ sensor, which was made with liquid metal (Galinstan) and soft lithography, enables real-time monitoring of the amount of mechanical stress (5%, 10%, 15%, 20%,
25%) placed on the film (cells). The thin film sensors we designed are highly sensitive, have a linear response, and can automatically adjust to temperature changes. These benefits are
achieved by harnessing the responsive nature of liquid metals and incorporating a design that uses embedded Wheatstone bridge circuits, which are known for their precise measurement
capabilities. Cardiomyocytes stimulated for 24 h on the aforementioned platform showed greater activity and induced the arrangement in cell organization. Our results indicated that
cardiomyocyte maturation is enhanced by mechanical stimulation in a strain magnitude-dependent manner. As we increased the amount of cyclic strain, we observed an increase in both the number
and cell area of cardiomyocytes, with the most favorable results occurring at 15% cyclic strain. After more than 2 days (60 h) of incubation, cell viability significantly increased under
15% cyclic strain, and the heterogeneity of cell direction growth was largely consistent under stretching conditions. Cellular activity corresponded to a faster rate of cell proliferation
and an increase in the area of individual cells; the alignment pattern corresponded to a tendency of skeletal proteins to align along the direction of stimulation. The experimental results
demonstrated that our embedded in situ sensor-on-chip is a useful platform for measuring the mechanical stimulation of cells. RESULTS AND DISCUSSION UNIAXIAL STRETCHING AND DEFORMATION
CONVERSION The paper studied a rectangular area (400 μm × 8000 μm) located in the middle of the air chamber in the culture chamber (shown in Fig. S2). This paper examined the direction of
the polydimethylsiloxane (PDMS) membrane stretching to define the specific stress of cells during mechanical stimulation. PS beads were encapsulated in a PDMS membrane (5 μm in diameter;
fluorescence: red) for a more precise quantitative study of deformation. The sequential image of particles at distinct stretching levels is shown in Fig. 1b from the initial condition to the
maximum range with a constant volume decrease of 500 μL. A displacement study of the stretched membrane, specifically for contrast in various directions (in the _x_-axis direction and the
_y_-axis direction), was processed using ImageJ. The reproducibility and consistency of the fabrication process were confirmed by using six sets of data from three separate chips. At a
maximum withdrawal of 3000 μL, there is an almost linear deformation tendency in the _x_-axis, with a magnitude of 2.5% in the PDMS membrane. However the deformation trend in the _y_-axis
tends to be steady. It is also proven that there is a general trend toward a linear rise in displacement from the membrane’s center to its edge. As shown in Fig. 1a, the stretching of the
entire uniaxial cell stretching region is simulated by COMSOL finite element simulation. Since the four borders of the rectangle are connected to the same chamber, the four borders of the
rectangle region will be subjected to the same pulling force when the syringe is withdrawn. The surface stress distribution of the whole cell culture area under this pressure was obtained
via simulation. The deeper the color is, the greater the stress. Therefore, combined with the displacement experiments and simulation results of the PS balls, the deformation of the film is
approximately uniaxial deformation. This study demonstrated that even with common syringes and injectors acting as an external input, pure uniaxial direction stimulation was produced with
our chip platform. It is challenging to observe a change of only 10 μm on a horizontal plane with the naked eye or even with a low-magnification microscope. The aforementioned
cell-stretching area, which reaches 2.5% at maximum extraction, is only 400 μm wide. Therefore, it is even more challenging to precisely and quantitatively measure the amount of stretching
of the film at various extraction levels. Since the deformation of the film in the upper layer of the air cavity, which is connected to the sensitive area, leads to the stretching
deformation of the film in the sensitive area when it is subjected to pressure, the measurement of the deformation of the film in the sensitive area can be converted into a measurement of
the degree of tension and compression of the suspended film in the upper layer of the vacuum cavity35,36. The deflections of the suspended PDMS films embedded with fluorescent particles were
observed and calibrated using a lateral microscope by Dou21 from the University of Toronto and Sato37 from Tokyo. Another method34 using electrical signals is crack-sensing to increase the
sensitivity of the sensor, but this method has high complexity and low repeatability in manufacturing. Since lateral microscopy is very demanding in terms of equipment and environment, we
used a microfluidic channel incorporated into the suspension film and liquid metal to obtain a highly accurate miniature in situ strain transducer35,36. This method allows us to obtain the
magnitude of tensile deformation in the sensitive region utilizing an on-chip integrated sensor coupled to an external circuit. The described in situ sensor on a chip is designed and
fabricated based on an embedded Galinstan microfluidic channel with a height of 20 μm and a width of 50 μm (Fig. 2c and Fig. S2). The fabrication process is shown in detail in Fig. 2b and
Fig. S1. The simulation diagram in Fig. 1f shows the displacement of the film above and below the liquid metal flow channel after the pressure is applied, and the bottom diagram in Fig. 1f
shows the results of the membrane deformation after the above pressure. It can be seen from the simulation results that the cross-section will not change greatly, but because the liquid
metal channel layer is a cavity below, the film will sag down with the liquid metal, and the length of the liquid metal will change greatly, so the resistance length will increase. According
to the resistance calculation formula: $$R=\frac{\rho L}{S}$$ (1) where _R_ is the resistance value of the sensor, _L_ is the length of the flow path, _S_ is the cross-section of the flow
path, and _ρ_ is the resistivity of the liquid metal is constant. As _L_ increases l, _S_ basically remains the same, and the resistance of the overall sensor increases during stretching.
The middle of the channel receives the largest deformation force, corresponding to the most significant strain variable _ε_max, given by $${\varepsilon }_{\max
}=\frac{3P{L}_{0}^{2}(1-{v}^{2})}{8{h}^{2}E}$$ (2) The assumed values are _P_, the pressure exerted on the film; _L__0_, the channel width; _h_, the diaphragm thickness; _v_, is the
Poisson’s ratio; and _E_, is Young’s modulus. The sides around the rotor are fixed. $${\varepsilon }_{\min }=0$$ (3) Assuming that the strain gauge factor of the liquid metal equals 2, for a
given input voltage _V_in, the theoretical output voltage _V_out of the sensor is approximated as $${V}_{\rm{out}}=0.75\frac{P{L}_{0}^{2}(1-{v}^{2})}{{h}^{2}E}{V}_{\rm{in}}$$ (4) After
calculation, it can be concluded that the pressure _P_ and the voltage are directly proportional to each other, which is also supported by the simulation results in Fig. 2a. The above
considerations are ideal states based on a number of assumptions of the calculation. The actual experimental results and theoretical calculations have some deviation but basically tend to be
proportional to the relationship. ON-CHIP STRAIN SENSING QUANTIFIED STIMULUS AMPLITUDE We established and assessed several critical performance characteristics in order to fully
characterize the performance of the microfluidic diaphragm sensor. Sensitivity, linearity, reproducibility, and stability are some of these indications. In contrast to the linearity obtained
from the sensitivity regression line, the sensitivity measures the effect of each unit of pressure on the sensor voltage. Since the use of sensors with resistive sensing mechanisms and
temperature sensitivity is a drawback of liquid metals38, stability generally relates to retaining the same resistance at different temperatures39. Figure 3a, b depicts two types of sensors:
a single-arm sensor and a Wheatstone bridge sensor with built-in temperature correction. The single-arm sensor underwent temperature sensitivity testing, as depicted in Fig. 3d. Since
liquid metals are sensitive to changes in ambient temperature, the lack of additional temperature correction has a substantial effect on the repeatability and stability of the sensor. By
measuring the difference in resistance change, a bridge sensor with temperature compensation is created to eliminate the impact of the environment and temperature. The physical and localized
perspective of the sensor is depicted in Fig. 3c. After that, we calibrated the connection between the external syringe extraction (perfusion) flow rate, the vacuum chamber pressure, and
the relative change in sensor voltage (compared to the voltage output in the state of no external pressure being applied) (Fig. 3e, f). We determined the internal pressure of the vacuum
chamber (5.55, 10.05, 16.47, 21.35, and 24.81 kPa) and the flow rate of the syringe withdrawal (200, 400, 600, 800, and 1000 μL). Figure 3g shows the real-time provenance voltage response
for three cycles of extraction and perfusion at different tensile stresses (5%, 10%, 15%, 20%, and 25%), demonstrating the repeatability and stability of the sensor. In addition, fatigue
testing was performed to assess the possible structural degradation of the sensor during prolonged use (Fig. 3h, i). Dynamic stimulation of the sensor for three days using maximum perfusion
(25%) did not significantly change either the sensor voltage or the air pressure, which verified the stability of the device under long-term mechanical stimulation. AREA AND INCREMENT
EXPRESSION ENHANCEMENT UNDER MECHANICAL STIMULATION Since the heart beats continuously and independently, it is constantly exposed to various physiological stimuli, primarily mechanical
stimuli40,41. As a result, mechanical stimuli generated by cardiomyocytes can be recognized as mechanical stimuli. We postulated that dynamic cardiomyocyte culture at various stimulation
levels would classify cardiac cell disease and physiology42,43. Cardiomyocytes were cultivated in the abovementioned chip with integrated in situ sensors and subjected to calibrated 5%, 10%,
15%, 20 and 25% cyclic stretch conditions for 24 h to confirm the notion mentioned above that cardiomyocytes respond to externally applied mechanical stimuli. The findings revealed that the
number of proliferating cells increased and that the size of the area changed slightly; however, more significantly, 15% mechanical stimulation promoted the overall alignment of the cells.
This phenomenon was more obvious during long-term cultivation (60 h). Using a microscope to observe the sensitive area inside the annular air cavity, we found that mechanical stimulation
significantly promoted cell growth (Fig. S5 and Fig. 4). Stimulation of cardiomyocytes on the film with different strain amplitudes (no stimulation, 5%, 10%, 15%, 20%, and 25%) resulted in a
rapid spreading of the cells and a corresponding increase in the cell area and number of cells. The changes in cell number and area after 24 h of continuous stimulation (control: cell
number growth rate of 25.16%, cell area growth rate of 3.52%; 5%: cell number growth rate of 51.34%, cell area growth rate of 7.57%; 10%: cell number growth rate of 78.13%, cell area growth
rate of 15.87%; 15%: cell number growth rate of 85.80% and cell area growth rate of 20.36%; 20%: cell number growth rate of 88.28% and cell area growth rate of 3.02%; 25%: cell number growth
rate of 55.17% and cell area growth rate of 0.46%) are shown in Fig. S5. At 15% mechanical strain, there was a stable and rapid increase in cell number and area as the amplitude of
mechanical stimulation increased. However, at higher mechanical strain levels, we noticed that the cells partially detached from the device mold and even started to cluster by death. We
evaluated the effect of mechanical stimulation on cell alignment by determining the angle and length of the long axis of the cells (Fig. S6 and Fig. 5). Compared with the control group, a
certain degree of mechanical stimulation was able to align the length and angle of the cells (_n_ = 99 cells in each case), and it has been shown that explicit mechanical strain leads to the
reorganization of the various cell types along the direction of the strain. Some scholars have shown that mechanical strain causes various cells to realign along the direction of the
strain, and for cardiomyocytes, the alignment behavior is regulated by mechanotransduction resulting in “strain avoidance44,45”. Through the above analysis, we can see a significant
difference between the strains applied to our chip in the transverse and longitudinal directions, which is likely the reason for the rearrangement of the cardiomyocytes. After the cells were
incubated for an extended period until the cell culture chamber was filled with cells under 15% stimulation, the consistency of our platform’s cell orientations became more obvious after 60
h of incubation. The design of the vacuum cavity needs to be improved to add uniaxial control in various directions, devices that can apply dynamically controllable anisotropic biaxial
strains to cells, and even devices with circumferentially oriented stresses to further investigate the impact of stress direction on cell alignment46. Fluorescence immunostaining is
performed to further characterize the response of cardiomyocytes to stimuli. The design of multimodal built-in sensors is required to obtain more multidimensional information about the
environment inside the chip (cell culture chamber) prior to further research on immediate testing47 of combined stimuli, such as electrical stimulation, to improve the simulation of the
cellular environment. CONCLUSION The research in this paper focused on the precise quantification of mechanical stimuli via embedded sensors in organ-on-a-chip. We developed a platform with
an integrated vacuum chamber to provide precise uniaxial tensile control of the biochemical and mechanical environment experienced by the in vivo microenvironment. Sensitive liquid metal
flexible sensors were integrated into the vacuum chamber to measure the deformation of the membrane in the sensitive region. The flexible strain sensors used a Wheatstone bridge design to
increase sensitivity, while temperature self-compensation kept the sensors free of external bias. Mechanical stimulation was applied to cells inside the organ-on-a-chip with strain
amplitudes ranging from 5 to 25% by using the abovementioned flexible sensors calibrated to the relationship between external stimulation pump flow, vacuum chamber pressure, and sensor
voltage changes. By applying different amplitudes of stimulation to cardiomyocytes, our experimental results showed that the cell number and area gradually increased with increasing
mechanical stimulation and gradually unified in terms of the consistency of cell alignment, which stabilized at 15%, with a more significant effect after 3 days of culturing. Therefore, our
in situ strain sensor on a chip is a good platform for measuring the mechanical stress on cells. The work in this paper has made innovations in the internal environment sensing of
organ-on-a-chips, which is expected to improve the precise control of the internal environment of microfluidic chips and organ-on-a-chip and promote the quantitative research on cell
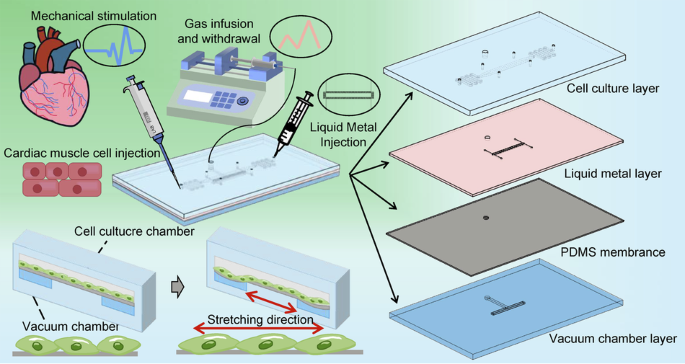
mechanical conduction results. MATERIALS AND METHODS CHIP STRUCTURE AND WORKING MECHANISMS We created a gas-chamber-surrounded chip with an integrated in situ sensor that consists of four
layers: a cell culture layer, a liquid metal layer, a PDMS membrane layer, and a vacuum chamber layer (Fig. 6b). All of the aforementioned layers are made of PDMS (Sylgard-184, Dow), which
has good light transmission characteristics and enables the microscopic examination of the chip’s interior structure and cells. The four layers of the chip are strongly bound together by
oxygen plasma (Diener Electronic, Zepto). The cell culture layer features a long channel (length: 1 cm, width: 400 μm) flanked by through-holes (depth: 2 mm, radius: 1 mm) for injecting cell
suspensions and cell culture medium. The bottom layer of the device contains the vacuum chamber, which also serves as the bottom of the cell culture chamber, allowing cells to attach and
grow against the membrane surface and stretch when the gas is extracted (Fig. 6c). The liquid metal channel (height: 20 μm, width: 50 μm), embedded in the elastic film in the middle layer,
is suspended above the vacuum chamber. The deformation of the suspended film inside can be precisely measured in real-time by an external circuit because the channel wrapped with liquid is
sensitive to film deformation. An annular vacuum chamber (length: 1 cm, width: 600 μm) surrounds a central raised platform (length: 800 μm, width: 400 μm), which is the cell stretching area
for observation. A cylindrical chamber (depth: 3 mm, radius: 1 mm) that is joined to a syringe (2 ml) and a micro control pump (LSP04-1A) is connected to the vacuum chamber. To mechanically
stimulate adherent cells, the syringe was infused, and the vacuum chamber was withdrawn to deform the suspended film layer. The integrated sensor above the air cavity is connected to an
external circuit to further process the signal, and finally, the amplitude of deformation in the sensitive area is displayed on the oscilloscope (Fig. 2a). CHIP MANUFACTURING Figure 2b shows
how the device was made. Soft lithography and negative adhesive (SU8 2050) were used to create PDMS molds for each layer of the channels. PDMS was then prepared in a 10:1 elastomer and
hardener ratio and carefully mixed before being poured onto the molds. The molds were placed in a vacuum to remove air bubbles, which enhanced the device’s observation ability. Two grams of
prepared liquid PDMS were placed in a dish and spun at 1000 rpm/min for 30 s using a spin coater (LEBO Science) to create a thin film of PDMS. The film was then dried at 65 °C for 2 h. After
demolding, the surface was cleaned of dust, through holes were punched with a 1 mm hole punch, and each layer was then adhered. The liquid metal was injected into the liquid metal channel
and sealed with a pin. SENSOR MANUFACTURING In the suspended film above the gas chamber, embedded microfluidic channels were built up, and Galinstan (a eutectic alloy of gallium, indium, and
tin) was injected into the channels. A flexible microfluidic sensor was created by filling the whole microfluidic channel with liquid metal because of its outstanding fluidity and
conductivity. This flexible microfluidic sensor can then be connected to an external circuit to determine the degree of deformation of the film. After the preparation of the chip and the
built-in sensor, the PDMS film was modified for cell culture. PIEZORESISTIVE WHEATSTONE BRIDGE SENSOR PRINCIPLE Volume changes caused by external mechanical deformation are the decisive
factor in changes in conductor resistance. A rectangular sensitive region has four edges that are covered by four sets of serpentine sensor grids, which make up the embedded strain resistor.
The four sensor grids (Fig. 1e) are connected end to end, and an equivalent Wheatstone bridge is created by using two nonadjacent segments for the reference voltage input and the other two
pairs for the voltage output. SENSOR QUANTIFICATION A barometer was utilized to calculate the pressures corresponding to various syringe extractions into the vacuum cavity to calibrate the
device. An additional part of the picture shows the tensile deformation of the film in the sensitive area for various extraction volumes (Fig. 1b). In COMSOL Multiphysics (Version 5.6,
COMSOL AB, Stockholm, Sweden), finite element analysis was used to simulate the membrane’s displacement under strain in both the transverse and longitudinal directions (Fig. 1a). The inputs
included the device’s dimensions, the suspended film’s characteristics, and the applied air pressure. It was expected that the PDMS material would have a Poisson’s ratio of 0.49 and be
isoelastic in all directions. The embedded liquid metal sensor collects resistance and voltage signals through a digital bridge (KEYSIGHT, E4980AL) and an external circuit coupled to an
oscilloscope (Tektronix, TBS 1202B). This showed the correlation between the sensor voltage and gas extraction volume. The relationship between the sensor voltage and the tensile force
applied to the film in the sensitive area was finally calibrated by combining the pressure–volume curve and the voltage–volume curve. CELL CULTURE Cells were grown in low-glucose DMEM
(Corning) supplemented with 10% fetal bovine serum (FBS, Gemini Bio Products), at 37 °C with 5% CO2 for experiments employing human cardiomyocyte (AC16), an immortalized organelle. The cells
(suspension at 0.8 × 106 cells/ml in a volume of 10 μL) were added to the cell culture chambers after Laminin (Gibco) was added to the chambers to encourage cell attachment and spreading.
This resulted in low-density monolayer cells that were ready for mechanical stimulation after 10 h. CELL IMAGING AND IMMUNOFLUORESCENCE STAINING Immunofluorescence staining of AC16
cardiomyocytes was performed to determine how mechanical stimulation affected cell maturation (Fig. S3). The cells were fixed with 4% paraformaldehyde (PFA) and permeabilized with 0.1% (w/v)
Triton X-100 for 10 min after stimulation for 24 h (60 h for long-term culture). To prevent nonspecific antibody binding after permeabilization, the cells were blocked with antibody diluent
(5% goat serum in TBST) for 90 min. The cells were incubated overnight at 4 °C with primary antibodies. Nuclei and intermediate filaments were stained with an anti-vinculin antibody
[EPR3776] and the cytoskeletal marker phalloidin (Actin), respectively. Subsequently, the cells were incubated with secondary antibodies for 2 h at room temperature. Alexa Fluor
594-conjugated secondary antibodies (goat anti-rabbit IgG, ab150077, Abcam) were used for the visualization of microtubules and intermediate filaments, respectively. The above antibodies
were diluted 1:1000 in antibody dilution buffer. To stain the microfilaments, the cells were incubated with phalloidin-tetramethylrhodamine isothiocyanate (TRITC, Sigma, diluted 3.5:500 in
PBS) solution for 30 min at room temperature. Additionally, 4′,6-diamino-2-phenylindole (DAPI, 41002, Beyotime, diluted 1:1000 in PBS) was used to stain the cell nuclei. Nikon microscope
(Nikon) fluorescence photos were taken, and ImageJ was used for analysis. The Morpholibj plugin for ImageJ was used for image processing throughout the analysis of the images. Cellulose was
used to aid in the graphical segmentation of cell bright field images to obtain more accurate findings. STATISTICAL ANALYSIS All studies in this study using various batches of devices or
cells were carried out in at least triplicate, and the results are shown as the mean ± standard error. Differences among the three groups were examined by one-way ANOVA. A _p_-value <
0.05 indicated statistical significance (ns = not significant). REFERENCES * Siddique, A.-B. et al. The effect of topographical and mechanical stimulation on the structural and functional
anisotropy of cardiomyocytes grown on a circular PDMS diaphragm. _Biosens. Bioelectron._ 204, 114017 (2022). Article Google Scholar * So, J. et al. Shape estimation of soft manipulator
using stretchable sensor. _Cyborg. Bionic Syst._ 2021, 9843894 (2021). Article Google Scholar * Yue, T. et al. A modular microfluidic system based on a multilayered configuration to
generate large-scale perfusable microvascular networks. _Microsyst. Nanoeng._ 7, 1–13 (2021). Article Google Scholar * Karbassi, E. et al. Cardiomyocyte maturation: advances in knowledge
and implications for regenerative medicine. _Nat. Rev. Cardiol._ 17, 341–359 (2020). Article Google Scholar * Dou, W. et al. Ultrathin and flexible bioelectronic arrays for functional
measurement of iPSC-cardiomyocytes under cardiotropic drug administration and controlled microenvironments. _Nano Lett._ 23, 2321–2331 (2023). Article Google Scholar * Hu, N. et al.
Intracellular recording of cardiomyocyte action potentials by nanobranched microelectrode array. _Biosens. Bioelectron._ 169, 112588 (2020). Article Google Scholar * Qian, F. et al.
Simultaneous electrical recording of cardiac electrophysiology and contraction on chip. _Lab Chip_ 17, 1732–1739 (2017). Article Google Scholar * Feng, Y. et al. Impedance-enabled
camera-free intrinsic mechanical cytometry. _Small Methods_ 6, 2270043 (2022). Article Google Scholar * Kreutzer, J. et al. Pneumatic unidirectional cell stretching device for
mechanobiological studies of cardiomyocytes. _Biomech. Model Mechanobiol._ 19, 291–303 (2020). Article Google Scholar * Hart, K. C. et al. An easy-to-fabricate cell stretcher reveals
density-dependent mechanical regulation of collective cell movements in epithelia. _Cel. Mol. Bioeng._ 14, 569–581 (2021). Article Google Scholar * Huang, L., Liang, F., Feng, Y., Zhao, P.
& Wang, W. On-chip integrated optical stretching and electrorotation enabling single-cell biophysical analysis. _Microsyst. Nanoeng._ 6, 1–14 (2020). Article Google Scholar * Chen, M.
et al. Three-dimensional stretchable sensor-hydrogel integrated platform for cardiomyocyte culture and mechanotransduction monitoring. _Anal. Chem._ 95, 12859–12866 (2023). Article Google
Scholar * Kamble, H. et al. An electromagnetically actuated double-sided cell-stretching device for mechanobiology research. _Micromachines_ 8, 256 (2017). Article Google Scholar * Feng,
Y. et al. Impedance-based multimodal electrical-mechanical intrinsic flow cytometry. _Small_ 19, 2303416 (2023). Article Google Scholar * Clerc, O. et al. Cardiomyocyte stretch mediates
the relation between left ventricular amyloid burden and adverse outcomes in light chain amyloidosis: a 18F-florbetapir positron emission tomography study. _Eur. Heart J._ 43, ehac544.319
(2022). Article Google Scholar * Saucerman, J. J., Tan, P. M., Buchholz, K. S., McCulloch, A. D. & Omens, J. H. Mechanical regulation of gene expression in cardiac myocytes and
fibroblasts. _Nat. Rev. Cardiol._ 16, 361–378 (2019). Article Google Scholar * Kim, J., Kim, S., Uddin, S., Lee, S. S. & Park, S. Microfabricated stretching devices for studying the
effects of tensile stress on cells and tissues. _BioChip J._ 16, 366–375 (2022). Article Google Scholar * Zhong, S. et al. Efficacy of biological and physical enhancement on targeted
muscle reinnervation. _Cyborg. Bionic Syst._ 2022, 9759265 (2022). Article Google Scholar * Gao, C. et al. 3D bioprinting for fabricating artificial skin tissue. _Colloids Surf. B_ 208,
112041 (2021). Article Google Scholar * Chai, H. et al. Capillarity enabled large-array liquid metal electrodes for compact and high-throughput dielectrophoretic microfluidics. _Adv.
Mater_. 36, 32310212 (2024). Google Scholar * Cho, K. W., Lee, W. H., Kim, B.-S. & Kim, D.-H. Sensors in heart-on-a-chip: a review on recent progress. _Talanta_ 219, 121269 (2020).
Article Google Scholar * He, Y., Mao, T., Gu, Y., Yang, Y. & Ding, J. A simplified yet enhanced and versatile microfluidic platform for cyclic cell stretching on an elastic polymer.
_Biofabrication_ 12, 045032 (2020). Article Google Scholar * Wong, T.-Y. et al. Mechanical stretching simulates cardiac physiology and pathology through mechanosensor Piezo1. _J. Clin.
Med._ 7, 410 (2018). Article Google Scholar * Gao, C. et al. Biofabrication of biomimetic undulating microtopography at the dermal-epidermal junction and its effects on the growth and
differentiation of epidermal cells. _Biofabrication_ 16, 025018 (2024). Article Google Scholar * Kumar, V. et al. An in vitro microfluidic alveolus model to study lung biomechanics.
_Front. Bioeng. Biotechnol._ 10, 848699 (2022). Article Google Scholar * Zheng, W. et al. A microfluidic flow-stretch chip for investigating blood vessel biomechanics. _Lab a Chip_ 12,
3441–3450 (2012). Article Google Scholar * Song, S. Y. et al. Cardiac-mimetic cell-culture system for direct cardiac reprogramming. _Theranostics_ 9, 6734–6744 (2019). Article Google
Scholar * Dou, W. et al. Microengineered platforms for characterizing the contractile function of in vitro cardiac models. _Microsyst. Nanoeng._ 8, 1–22 (2022). Article Google Scholar *
Schürmann, S. et al. The IsoStretcher: an isotropic cell stretch device to study mechanical biosensor pathways in living cells. _Biosens. Bioelectron._ 81, 363–372 (2016). Article Google
Scholar * Oyunbaatar, N.-E. et al. Development of a next-generation biosensing platform for simultaneous detection of mechano- and electrophysiology of the drug-induced cardiomyocytes. _ACS
Sens._ 4, 2623–2630 (2019). Article Google Scholar * Sakamiya, M., Fang, Y., Mo, X., Shen, J. & Zhang, T. A heart-on-a-chip platform for online monitoring of contractile behavior via
digital image processing and piezoelectric sensing technique. _Med. Eng. Phys._ 75, 36–44 (2020). Article Google Scholar * Lee, S.-Y., Kim, D.-S., Kim, E.-S. & Lee, D.-W. Nano-textured
polyimide cantilever for enhancing the contractile behavior of cardiomyocytes and its application to cardiac toxicity screening. _Sens. Actuators B_ 301, 126995 (2019). Article Google
Scholar * Yoon, J.-K. et al. Stretchable piezoelectric substrate providing pulsatile mechanoelectric cues for cardiomyogenic differentiation of mesenchymal stem cells. _ACS Appl. Mater.
Interfaces_ 9, 22101–22111 (2017). Article Google Scholar * Wang, L. et al. Crack sensing of cardiomyocyte contractility with high sensitivity and stability. _ACS Nano_ 16, 12645–12655
(2022). Article Google Scholar * Gao, Y. et al. Wearable microfluidic diaphragm pressure sensor for health and tactile touch monitoring. _Adv. Mater._ 29, 1701985 (2017). Article Google
Scholar * Lin, C.-H. et al. Measurement of in-plane elasticity of live cell layers using a pressure sensor embedded microfluidic device. _Sci. Rep._ 6, 36425 (2016). Article Google Scholar
* Sato, K., Nitta, M. & Ogawa, A. A microfluidic cell stretch device to investigate the effects of stretching stress on artery smooth muscle cell proliferation in pulmonary arterial
hypertension. _Inventions_ 4, 1 (2019). Article Google Scholar * Babaei, N., Hannani, N., Dabanloo, N. J. & Bahadori, S. A Systematic review of the use of commercial wearable activity
trackers for monitoring recovery in individuals undergoing total hip replacement surgery. _Cyborg. Bionic Syst._ https://doi.org/10.34133/2022/9794641 (2022). * Choi, S. et al. Highly
conductive, stretchable and biocompatible Ag–Au core–sheath nanowire composite for wearable and implantable bioelectronics. _Nat. Nanotech._ 13, 1048–1056 (2018). Article Google Scholar *
Man, K. et al. Dimensionality-dependent mechanical stretch regulation of cell behavior. _ACS Appl. Mater. Interfaces_ 14, 17081–17092 (2022). Article Google Scholar * Pitoulis, F. G. et
al. Remodelling of adult cardiac tissue subjected to physiological and pathological mechanical load in vitro. _Cardiovasc. Res._ 118, 814–827 (2022). Article Google Scholar * Lim, G. B.
Piezo1 senses pressure overload and initiates cardiac hypertrophy. _Nat. Rev. Cardiol._ 19, 503–503 (2022). Article Google Scholar * Li, J. et al. Stretch harmonizes sarcomere strain
across the cardiomyocyte. _Circ. Res._ 133, 255–270 (2023). Article Google Scholar * Alhmoud, H., Alkhaled, M., Kaynak, B. E. & Hanay, M. S. Leveraging the elastic deformability of
polydimethylsiloxane microfluidic channels for efficient intracellular delivery. _Lab Chip_ 23, 714–726 (2023). Article Google Scholar * Gavara, N., Roca-Cusachs, P., Sunyer, R., Farré, R.
& Navajas, D. Mapping cell-matrix stresses during stretch reveals inelastic reorganization of the cytoskeleton. _Biophys. J._ 95, 464–471 (2008). Article Google Scholar * Chen, X. et
al. A clamp-free micro-stretching system for evaluating the viscoelastic response of cell-laden microfibers. _Biosens. Bioelectron._ 214, 114517 (2022). Article Google Scholar * Wang, E.
Y. et al. An organ-on-a-chip model for pre-clinical drug evaluation in progressive non-genetic cardiomyopathy. _J. Mol. Cell. Cardiol._ 160, 97–110 (2021). Article Google Scholar Download
references ACKNOWLEDGEMENTS This work was supported by grants from the National Natural Science Foundation of China (Nos. 62373235, 62303290, and 62073208), the Shanghai Science and
Technology Committee Natural Science Foundation (No. 23ZR1423700), and the Shanghai Municipal Education Innovation Project 2021-09-E00113. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
School of Mechatronics Engineering and Automation, Shanghai University, Shanghai, China Yuyin Zhang, Hongze Yin, Jiahao Wang, Na Liu, Songyi Zhong, Long Li, Quan Zhang & Tao Yue * School
of Future Technology, Shanghai University, Shanghai, China Yue Wang, Songyi Zhong, Quan Zhang & Tao Yue * Key Laboratory of Advanced Manufacturing Technology of Zhejiang Province,
School of Mechanical Engineering, Zhejiang University, Hangzhou, China Yue Wang * Shanghai Key Laboratory of Intelligent Manufacturing and Robotics, Shanghai University, Shanghai, China Na
Liu, Long Li, Quan Zhang & Tao Yue * Shanghai Institute of Intelligent Science and Technology, Tongji University, Shanghai, China Na Liu & Tao Yue Authors * Yuyin Zhang View author
publications You can also search for this author inPubMed Google Scholar * Yue Wang View author publications You can also search for this author inPubMed Google Scholar * Hongze Yin View
author publications You can also search for this author inPubMed Google Scholar * Jiahao Wang View author publications You can also search for this author inPubMed Google Scholar * Na Liu
View author publications You can also search for this author inPubMed Google Scholar * Songyi Zhong View author publications You can also search for this author inPubMed Google Scholar *
Long Li View author publications You can also search for this author inPubMed Google Scholar * Quan Zhang View author publications You can also search for this author inPubMed Google Scholar
* Tao Yue View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.Z. conceived, designed, performed experiments, analyzed data and wrote the
paper; Y.W. conceived, designed the experiments and wrote the paper; N.L. analyzed experimental data and reviewed the paper; S.Z., L.L. and Q.Z. reviewed the paper; T.Y. conceived, designed
the experiments, analyzed data and wrote the paper. CORRESPONDING AUTHORS Correspondence to Yue Wang or Tao Yue. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare no competing
interests. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which
permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to
the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhang, Y., Wang, Y., Yin, H. _et al._ Strain sensor on a chip for quantifying the magnitudes of tensile stress on cells. _Microsyst
Nanoeng_ 10, 88 (2024). https://doi.org/10.1038/s41378-024-00719-z Download citation * Received: 02 February 2024 * Revised: 08 April 2024 * Accepted: 23 April 2024 * Published: 25 June 2024
* DOI: https://doi.org/10.1038/s41378-024-00719-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link
is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative