Novel prmt7 mutation in a rare case of dysmorphism and intellectual disability
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
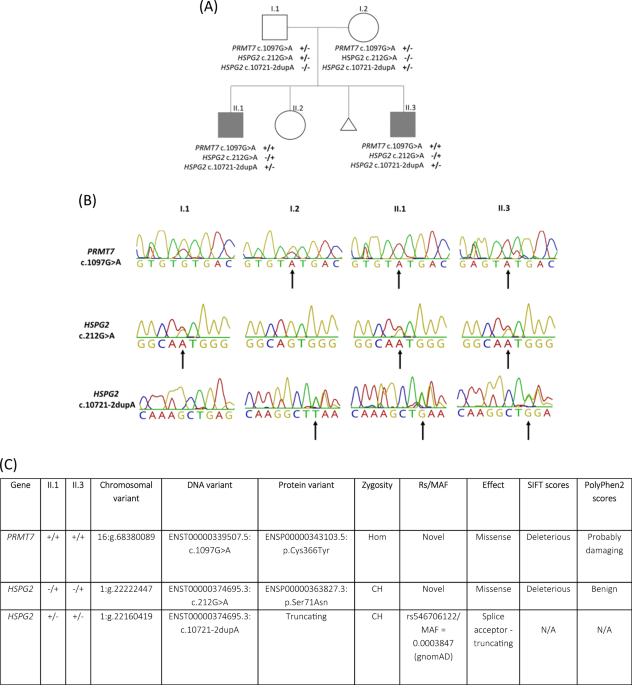
ABSTRACT Protein arginine N-methyltransferase 7 (_PRMT7_) encodes an arginine methyltransferase central to a number of fundamental biological processes, mutations in which result in an
autosomal recessive developmental disorder characterized by short stature, brachydactyly, intellectual developmental disability and seizures (SBIDDS). To date, fewer than 15 patients with
biallelic mutations in _PRMT7_ have been documented. Here we report brothers from a consanguineous Iraqi family presenting with a developmental disorder characterized by global developmental
delay, shortened stature, facial dysmorphisms, brachydactyly, and kidney dysfunction. In both affected brothers, whole genome sequencing (WGS) identified a novel homozygous substitution in
_PRMT7_ (ENST00000339507.5), c.1097 G > A (p.Cys366Tyr), considered to account for the majority of the phenotypic presentation. Rare compound heterozygous mutations in the
dysplasia-associated perlecan-encoding _HSPG2_ gene (ENST00000374695.3) were also found (c.10721-2dupA, p.Ser71Asn and c.212 G > A), potentially accounting for the kidney dysfunction. In
addition to expanding the known mutational spectrum of variably expressive _PRMT7_ mutations alongside potential digenic inheritance with _HSPG2_, this report underlines the diagnostic
utility of a WGS-guided analysis in the detection of rare genetic disorders. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS BIALLELIC _ZNF407_ MUTATIONS IN A NEURODEVELOPMENTAL DISORDER WITH
ID, SHORT STATURE AND VARIABLE MICROCEPHALY, HYPOTONIA, OCULAR ANOMALIES AND FACIAL DYSMORPHISM Article 31 July 2020 A NOVEL _RLIM/RNF12_ VARIANT DISRUPTS PROTEIN STABILITY AND FUNCTION TO
CAUSE SEVERE TONNE–KALSCHEUER SYNDROME Article Open access 05 May 2021 BIALLELIC _ATP2B1_ VARIANTS AS A LIKELY CAUSE OF A NOVEL NEURODEVELOPMENTAL MALFORMATION SYNDROME WITH PRIMARY
HYPOPARATHYROIDISM Article Open access 06 November 2023 REFERENCES * Krause CD, Yang Z-H, Kim Y-S, Lee J-H, Cook JR, Pestka S. Protein arginine methyltransferases: evolution and assessment
of their pharmacological and therapeutic potential. Pharm Ther. 2007;113:50–87. Article CAS Google Scholar * Carithers LJ, Moore HM. The Genotype-Tissue Expression (GTEx) Project.
Biopreserv Biobank. 2015;13:307–8. Article PubMed PubMed Central Google Scholar * Migliori V, Müller J, Phalke S, Low D, Bezzi M, Mok WC, et al. Symmetric dimethylation of H3R2 is a
newly identified histone mark that supports euchromatin maintenance. Nat Struct Mol Biol. 2012;19:136–44. Article CAS PubMed Google Scholar * Di Lorenzo A, Bedford MT. Histone arginine
methylation. FEBS Lett. 2011;585:2024–31. Article PubMed Google Scholar * Szewczyk MM, Ishikawa Y, Organ S, Sakai N, Li F, Halabelian L, et al. Pharmacological inhibition of PRMT7 links
arginine monomethylation to the cellular stress response. Nat Commun. 2020;11:2396. Article CAS PubMed PubMed Central Google Scholar * Li WJ, He YH, Yang JJ, Hu GS, Lin YA, Ran T, et
al. Profiling PRMT methylome reveals roles of hnRNPA1 arginine methylation in RNA splicing and cell growth. Nat Commun. 2021;12:1946. Article PubMed PubMed Central Google Scholar *
Kernohan KD, McBride A, Xi Y, Martin N, Schwartzentruber J, Dyment DA, et al. Loss of the arginine methyltranserase PRMT7 causes syndromic intellectual disability with microcephaly and
brachydactyly. Clin Genet. 2017;91:708–16. Article CAS PubMed Google Scholar * Birnbaum R, Yosha-Orpaz N, Yanoov-Sharav M, Kidron D, Gur H, Yosovich K, et al. Prenatal and postnatal
presentation of _PRMT7_ related syndrome: expanding the phenotypic manifestations. Am J Med Genet Part A. 2019;179:78–84. Article CAS PubMed Google Scholar * Akawi N, McRae J, Ansari M,
Balasubramanian M, Blyth M, Brady AF, et al. Discovery of four recessive developmental disorders using probabilistic genotype and phenotype matching among 4,125 families. Nat Genet.
2015;47:1363–9. Article CAS PubMed PubMed Central Google Scholar * Jeong HJ, Lee HJ, Vuong TA, Choi KS, Choi D, Koo SH, et al. Prmt7 deficiency causes reduced skeletal muscle oxidative
metabolism and age-related obesity. Diabetes. 2016;65:1868–82. Article CAS PubMed Google Scholar * Blanc RS, Vogel G, Chen T, Crist C, Richard S. PRMT7 Preserves Satellite Cell
Regenerative Capacity. Cell Rep. 2016;14:1528–39. Article CAS PubMed Google Scholar * Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform.
Bioinformatics. 2009;25:1754–60. Article CAS PubMed PubMed Central Google Scholar * Broad Institute. Picard tools [Internet]. https://broadinstitute.github.io/picard/. 2016. * Van der
Auwera GA, Carneiro MO, Hartl C, Poplin R, del Angel G, Levy-Moonshine A, et al. From FastQ Data to High-Confidence Variant Calls: the Genome Analysis Toolkit Best Practices Pipeline. In:
Current Protocols in Bioinformatics. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2013. p.11.10.1–33. * Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, del Angel G, Levy-Moonshine A, et
al. From fastQ data to high-confidence variant calls: The genome analysis toolkit best practices pipeline. Curr Protoc Bioinforma. 2013;43:11.10.1–33. Google Scholar * Chen K, Wallis JW,
McLellan MD, Larson DE, Kalicki JM, Pohl CS, et al. BreakDancer: an algorithm for high-resolution mapping of genomic structural variation. Nat Methods. 2009;6:677–81. Article CAS PubMed
PubMed Central Google Scholar * Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature.
2016;536:285–91. Article CAS PubMed PubMed Central Google Scholar * 1000 Genomes. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–73. Article
Google Scholar * Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform.
2013;14:178–92. Article PubMed Google Scholar * Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase
chain reaction. BMC Bioinforma. 2012;13:134. Article CAS Google Scholar * McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GRS, Thormann A, et al. The Ensembl Variant Effect Predictor. Genome
Biol. 2016;17:122. Article PubMed PubMed Central Google Scholar * Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–4.
Article CAS PubMed PubMed Central Google Scholar * Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet.
2013;7:Unit7.20. PubMed Google Scholar * Lopes MC, Joyce C, Ritchie GRS, John SL, Cunningham F, Asimit J, et al. A combined functional annotation score for non-synonymous variants. Hum
Hered. 2012;73:47–51. Article CAS PubMed Google Scholar * Blanchette M, Kent WJ, Riemer C, Elnitski L, Smit AFA, Roskin KM, et al. Aligning Multiple Genomic Sequences With the Threaded
Blockset Aligner. Genom Res. 2004;14:708–15. Article CAS Google Scholar * Jaganathan K, Kyriazopoulou Panagiotopoulou S, McRae JF, Darbandi SF, Knowles D, Li YI, et al. Predicting
Splicing from Primary Sequence with Deep Learning. Cell. 2019;176:535–48. Article CAS PubMed Google Scholar * Yeo G, Burge CB. Maximum Entropy Modeling of Short Sequence Motifs with
Applications to RNA Splicing Signals. J Comput Biol. 2004;11:377–94. Article CAS PubMed Google Scholar * Leman R, Gaildrat P, Gac GL, Ka C, Fichou Y, Audrezet MP, et al. Novel diagnostic
tool for prediction of variant spliceogenicity derived from a set of 395 combined in silico/in vitro studies: an international collaborative effort. Nucleic Acids Res. 2018;46:7913–23.
Article CAS PubMed PubMed Central Google Scholar * Desmet FO, Hamroun D, Lalande M, Collod-Bëroud G, Claustres M, Béroud C. Human Splicing Finder: an online bioinformatics tool to
predict splicing signals. Nucleic Acids Res. 2009;37:e67. Article PubMed PubMed Central Google Scholar * Wang M, Marín A. Characterization and prediction of alternative splice sites.
Gene. 2006;366:219–27. Article CAS PubMed Google Scholar * Ferreira CR. The burden of rare diseases. Am J Med Genet A. 2019;179:885–92. Article PubMed Google Scholar * Boycott KM,
Vanstone MR, Bulman DE, MacKenzie AE. Rare-disease genetics in the era of next-generation sequencing: discovery to translation. Nat Rev Genet. 2013;14:681–91. Article CAS PubMed Google
Scholar * Katsanis SH, Katsanis N. Molecular genetic testing and the future of clinical genomics. Nat Rev Genet. 2013;14:415–26. Article CAS PubMed PubMed Central Google Scholar *
Musante L, Ropers HH. Genetics of recessive cognitive disorders. Trends Genet. 2014;30:32–9. Article CAS PubMed Google Scholar * Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH,
Zahler AM, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. Article CAS PubMed PubMed Central Google Scholar * Berry C, Thomas M, Langley B, Sharma M, Kambadur R.
Single cysteine to tyrosine transition inactivates the growth inhibitory function of Piedmontese myostatin. Am J Physiol Cell Physiol. 2002;283:C135–41. Article CAS PubMed Google Scholar
* Kernohan KD, McBride A, Xi Y, Martin N, Schwartzentruber J, Dyment DA, et al. Loss of the arginine methyltranserase PRMT7 causes syndromic intellectual disability with microcephaly and
brachydactyly. Clin Genet. 2017;91:708–16. Article CAS PubMed Google Scholar * Agolini E, Dentici ML, Bellacchio E, Alesi V, Radio FC, Torella A, et al. Expanding the clinical and
molecular spectrum of _PRMT7_ mutations: 3 additional patients and review. Clin Genet. 2018;93:675–81. Article CAS PubMed Google Scholar * Valenzuela I, Segura-Puimedon M,
Rodríguez-Santiago B, Fernández-Alvarez P, Vendrell T, Armengol L, et al. Further delineation of the phenotype caused by loss of function mutations in PRMT7. Eur J Med Genet. 2019;62:182–5.
Article PubMed Google Scholar * Basalom S, Trakadis Y, Shear R, Azouz ME, De Bie I. Dyssegmental dysplasia, Silverman-Handmaker type: a challenging antenatal diagnosis in a dizygotic twin
pregnancy. Mol Genet Genom Med. 2018;6:452–6. Article CAS Google Scholar * Arikawa-Hirasawa E, Wilcox WR, Le AH, Silverman N, Govindraj P, Hassell JR, et al. Dyssegmental dysplasia,
Silverman-Handmaker type, is caused by functional null mutations of the perlecan gene. Nat Genet. 2001;27:431–4. Article CAS PubMed Google Scholar * Arikawa-Hirasawa E, Le AH, Nishino I,
Nonaka I, Ho NC, Francomano CA, et al. Structural and Functional Mutations of the Perlecan Gene Cause Schwartz-Jampel Syndrome, with Myotonic Myopathy and Chondrodysplasia. Am J Hum Genet.
2002;70:1368–75. Article CAS PubMed PubMed Central Google Scholar * Izzi B, Francois I, Labarque V, Thys C, Wittevrongel C, Devriendt K, et al. Methylation defect in imprinted genes
detected in patients with an albright’s hereditary osteodystrophy like phenotype and platelet gs hypofunction. PLoS ONE. 2012;7:e38579. Article CAS PubMed PubMed Central Google Scholar
* Cianferotti L, Brandi ML. Pseudohypoparathyroidism. Minerva Endocrinol. 2018;43:156–67. Article PubMed Google Scholar * Mantovani G, De Sanctis L, Barbieri AM, Elli FM, Bollati V, Vaira
V, et al. Pseudohypoparathyroidism and GNAS epigenetic defects: clinical evaluation of Albright hereditary osteodystrophy and molecular analysis in 40 patients. J Clin Endocrinol Metab.
2010;95:651–8. Article CAS PubMed Google Scholar * Linglart A, Maupetit-Méhouas S, Silve C. GNAS-related loss-of-function disorders and the role of imprinting. Horm Res Paediatr.
2013;79:119–29. Article CAS PubMed Google Scholar * Cooper GM, Shendure J. Needles in stacks of needles: finding disease-causal variants in a wealth of genomic data. Nat Rev Genet.
2011;12:628–40. Article CAS PubMed Google Scholar * Stavropoulos DJ, Merico D, Jobling R, Bowdin S, Monfared N, Thiruvahindrapuram B, et al. Whole-genome sequencing expands diagnostic
utility and improves clinical management in paediatric medicine. NPJ Genom Med. 2016;1:15012. Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS The
families are gratefully acknowledged for their participation in the study. The study was supported by the New Zealand eScience Infrastructure. FUNDING JCJ is supported by a government-funded
Rutherford Discovery Fellowship administered by the Royal Society of New Zealand. The research was funded by the IHC Foundation. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School of
Biological Sciences, The University of Auckland, Auckland, New Zealand Jessie Poquérusse, Whitney Whitford, Russell G. Snell, Klaus Lehnert & Jessie C. Jacobsen * Centre for Brain
Research, The University of Auckland, Auckland, New Zealand Jessie Poquérusse, Whitney Whitford, Russell G. Snell, Klaus Lehnert & Jessie C. Jacobsen * Genetic Health Service New
Zealand, Auckland City Hospital, Auckland, New Zealand Juliet Taylor & Salam Alburaiky Authors * Jessie Poquérusse View author publications You can also search for this author inPubMed
Google Scholar * Whitney Whitford View author publications You can also search for this author inPubMed Google Scholar * Juliet Taylor View author publications You can also search for this
author inPubMed Google Scholar * Salam Alburaiky View author publications You can also search for this author inPubMed Google Scholar * Russell G. Snell View author publications You can also
search for this author inPubMed Google Scholar * Klaus Lehnert View author publications You can also search for this author inPubMed Google Scholar * Jessie C. Jacobsen View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS JCJ, KL and RGS conceived the experiments. JP and WW performed DNA-based laboratory experiments. JP,
WW, KL, RGS and JCJ performed data and bioinformatic analysis. JT and SA conducted the clinical evaluation. JP and JCJ wrote the paper. All authors reviewed the paper. CORRESPONDING AUTHOR
Correspondence to Jessie C. Jacobsen. ETHICS DECLARATIONS ETHICAL APPROVAL Ethical approval was obtained by the Northern B Health and Disability Ethics Committee (12/NTB/59) prior to
acquiring, sequencing, and analyzing all human genetic information. All procedures were performed in accordance with the ethical standards of the institutional and national responsible
committees on human experimentation and with the 1975 Helsinki Declaration (as revised in 2000). PATIENT CONSENT Consent was obtained from the patient’s family for publication of this
report. COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Poquérusse, J., Whitford, W., Taylor, J. _et al._ Novel _PRMT7_ mutation in a rare case of dysmorphism and intellectual disability. _J Hum Genet_ 67, 19–26 (2022).
https://doi.org/10.1038/s10038-021-00955-5 Download citation * Received: 05 April 2021 * Revised: 05 June 2021 * Accepted: 20 June 2021 * Published: 09 July 2021 * Issue Date: January 2022 *
DOI: https://doi.org/10.1038/s10038-021-00955-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative