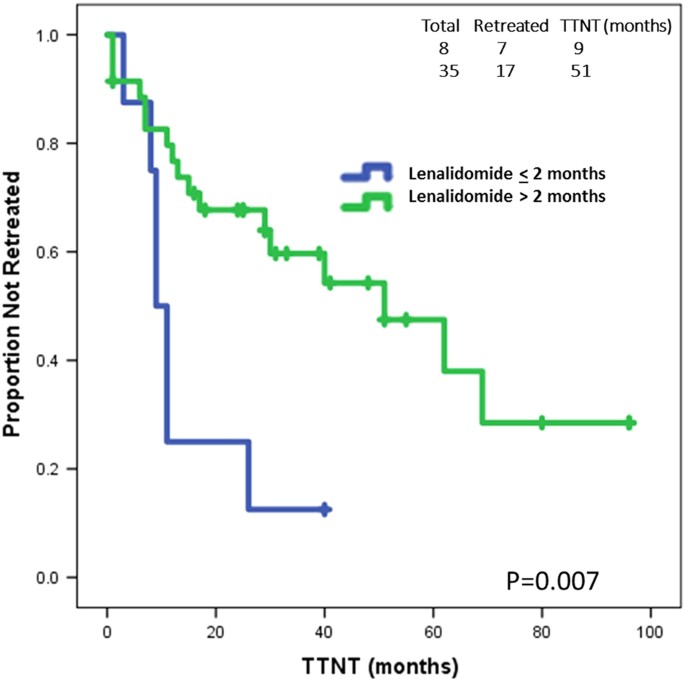
Sustained long-lasting responses after lenalidomide discontinuation in patients with chronic lymphocytic leukemia
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Toxicity prompting treatment discontinuation remains a major challenge in the management of chronic lymphocytic leukemia (CLL), both with chemoimmunotherapy and with novel biological agents
[1,2,3]. Toxicity-related treatment discontinuation was reported in up to 60% of CLL patients treated with the combination of fludarabine, cyclophosphamide, and rituximab [4, 5]. In patients
treated with chemoimmunotherapy, treatment discontinuation can result in lower response rates and shorter time to next treatment (TTNT) [6]. Toxicity-related treatment discontinuation has
been reported in about 10 to 20% of CLL patients treated with ibrutinib [7] and up to 40% of those treated with idelalisib plus rituximab [8]; typically, following discontinuation, the
disease rapidly progresses with aggressive biological and clinical features and an overall unfavorable outcome [7, 8].
Lenalidomide is an immunomodulatory agent with well-established clinical benefits in CLL [9]. Unfortunately, its development has been slowed by the results of a phase III front-line clinical
trial comparing single-agent lenalidomide with single-agent chlorambucil in elderly patients with CLL, which was stopped early because of higher rate of toxicity-related deaths in the
lenalidomide arm [10].
We thank Stephanie P Deming, Senior Scientific Editor, for revising the grammatical and stylistic correctness of this manuscript. This research is supported in part by the MD Anderson Cancer
Center Support Grant P30 CA016672.
Z.E. designed, performed, and analyzed the study, provided clinical care to patients, and wrote the paper; P.S. analyzed data, performed statistical analysis, and wrote the paper; A.F.,
W.G.W., N.J., P.A.T., S.M.O., H.K., J.A.B., C.H., K.R. and M.J.K. provided clinical care to patients and coauthored the paper.
M.J.K. was a consultant for Celgene Corporation; W.G.W. was a consultant/advisory board member for Celgene Corporation; S.M.O. was a consultant for Celgene Corporation; and A.F. received
research support from Celgene Corporation. The remaining authors declare no competing financial interests.
Anyone you share the following link with will be able to read this content: