Assessing the difference in contamination of retail meat with multidrug-resistant bacteria using for-consumer package label claims that indicate on-farm antibiotic use practices— united states, 2016–2019
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND Antibiotic use in food-producing animals can select for antibiotic resistance in bacteria that can be transmitted to people through contamination of food products during
meat processing. Contamination resulting in foodborne illness contributes to adverse health outcomes. Some livestock producers have implemented antibiotic use reduction strategies marketed
to consumers on regulated retail meat packaging labels (“label claims”). OBJECTIVE We investigated whether retail meat label claims were associated with isolation of multidrug-resistant
organisms (MDROs, resistant to ≥3 classes of antibiotics) from U.S. meat samples. METHODS We utilized retail meat data from the U.S. Food and Drug Administration National Antimicrobial
Resistance Monitoring System (NARMS) collected during 2016–2019 for bacterial contamination of chicken breast, ground turkey, ground beef, and pork chops. We used modified Poisson regression
models to compare the prevalence of MDRO contamination among meat samples with any antibiotic restriction label claims versus those without such claims (i.e., conventionally produced).
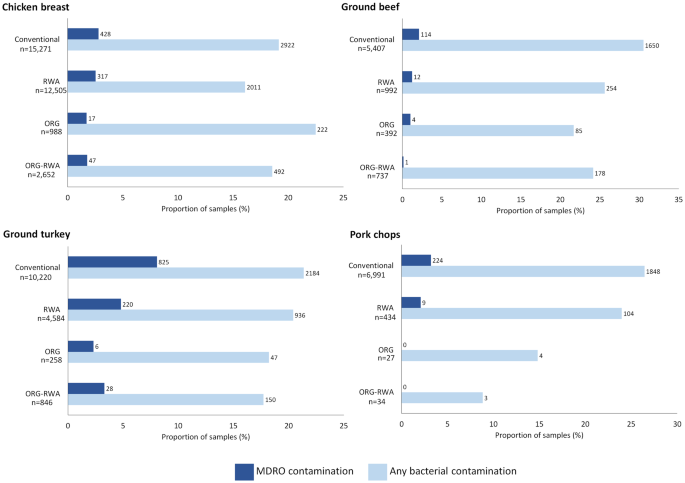
RESULTS In NARMS, 62,338 meat samples were evaluated for bacterial growth from 2016–2019. Of these, 24,446 (39%) samples had label claims that indicated antibiotic use was restricted during
animal production. MDROs were isolated from 2252 (4%) meat samples, of which 71% (_n_ = 1591) were conventionally produced, and 29% (_n_ = 661) had antibiotic restriction label claims.
Compared with conventional samples, meat with antibiotic restriction label claims had a statistically lower prevalence of MDROs (adjusted prevalence ratio: 0.66; 95% CI: 0.61, 0.73). This
relationship was consistent for the outcome of any bacterial growth. IMPACT * This repeated cross-sectional analysis of a nationally representative retail meat surveillance database in the
United States supports that retail meats labeled with antibiotic restriction claims were less likely to be contaminated with MDROs compared with retail meat without such claims during
2016–2019. * These findings indicate the potential for the public to become exposed to bacterial pathogens via retail meat and emphasizes a possibility that consumers could reduce their
exposure to environmental reservoirs of foodborne pathogens that are resistant to antibiotics. Access through your institution Buy or subscribe This is a preview of subscription content,
access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 6 print issues and online access $259.00 per year only $43.17 per issue Learn
more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS
OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS DISTANCE AND DESTINATION OF RETAIL MEAT ALTER
MULTIDRUG RESISTANT CONTAMINATION IN THE UNITED STATES FOOD SYSTEM Article Open access 29 November 2023 ANTIMICROBIAL RESISTANT BACTERIA RECOVERED FROM RETAIL GROUND MEAT PRODUCTS IN THE US
INCLUDE A _RAOULTELLA ORNITHINOLYTICA_ CO-HARBORING _BLA_KPC-2 AND _BLA_NDM-5 Article Open access 07 July 2021 ANTIBIOTIC RESIDUES CORRELATE WITH ANTIBIOTIC RESISTANCE OF _SALMONELLA
TYPHIMURIUM_ ISOLATED FROM EDIBLE CHICKEN MEAT Article Open access 30 April 2025 DATA AVAILABILITY The data used in this study are publicly available via the National Antimicrobial
Resistance Monitoring System website [35]. REFERENCES * Tack DM, Ray L, Griffin PM, Cieslak PR, Dunn J, Rissman T, et al. Preliminary incidence and trends of infections with pathogens
transmitted commonly through food - foodborne diseases active surveillance network, 10 U.S. Sites, 2016-2019. Morb Mortal Wkly Rep. 2020;69:509–14. Article Google Scholar * Collier SA,
Deng L, Adam EA, Benedict KM, Beshearse EM, Blackstock AJ, et al. Estimate of burden and direct healthcare cost of infectious waterborne disease in the United States. Emerg Infect Dis.
2021;27:140–9. Article PubMed PubMed Central Google Scholar * CDC. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services,
CDC; 2019. * Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, et al. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the diagnosis and management of
infectious diarrhea. Clin Infect Dis. 2017;65:e45–e80. Article PubMed PubMed Central Google Scholar * Parisi A, Crump JA, Glass K, Howden BP, Furuya-Kanamori L, Vilkins S, et al. Health
outcomes from multidrug-resistant salmonella infections in high-income countries: a systematic review and meta-analysis. Foodborne Pathog Dis. 2018;15:428–36. Article PubMed Google Scholar
* Varma JK, Mølbak K, Barrett TJ, Beebe JL, Jones TF, Rabatsky-Ehr T, et al. Antimicrobial-resistant nontyphoidal salmonella is associated with excess bloodstream infections and
hospitalizations. J Infect Dis. 2005;191:554–61. Article PubMed Google Scholar * Innes GK, Nachman KE, Abraham AG, Casey JA, Patton AN, Price LB, et al. Contamination of retail meat
samples with multidrug-resistant organisms in relation to organic and conventional production and processing: a cross-sectional analysis of data from the United States National Antimicrobial
Resistance Monitoring System, 2012-2017. Environ Health Perspect. 2021;129:57004. Article PubMed Google Scholar * Yin X, M’Ikanatha NM, Nyirabahizi E, McDermott PF, Tate H. Antimicrobial
resistance in non-Typhoidal Salmonella from retail poultry meat by antibiotic usage-related production claims - United States, 2008-2017. Int J Food Microbiol. 2021;342:109044. Article CAS
PubMed Google Scholar * Berry ED, Wells JE, Kniel K, Thakur S. Reducing foodborne pathogen persistence and transmission in animal production environments: challenges and opportunities.
Microbiol Spectrum. 2016;4:56. https://doi.org/10.1128/microbiolspec.PFS-0006-2014. * Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant,
extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81.
Article CAS PubMed Google Scholar * Mathew AG, Cissell R, Liamthong S. Antibiotic resistance in bacteria associated with food animals: a united states perspective of livestock
production. Foodborne Pathog Dis. 2007;4:115–33. Article CAS PubMed Google Scholar * Innes GK, Markos A, Dalton KR, Gould CA, Nachman KE, Fanzo J, et al. How animal agriculture
stakeholders define, perceive, and are impacted by antimicrobial resistance: challenging the Wellcome Trust’s Reframing Resistance principles. Agric Hum Values. 2021;38:893–909. Article
Google Scholar * Patel SJ, Wellington M, Shah RM, Ferreira MJ. Antibiotic stewardship in food-producing animals: challenges, progress, and opportunities. Clin Ther. 2020;42:1649–58. Article
PubMed PubMed Central Google Scholar * Barrett JR, Innes GK, Johnson KA, Lhermie G, Ivanek R, Greiner Safi A, et al. Consumer perceptions of antimicrobial use in animal husbandry: a
scoping review. PLoS ONE. 2021;16:e0261010. Article CAS PubMed PubMed Central Google Scholar * FDA. Veterinary feed directive 2019.
https://www.fda.gov/animal-veterinary/development-approval-process/veterinary-feed-directive-vfd. * Wallinga D, Smit LAM, Davis MF, Casey JA, Nachman KE. A review of the effectiveness of
current US policies on antimicrobial use in meat and poultry production. Curr Environ Health Rep. 2022;9:339–54. Article PubMed PubMed Central Google Scholar * FDA. 2022 Summary report
on antimicrobials sold or distributed for use in food-producing animals. 2022.
https://www.fda.gov/animal-veterinary/antimicrobial-resistance/2022-summary-report-antimicrobials-sold-or-distributed-use-food-producing-animals. * Tiseo K, Huber L, Gilbert M, Robinson TP,
Van Boeckel TP. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics. 2020;9:918. Article PubMed PubMed Central Google Scholar * Spain CV, Freund D,
Mohan-Gibbons H, Meadow RG, Beacham L. Are they buying it? United States consumers’ changing attitudes toward more humanely raised meat, eggs, and dairy. Animals. 2018;8:128. Article PubMed
PubMed Central Google Scholar * Ritter GD. Using meat labels to communicate the risk of antimicrobial-resistant bacterial infections from foods of animal origin:: the case for a balanced
one health approach to raising food animals. Dela J Public Health. 2021;7:32–6. Article PubMed PubMed Central Google Scholar * Karavolias J, Salois MJ, Baker KT, Watkins K. Raised
without antibiotics: impact on animal welfare and implications for food policy. Transl Anim Sci. 2018;2:337–48. Article PubMed PubMed Central Google Scholar * Sutherland MA, Webster J,
Sutherland I. Animal health and welfare issues facing organic production systems. Animals. 2013;3:1021–35. Article PubMed PubMed Central Google Scholar * Bowman M, Marshall KK, Kuchler
F, Lynch L. Raised without antibiotics: lessons from voluntary labeling of antibiotic use practices in the broiler industry. Am J Agric Econ. 2016;98:622–42. Article Google Scholar * USDA.
Food Safety and Inspection Service labeling guideline on documentation needed to substantiate animal raising claims for label submissions 2019.
https://www.fsis.usda.gov/sites/default/files/media_file/2020-08/RaisingClaims_1.pdf. * USDA. 7 CFR Part 205. 2022
https://www.ecfr.gov/current/title-7/subtitle-B/chapter-I/subchapter-M/part-205?toc=1. * Cui S, Ge B, Zheng J, Meng J. Prevalence and antimicrobial resistance of Campylobacter spp. and
Salmonella serovars in organic chickens from Maryland retail stores. Appl Environ Microbiol. 2005;71:4108–11. Article CAS PubMed PubMed Central Google Scholar * Davis GS, Waits K,
Nordstrom L, Grande H, Weaver B, Papp K, et al. Antibiotic-resistant Escherichia coli from retail poultry meat with different antibiotic use claims. BMC Microbiol. 2018;18:174. Article CAS
PubMed PubMed Central Google Scholar * Kilonzo-Nthenge A, Brown A, Nahashon SN, Long D. Occurrence and antimicrobial resistance of enterococci isolated from organic and conventional
retail chicken. J Food Prot. 2015;78:760–6. Article CAS PubMed Google Scholar * Millman JM, Waits K, Grande H, Marks AR, Marks JC, Price LB, et al. Prevalence of antibiotic-resistant E.
coli in retail chicken: comparing conventional, organic, kosher, and raised without antibiotics. F1000Res. 2013;2:155. Article PubMed PubMed Central Google Scholar * Lestari SI, Han F,
Wang F, Ge B. Prevalence and antimicrobial resistance of Salmonella serovars in conventional and organic chickens from Louisiana retail stores. J Food Prot. 2009;72:1165–72. Article PubMed
Google Scholar * Mazengia E, Samadpour M, Hill H, Greeson K, Tenney K, Liao G, et al. Prevalence, concentrations, and antibiotic sensitivities of Salmonella serovars in poultry from
retail establishments in Seattle, Washington. J Food Prot. 2014;77:885–93. Article CAS PubMed Google Scholar * Schmidt JW, Vikram A, Doster E, Thomas K, Weinroth MD, Parker J, et al.
Antimicrobial resistance in U.S. retail ground beef with and without label claims regarding antibiotic use. J Food Prot. 2021;84:827–42. Article CAS PubMed Google Scholar * Vikram A,
Miller E, Arthur TM, Bosilevac JM, Wheeler TL, Schmidt JW. Similar levels of antimicrobial resistance in U.S. food service ground beef products with and without a “raised without
antibiotics” claim. J Food Prot. 2018;81:2007–18. Article CAS PubMed Google Scholar * Vikram A, Miller E, Arthur TM, Bosilevac JM, Wheeler TL, Schmidt JW. Food service pork chops from
three U.S. regions harbor similar levels of antimicrobial resistance regardless of antibiotic use claims. J Food Prot. 2019;82:1667–76. Article CAS PubMed Google Scholar * FDA. The
National Antimicrobial Resistance Monitoring System 2022. https://www.fda.gov/animal-veterinary/antimicrobial-resistance/national-antimicrobial-resistance-monitoring-system. * FDA. FDA NARMS
methodology. 2021. https://www.fda.gov/media/101741/download. * FDA. National Antimicrobial Resistance Monitoring System (NARMS) retail meat surveillance laboratory protocol 2021.
https://www.fda.gov/media/93332/download. * NARMS. The National Antimicrobial Resistance Monitoring System manual of laboratory methods 2021. https://www.fda.gov/media/101423/download. *
Nachman KE, Love DC, Baron PA, Nigra AE, Murko M, Raber G, et al. Nitarsone, inorganic arsenic, and other arsenic species in turkey meat: exposure and risk assessment based on a 2014 U.S.
market basket sample. Environ Health Perspect. 2017;125:363–9. Article CAS PubMed Google Scholar * Nachman KE, Baron PA, Raber G, Francesconi KA, Navas-Acien A, Love DC. Roxarsone,
inorganic arsenic, and other arsenic species in chicken: A U.S.-based market basket sample. Environ Health Perspect. 2013;121:818–24. Article PubMed PubMed Central Google Scholar * CLSI.
Performance standards for antimicrobial susceptibility testing CLSI supplement M100. Clinical and Laboratory Standards Institute; 2021. * FDA. Interpretative criteria for susceptibility
testing 2021. https://www.fda.gov/media/108180/download. * FDA. NARMS now: integrated data 2023.
https://www.fda.gov/animal-veterinary/national-antimicrobial-resistance-monitoring-system/narms-now-integrated-data. * Tamhane AR, Westfall AO, Burkholder GA, Cutter GR. Prevalence odds
ratio versus prevalence ratio: choice comes with consequences. Stat Med. 2016;35:5730–5. Article PubMed PubMed Central Google Scholar * American Academy of Pediatric. Red book: report of
the committee on infectious diseases. 32nd ed. Itasca, IL: American Academy of Pediatrics. 2021. * Abrams KM, Meyers CA, Irani TA. Naturally confused: consumers’ perceptions of all-natural
and organic pork products. Agric Hum Values. 2010;27:365–74. Article Google Scholar * Price L, Rogers L, Lo K. Policy reforms for antibiotic use claims in livestock. Science.
2022;376:130–2. Download references ACKNOWLEDGEMENTS The authors would like to acknowledge the National Antimicrobial Resistance Monitoring System and all who worked to collect these data
and make them available to the public. The authors also acknowledge Dr. Alison G. Abraham for her invaluable contribution upon which some of this work was built. FUNDING The authors
acknowledge the following funding sources: The National Institute of Allergy and Infectious Diseases (R01AI130066; GSS, JAC, KEN, MFD, SYT). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Environmental Health and Engineering, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA G. Sean Stapleton, Keeve E. Nachman & Meghan F. Davis * Center for
Livable Future, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA G. Sean Stapleton & Keeve E. Nachman * Yuma Center for Excellence in Desert Agriculture, Yuma, AZ,
USA Gabriel K. Innes * Risk Sciences and Public Policy Institute, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA Keeve E. Nachman * Department of Environmental and
Occupational Health Sciences, University of Washington School of Public Health, Seattle, WA, USA Joan A. Casey * Geospatial Analysis Lab, University of San Francisco, Harney Science Center,
San Francisco, CA, USA Andrew N. Patton * Milken Institute School of Public Health, The George Washington University, Washington, DC, USA Lance B. Price * Department of Research &
Evaluation, Kaiser Permanente Southern California, Pasadena, CA, USA Sara Y. Tartof * Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena,
CA, USA Sara Y. Tartof * Department of Molecular and Comparative Pathobiology & Division of Infectious Diseases, Johns Hopkins School of Medicine, Baltimore, MD, USA Meghan F. Davis
Authors * G. Sean Stapleton View author publications You can also search for this author inPubMed Google Scholar * Gabriel K. Innes View author publications You can also search for this
author inPubMed Google Scholar * Keeve E. Nachman View author publications You can also search for this author inPubMed Google Scholar * Joan A. Casey View author publications You can also
search for this author inPubMed Google Scholar * Andrew N. Patton View author publications You can also search for this author inPubMed Google Scholar * Lance B. Price View author
publications You can also search for this author inPubMed Google Scholar * Sara Y. Tartof View author publications You can also search for this author inPubMed Google Scholar * Meghan F.
Davis View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors contributed to conceptualization, data analysis and interpretation, and
writing and revising this manuscript. CORRESPONDING AUTHOR Correspondence to G. Sean Stapleton. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ETHICAL
APPROVAL This study used no human or animal subjects. Therefore, ethical approval was not required. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a
society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript
version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Stapleton, G.S., Innes,
G.K., Nachman, K.E. _et al._ Assessing the difference in contamination of retail meat with multidrug-resistant bacteria using for-consumer package label claims that indicate on-farm
antibiotic use practices— United States, 2016–2019. _J Expo Sci Environ Epidemiol_ 34, 917–926 (2024). https://doi.org/10.1038/s41370-024-00649-y Download citation * Received: 29 May 2023 *
Revised: 24 January 2024 * Accepted: 24 January 2024 * Published: 19 February 2024 * Issue Date: November 2024 * DOI: https://doi.org/10.1038/s41370-024-00649-y SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative KEYWORDS * Retail meat * Label claims * Antimicrobial resistance * Foodborne bacteria