Pentoxifylline and prevention of hyperoxia-induced lung injury in neonatal rats
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT INTRODUCTION: Oxygen exposure plays an important role in the pathogenesis of bronchopulmonary dysplasia (BPD). The phosphodiesterase inhibitor pentoxifylline (PTX) has
anti-inflammatory and antifibrotic effects in multiple organs. It was hypothesized that PTX would have a protective effect on hyperoxia-induced lung injury (HILI). METHODS: Newborn
Sprague-Dawley rats were exposed to >95% oxygen (O2) and injected subcutaneously with normal saline (NS) or PTX (75 mg/kg) twice a day for 9 d. NS-injected, room air–exposed pups were
controls. At days 4 and 9, lung tissue was collected to assess edema, antioxidant enzyme (AOE) activities, and vascular endothelial growth factor (VEGF) expression. At day 9, pulmonary
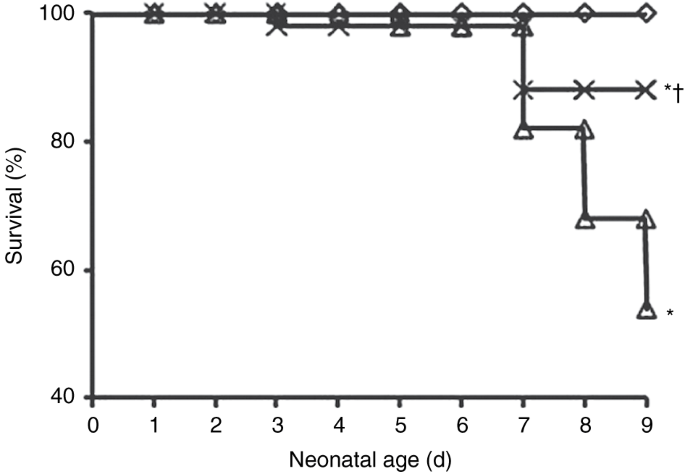
macrophage infiltration, vascularization, and alveolarization were also examined. RESULTS: At day 9, treatment with PTX significantly increased survival from 54% to 88% during hyperoxia.
Treatment with PTX significantly decreased lung edema and macrophage infiltration. PTX treatment increased lung AOE activities including those of superoxide dismutase (SOD), catalase (CAT),
and glutathione peroxidase (GPX). Furthermore, PTX treatment also increased the gene expression of VEGF189 and VEGF165, increased VEGF protein expression, and improved pulmonary
vascularization. DISCUSSION: These data indicate that the reduced lung edema and inflammation, increased AOE activities, and improved vascularization may be responsible for the improved
survival with PTX during hyperoxia. PTX may be a potential therapy in reducing some of the features of BPD in preterm newborns. SIMILAR CONTENT BEING VIEWED BY OTHERS EFFECTS OF PGE1 ON THE
ERS PATHWAY IN NEONATAL RATS WITH HYPEROXIC LUNG INJURY Article 16 July 2024 NINTEDANIB PRESERVES LUNG GROWTH AND PREVENTS PULMONARY HYPERTENSION IN A HYPEROXIA-INDUCED LUNG INJURY MODEL
Article Open access 11 October 2024 PRECONDITIONING THE IMMATURE LUNG WITH ENHANCED NRF2 ACTIVITY PROTECTS AGAINST OXIDANT-INDUCED HYPOALVEOLARIZATION IN MICE Article Open access 04 November
2020 MAIN Despite improvements in neonatal intensive care, bronchopulmonary dysplasia (BPD), the chronic lung disease of premature infants, is a major cause of long-term hospitalization,
recurrent respiratory illnesses, and mortality. The etiology of BPD is complex and has been linked to oxidative stress, mechanical ventilation, infection, and inflammation, as well as
genetic susceptibility (1). The pathogenesis of BPD is poorly understood and no effective therapy has yet been developed. Oxygen toxicity in the developing lung is well known for its
contribution to the pathogenesis of BPD. Evidence suggests that one important mechanism involved in lung injury during hyperoxia is direct oxidative damage through increased production of
reactive oxygen species. The pulmonary antioxidant enzyme (AOE) system, specifically superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX), is a protective mechanism
that confers resistance and minimizes toxicity from hyperoxia-induced reactive oxygen species (2,3,4). However, even though newborn animals show marked tolerance to hyperoxia as compared
with adults (5,6), premature newborns have deficient endogenous AOE activities (7) and limited capacity to augment their levels during oxygen exposure (8). Induction or replacement of AOE
activity may be part of the therapeutic approach to minimizing reactive oxygen species damage during hyperoxia. Evidence has demonstrated that downregulation of vascular endothelial growth
factor (VEGF) expression during hyperoxia is another important mechanism involved in lung injury and BPD (9). VEGF promotes vessel growth and remodeling, improves endothelial survival, and
contributes to the maintenance of alveolar structures (10). VEGF A, in particular, is a major regulator of angiogenesis. VEGF121, VEGF165, and VEGF189 represent the predominant isoforms of
VEGF A in the lung. They show different expressions and responses during lung development and injury, and their role in pulmonary vascularization is yet to be completely understood (11,12).
Treatment with recombinant VEGF has improved alveolarization during hyperoxia (13), suggesting that enhancing VEGF expression may be an important strategy to prevent hyperoxia-induced lung
injury (HILI) in neonates. Pentoxifylline (PTX), a methylxanthine derivative and phosphodiesterase inhibitor, has immunomodulatory and antifibrotic properties. It is proposed to have a
therapeutic role in attenuating tissue injury associated with sepsis and shock in animal models and humans, including neonates (14,15,16). Studies in rabbits have shown increased levels of
CAT, SOD, and GPX during ischemia and reperfusion injury after PTX treatment (17). Studies have also shown that PTX is able to attenuate lung injury related to mechanical ventilation and
meconium (18,19). In neonatal HILI models, PTX treatment decreased IL-6 concentration in bronchoalveolar lavage (20), reduced alveolar fibrin deposition, and prolonged survival (21).
However, whether PTX treatment affects AOE and VEGF expression in neonatal HILI is unknown. In this study, we hypothesized that PTX administration would improve survival in hyperoxia, which
would be associated with enhancement of AOE activities and upregulation of pulmonary VEGF expression in a newborn rat model of HILI. Our findings suggest a therapeutic potential of PTX in
reducing some of the features of BPD in preterm newborns. RESULTS PTX IMPROVES SURVIVAL Pups exposed to hyperoxia for 9 d had a significantly increased mortality as compared with those
exposed to room air (RA). However, treatment with PTX dramatically improved survival from 54% to 88% during hyperoxia (_P_ < 0.001; FIGURE 1 ). Survival between O2 + normal saline (NS)
and O2 + PTX groups was similar until day 6. After day 7, mortality in the O2 + PTX group remained stable until the experiment ended on day 9, whereas the mortality in the O2 + NS group
continued to increase. PTX REDUCES LUNG EDEMA By day 4, there was no difference in lung wet-to-dry-weight ratio between animals raised in RA and animals exposed to O2. At day 9, lung
wet-to-dry-weight ratio was significantly increased in O2-exposed groups. However, animals treated with PTX showed a significantly lower lung wet-to-dry-weight ratio as compared with the O2
+ NS group ( FIGURE 2 ), suggesting less pulmonary edema in the PTX-treated group. PTX DECREASES MACROPHAGE INFILTRATION At day 9, macrophages were barely detected in RA + NS lungs. In
contrast, in O2 + NS lungs, there were many macrophages in alveolar airspaces, whereas fewer macrophages were detected in O2 + PTX lungs ( FIGURE 3 ). Quantification of macrophages
demonstrated an eightfold increase in O2 + NS lungs as compared with RA + NS lungs. Treatment with PTX produced significantly less macrophage infiltration as compared with O2 + NS lungs.
These results suggest that PTX is protective against hyperoxia-induced macrophage infiltration. PTX ENHANCES AOE ACTIVITY To assess the effect of hyperoxia and PTX treatment on the pulmonary
antioxidant defense system against free radicals, we studied CAT, SOD, and GPX activities. No differences were observed in these enzyme activities among the three study groups at day 4 (
FIGURE 4A ). At day 9, as compared with the RA + NS group, pups in the O2 + PTX group showed significant increases in CAT (236.2 ± 59.7 vs. 416.2 ± 91.1, _P_ < 0.001), total SOD (11,631.4
± 4,282.6 vs. 55,758 ± 9,579.1, _P_ < 0.001), and GPX (0.26 ± 0.1 vs. 1.03 ± 0.4, _P_ < 0.001) all expressed as activity units/mg DNA ( FIGURE 4B ). CAT activity was also
significantly elevated in the O2 + PTX group as compared with the O2 + NS group (416.2 ± 91.1 vs. 292.5 ± 52.4, _P_ < 0.001; FIGURE 4B ). These changes were not a result of differences in
lung protein or DNA content between the O2 + PTX and O2 + NS groups (data not shown). PTX INCREASES VEGF EXPRESSION AND IMPROVES VASCULAR DEVELOPMENT We investigated lung VEGF189, VEGF165,
and VEGF121 expression using semiquantitative reverse-transcriptase PCR. There was no difference in the expression of the three isoforms at day 4 among the three study groups (data not
shown). In contrast, at day 9, the O2 + NS group had significantly decreased expression of VEGF189 and VEGF165 as compared with the RA + NS group (VEGF189: 0.31 ± 0.07 vs. 0.4 ± 0.02, _P_
< 0.05; VEGF165: 0.29 ± 0.06 vs. 0.37 ± 0.01, _P_ = 0.001). However, PTX treatment significantly increased the expression of these two isoforms as compared with O2 + NS pups (VEGF189:
0.61 ± 0.005 vs. 0.31 ± 0.07, _P_ < 0.001; VEGF165: 0.37 ± 0.02 vs. 0.29 ± 0.06, _P_ < 0.05) ( FIGURE 5A ). There was no change in the expression of VEGF121 after hyperoxia or exposure
to PTX. To evaluate the correlation between VEGF isoform expression and protein expression, we performed enzyme-linked immunosorbent assay at day 9. Although there was no significant
decrease in VEGF concentration when comparing the O2 + NS and the RA + NS groups, treatment with PTX significantly increased VEGF expression in comparison with NS-exposed animals during
hyperoxia (106.8 ± 39.8 vs. 49.8 ± 41.4, _P_ < 0.01; FIGURE 5B ). To determine whether increased VEGF expression by PTX leads to improved vascular development, we assessed vascular
density on von Willebrand factor–stained lung tissue sections. The lungs from the O2 + NS group had 65% decreased vascular density as compared with the normoxia group. In contrast, treatment
with PTX significantly increased vascular density during hyperoxia as compared with the O2 + NS group (4.7 ± 0.5 vs. 3.4 ± 0.8, _P_ < 0.01; FIGURE 5C ). EFFECTS OF PTX ON ALVEOLAR
STRUCTURE AND FIBROSIS To evaluate the effect of PTX treatment on alveolar development, we performed lung histology and morphometry on day 9. On histological examination, lungs in the
normoxia group displayed normal alveolarization. In contrast, lungs from hyperoxia groups, both NS and PTX, demonstrated distal airspace enlargement, decreased septation, and a reduction in
complexity (data not shown). Morphometric analysis demonstrated a significant decrease in radial alveolar count and a significant increase in mean linear intercept in the O2 groups (as
compared with the RA group) and there was no significant difference between the O2 + NS and O2 + PTX groups (data not shown). We assessed connective tissue growth factor (CTGF) and α-smooth
muscle actin (α-SMA) expression as markers for fibrosis by western blot analysis on days 4 and 9. On day 4, there was no difference in CTGF and α-SMA expression among the groups. On day 9,
hyperoxia exposure significantly increased CTGF and α-SMA expression as compared with normoxia exposure, but there was no significant difference between O2 + NS and O2 + PTX groups (data not
shown). DISCUSSION Consistent with our hypothesis, we have found that therapy with PTX (a phosphodiesterase inhibitor) during 9 d of hyperoxia exposure increased AOE activity, increased
VEGF expression, improved vascular formation, decreased pulmonary edema and macrophage infiltration, and improved survival in newborn rats. These data highlight some of the potential
mechanisms by which PTX protects against neonatal HILI. The lung’s ability to respond to oxidative stress depends largely on its capacity to upregulate protective antioxidants. Newborn
experimental animals are more tolerant than adults to hyperoxia. However, preterm experimental animals and presumably newborn infants as well have deficient endogenous AOE activity and
limited capacity to augment their levels of protective AOEs during oxygen exposure to overcome oxidative stress (7,8). Studies have established positive correlations between relative
resistance to hyperoxia and increase of some or all of the pulmonary AOEs in newborn animals and humans (22,23). Bucher _et al._ demonstrated a significant increase in GPX and SOD but not in
CAT activity in newborn rats exposed to O2 >95% for 6 d and in all three AOEs when exposed for 12 d (2). Ilizarov _et al._ demonstrated that overexpression of manganese-SOD improves
survival of pulmonary epithelial cells during hyperoxia and CAT offers additional protection when coexpressed with manganese-SOD (24). Treatment with PTX has been shown to increase AOE
levels during ischemia and reperfusion injury in different tissues and organs (17); however, no studies have been reported on its effect on AOE levels during prolonged hyperoxia. Although
the absolute AOE activities at day 4 were found to actually be higher than on day 9, we found a significant increase in GPX activity after 9 d of hyperoxia in control rats, whereas treatment
with PTX increased the AOE activities of CAT, SOD, and GPX, indicating that PTX may increase tolerance to hyperoxia by improving cellular antioxidant defense mechanisms. To the best of our
knowledge, this is the first study showing a PTX effect on lung AOE activity levels during prolonged hyperoxia. VEGF plays a central role in normal lung development. Inhibition of
angiogenesis reduces alveolarization (25) and VEGF expression is decreased in infants dying of BPD (26). As previously reported (27), we found suppression of VEGF expression in newborn rat
lungs during hyperoxia. VEGF is a potent endothelial cell mitogen that stimulates angiogenesis, promotes vessel remodeling, enhances endothelial survival, and maintains alveolar structures.
Different isoforms have different affinities for heparin and receptors, and distinct tempo-spatial expression of these isoforms suggests different function. Mice expressing only VEGF121 had
impaired lung vascular and airspace formation, indicating an essential role for the heparin sulfate–binding VEGF165 and VEGF189 isoforms in lung development (28). In our study, we
demonstrated that PTX protects against hyperoxia-induced downregulation of VEGF189 and VEGF165 isoform expression as well as downregulation of VEGF protein expression. Kunig _et al._
reported enhanced vascularization in animals treated with VEGF during recovery after hyperoxia (13). Our study demonstrated that PTX treatment during hyperoxia is able to markedly increase
pulmonary vascular density, which may be induced by increased VEGF expression. We found that VEGF121 expression was constant in all three study groups, indicating that VEGF121 may not be
influenced by oxygen stress as seen in a rabbit model (28). Previous studies have shown that VEGF121 is a predominant form before embryonic day 14, indicating that this isoform has a unique
role early in lung development (29), with perhaps a less important role toward birth, leading to a decreased expression and a lack of response during oxygen exposure. As expected, lung edema
was decreased in rat pups treated with PTX during hyperoxia. This decreased lung edema may be associated with increased activity in the AOE system as a result of PTX, which could lead to
decreases in reactive oxygen species tissue damage and inflammation, thus resulting in decreased microvascular permeability. In addition, upregulation of VEGF expression by PTX may stabilize
the endothelium, preventing further development of pulmonary leakage. Previous studies have shown that hyperoxia induces fibrotic gene expression such as that of CTGF (30). Furthermore,
anti-CTGF therapy attenuates hyperoxia-induced alveolar damage and vascular remodeling (31). Multiple studies in kidney and liver have demonstrated that PTX downregulates the expression of
CTGF (32,33); therefore, we expected that PTX would decrease CTGF expression and lung fibrosis during hyperoxia. Consistent with previous studies, we found that hyperoxia increases CTGF and
α-SMA expression. However, we failed to observe downregulation of CTGF and α-SMA expression by PTX. We also did not observe a beneficial effect of PTX on alveolarization during hyperoxia.
Beneficial effects of PTX treatment on survival have been reported in sepsis and in HILI models (21,34). Our study demonstrated survival improvement from 54% to 88% with PTX. Our results,
showing a positive effect of PTX on increasing AOE activity, upregulating VEGF, and decreasing pulmonary edema during oxygen exposure, show that PTX may be sufficient to improve survival but
inadequate to blunt the effect of oxygen on alveolarization and fibrosis. However, other unexplored factors might have also accounted for the improved survival of newborn rats treated with
PTX, including reduction in activation of nuclear factor-κβ and production of tumor necrosis factor-α, leading to attenuated lung injury (35), and the release of endothelium-derived nitric
oxide by PTX in the pulmonary vascular bed, resulting in improved oxygenation (36). There are several potential limitations of this study. First, different routes of administration, dosage,
and length of treatment from hours to weeks for PTX have been used in numerous studies. The twice-a-day dosing used in this experiment might have not been optimal to exert all the potential
benefits of PTX in the lungs of the newborn. However, this dosing was already found to be safe in preterm rats and beneficial in HILI as reported by ter Horst _et al._ (21). Second, the
animals most susceptible to oxygen-induced injury were not analyzed due to their death. Whether PTX treatment during hyperoxia would have had similar effects on lungs of the pups that died
remains unknown. In summary, we found that PTX increases AOE activities, upregulates VEGF expression, improves lung vascularization, and decreases pulmonary edema and macrophage infiltration
in newborn rats with hyperoxic lung injury. These enzymatic and molecular changes may ultimately have led to the improved survival during 9 d of hyperoxia. Further studies are needed to
determine other mechanisms of PTX in lung protection as well as the most appropriate dose to obtain the maximal response. Nonetheless, these findings suggest that PTX therapy may play a role
in the reduction of some of the features of BPD in premature infants. METHODS ANIMAL MODEL AND EXPERIMENTAL PROTOCOL Timed pregnant Sprague-Dawley rats were obtained from Charles River
(Portage, MI). Pups were delivered naturally at term gestation, pooled, and randomly assigned to three groups to receive RA plus placebo, NS (RA + NS); hyperoxia (>95% O2) plus NS (O2 +
NS); or hyperoxia plus PTX (O2 + PTX; Aventis Pharma, Mexico City, Mexico). Hyperoxia was achieved in a sealed Plexiglas chamber as previously described (31). PTX (75 mg/kg equivalent to
3.75 ml/kg) based on the efficacy and safety data previously reported by ter Horst _et al._ (21) or NS (same volume) was given via subcutaneous injection before O2 exposure and then twice a
day during continuous exposure to RA or O2 for 4–9 d. Dams were rotated daily between RA and O2 to avoid oxygen toxicity. Animals were killed on day 4 and 9 with intraperitoneal injections
of Eutasol (0.15 ml/kg; Virbac AH, Fort Worth, TX) for subsequent studies. The research protocol and procedures were reviewed and approved by the Animal Care and Use Committee at the
University of Miami. For biochemical assays and molecular analyses, lungs were perfused with ice-cold NS via the right ventricle until white. The perfused lungs were then excised, trimmed of
extraparenchymal tracheal–bronchial and vascular tissue, weighed, frozen in liquid nitrogen, and stored in a −80 °C freezer. For histological and morphometric analyses, lungs were infused
with 4% paraformaldehyde in phosphate-buffered saline via a tracheal catheter under 20 cm H2O pressure for 5 min and then fixed in 4% paraformaldehyde overnight at 4 °C. Dehydrated lung
tissues were paraffin-embedded and 5-µm tissue sections were prepared. Unperfused lungs were excised _en bloc_ and dissected away from the heart and thymus. The right upper lobe was
immediately removed, blotted dry, and weighed immediately after removal. The lungs were then dried for 4 d in an oven at 60 °C and reweighed. The wet-to-dry-weight ratio was then calculated.
Lung tissue sections were immunostained for Mac3, a macrophage-specific marker (BD Biosciences, San Jose, CA). The number of Mac3-positive cells in the alveolar airspaces was counted from
10 random images taken with the ×40 objective on each slide. Frozen lung tissue was pooled in groups from two rat pups and homogenized in ice-cold phosphate-buffered saline. The lung
homogenates were assayed by standard spectrophotometric techniques for activities of total SOD with the xanthine/xanthine oxidase method (37), of CAT using the rate of reduction of hydrogen
peroxide (38), and of GPX using the rate of oxidation of nicotinamide adenine dinucleotide phosphate using cumene hydroxyperoxide as substrate (39). Lung homogenates were also assayed for
total DNA content according to the Schmit–Thannhauser–Schneider method (40). Purified reference standards for all these assays were obtained commercially (Sigma Chemical, St Louis, MO).
Alveolarization was assessed on hematoxylin–eosin stained tissue sections by radial alveolar count and by mean linear intercept as previously described (41,42). To assess vascular
development, immunofluorescence staining for von Willebrand factor (Dako, Carpinteria, CA), an endothelial-specific marker, was performed. Ten random images were taken with the ×20 objective
on each von Willebrand factor–stained slide. The vascular density was expressed as the average number of von Willebrand factor–positive vessels (15–50 µm) counted per high-power field as
previously described (42). RNA ISOLATION AND SEMIQUANTITATIVE REVERSE-TRANSCRIPTASE PCR OF VEGF SPLICE VARIANTS Total RNA isolation and cDNA reverse transcription were performed as
previously described (42). Reverse-transcriptase PCR was performed using a pair of rat VEGF primers: sense, 5′-CCAGCACATAGGAGAGATGAGCTTC-3′ and antisense, 5′-GGTGTGGTGGTGACATGGTTAATC-3′,
which resulted in three bands (262, 394, and 466 bp) corresponding to the three principal VEGF isoforms VEGF121, VEGF165, and VEGF189, respectively, expressed in rats (43). As a control,
reverse-transcriptase PCR was also performed with β-actin-specific primers. The amplified cDNA fragments were then separated on 2% agarose gels and visualized by ethidium bromide staining.
The intensity of the cDNA products was determined with the Quantity One Imaging Analysis Program (Bio-Rad, Hercules, CA). The relative mRNA levels of each VEGF splicing variant were
determined after normalization to β-actin. MEASUREMENT OF VEGF CONCENTRATION AND WESTERN BLOT ANALYSIS Total VEGF protein concentration in lung homogenates was analyzed using a commercial
enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). Total protein isolation and western blot analysis were performed as
previously described (42). STATISTICAL ANALYSIS Data are expressed as mean ± SD. Comparison among the groups was performed by ANOVA followed by the Holm–Sidak method as a _post hoc_
analysis. For comparison of survival curves, Kaplan–Meier analysis followed by a log-rank test was used. A _P_ <0.05 was considered significant. STATEMENT OF FINANCIAL DISCLOSURE This
study was supported by Forest Pharmaceuticals and INO Therapeutics, through the Advancing Newborn Medicine Fellowship Grant Program and Project Newborn from the University of Miami.
REFERENCES * Jobe AH, Bancalari E . Bronchopulmonary dysplasia. _Am J Respir Crit Care Med_ 2001;163:1723–9. Article CAS Google Scholar * Bucher JR, Roberts RJ . The development of the
newborn rat lung in hyperoxia: a dose-response study of lung growth, maturation, and changes in antioxidant enzyme activities. _Pediatr Res_ 1981;15:999–1008. Article CAS Google Scholar *
Koo HC, Davis JM, Li Y, et al. Effects of transgene expression of superoxide dismutase and glutathione peroxidase on pulmonary epithelial cell growth in hyperoxia. _Am J Physiol Lung Cell
Mol Physiol_ 2005;288:L718–26. Article CAS Google Scholar * Wilborn AM, Evers LB, Canada AT . Oxygen toxicity to the developing lung of the mouse: role of reactive oxygen species.
_Pediatr Res_ 1996;40:225–32. Article CAS Google Scholar * Yam J, Frank L, Roberts RJ . Oxygen toxicity: comparison of lung biochemical responses in neonatal and adult rats. _Pediatr Res_
1978;12:115–9. Article CAS Google Scholar * Frank L, Groseclose EE . Preparation for birth into an O2-rich environment: the antioxidant enzymes in the developing rabbit lung. _Pediatr
Res_ 1984;18:240–4. Article CAS Google Scholar * Gerdin E, Tydén O, Eriksson UJ . The development of antioxidant enzymatic defense in the perinatal rat lung: activities of superoxide
dismutase, glutathione peroxidase, and catalase. _Pediatr Res_ 1985;19:687–91. Article CAS Google Scholar * Frank L, Sosenko IR . Failure of premature rabbits to increase antioxidant
enzymes during hyperoxic exposure: increased susceptibility to pulmonary oxygen toxicity compared with term rabbits. _Pediatr Res_ 1991;29:292–6. Article CAS Google Scholar * Maniscalco
WM, Watkins RH, D’Angio CT, Ryan RM . Hyperoxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. _Am J Respir Cell
Mol Biol_ 1997;16:557–67. Article CAS Google Scholar * Thébaud B, Abman SH . Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic
lung disease. _Am J Respir Crit Care Med_ 2007;175:978–85. Article Google Scholar * Watkins RH, D’Angio CT, Ryan RM, Patel A, Maniscalco WM . Differential expression of VEGF mRNA splice
variants in newborn and adult hyperoxic lung injury. _Am J Physiol_ 1999;276:L858–67. CAS PubMed Google Scholar * Hsia CCW, Berberich MA, Driscoll B, et al. American Thoracic Society
Documents. Mechanisms and limits of induced postnatal lung growth. _Am J Respir Crit Care Med_ 2004;170:319–43. Article Google Scholar * Kunig AM, Balasubramaniam V, Markham NE, Seedorf G,
Gien J, Abman SH . Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. _Am J Physiol Lung Cell Mol Physiol_
2006;291:L1068–78. Article CAS Google Scholar * Harris E, Schulzke SM, Patole SK . Pentoxifylline in preterm neonates: a systematic review. _Paediatr Drugs_ 2010;12:301–11. Article
Google Scholar * Michetti C, Coimbra R, Hoyt DB, Loomis W, Junger W, Wolf P . Pentoxifylline reduces acute lung injury in chronic endotoxemia. _J Surg Res_ 2003;115:92–9. Article CAS
Google Scholar * Yada-Langoi MM, Macae M, Coimbra R, et al. Hypertonic saline and pentoxifylline prevent lung injury and bacterial translocation after hemorrhagic shock. _Shock_
2000;14:594–8. Article Google Scholar * Savas S, Delibas N, Savas C, Sütçü R, Cindas A . Pentoxifylline reduces biochemical markers of ischemia-reperfusion induced spinal cord injury in
rabbits. _Spinal Cord_ 2002;40:224–9. Article CAS Google Scholar * Smalling WE Jr, Suguihara C, Huang J, Rodriguez MM, Bancalari E . Protective effect of pentoxifylline on volume-induced
lung injury in newborn piglets. _Biol Neonate_ 2004;86:15–21. Article CAS Google Scholar * Korhonen K, Kiuru A, Svedström E, Kääpä P . Pentoxifylline reduces regional inflammatory and
ventilatory disturbances in meconium-exposed piglet lungs. _Pediatr Res_ 2004;56:901–6. Article CAS Google Scholar * Lindsey HJ, Kisala JM, Ayala A, Lehman D, Herdon CD, Chaudry IH .
Pentoxifylline attenuates oxygen-induced lung injury. _J Surg Res_ 1994;56:543–8. Article CAS Google Scholar * ter Horst SA, Wagenaar GT, de Boer E, et al. Pentoxifylline reduces fibrin
deposition and prolongs survival in neonatal hyperoxic lung injury. _J Appl Physiol_ 2004;97:2014–9. Article CAS Google Scholar * Davis JM . Superoxide dismutase: a role in the prevention
of chronic lung disease. _Biol Neonate_ 1998;74:Suppl 1:29–34. Article CAS Google Scholar * Tanswell AK, Freeman BA . Pulmonary antioxidant enzyme maturation in the fetal and neonatal
rat. I. Developmental profiles. _Pediatr Res_ 1984;18:584–7. Article CAS Google Scholar * Ilizarov AM, Koo HC, Kazzaz JA, et al. Overexpression of manganese superoxide dismutase protects
lung epithelial cells against oxidant injury. _Am J Respir Cell Mol Biol_ 2001;24:436–41. Article CAS Google Scholar * Jakkula M, Le Cras TD, Gebb S, et al. Inhibition of angiogenesis
decreases alveolarization in the developing rat lung. _Am J Physiol Lung Cell Mol Physiol_ 2000;279:L600–7. Article CAS Google Scholar * Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay
LA, Maniscalco WM . Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. _Am J Respir
Crit Care Med_ 2001;164:1971–80. Article CAS Google Scholar * Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H . Angiogenic factors and alveolar vasculature: development
and alterations by injury in very premature baboons. _Am J Physiol Lung Cell Mol Physiol_ 2002;282:L811–23. Article CAS Google Scholar * Galambos C, Ng YS, Ali A, et al. Defective
pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. _Am J Respir Cell Mol Biol_ 2002; 27:194–203. Article CAS Google Scholar * Greenberg
JM, Thompson FY, Brooks SK, et al. Mesenchymal expression of vascular endothelial growth factors d and A defines vascular patterning in developing lung. _Dev Dyn_ 2002;224:144–53. Article
CAS Google Scholar * Chen CM, Wang LF, Chou HC, Lang YD, Lai YP . Up-regulation of connective tissue growth factor in hyperoxia-induced lung fibrosis. _Pediatr Res_ 2007;62:128–33. Article
CAS Google Scholar * Alapati D, Rong M, Chen S, et al. Connective tissue growth factor antibody therapy attenuates hyperoxia-induced lung injury in neonatal rats. _Am J Respir Cell Mol
Biol_ 2011;45:1169–77. Article CAS Google Scholar * Lin SL, Chen RH, Chen YM, et al. Pentoxifylline attenuates tubulointerstitial fibrosis by blocking Smad3/4-activated transcription and
profibrogenic effects of connective tissue growth factor. _J Am Soc Nephrol_ 2005;16:2702–13. Article CAS Google Scholar * Raetsch C, Jia JD, Boigk G, et al. Pentoxifylline downregulates
profibrogenic cytokines and procollagen I expression in rat secondary biliary fibrosis. _Gut_ 2002;50:241–7. Article CAS Google Scholar * Yang S, Zhou M, Koo DJ, Chaudry IH, Wang P .
Pentoxifylline prevents the transition from the hyperdynamic to hypodynamic response during sepsis. _Am J Physiol_ 1999;277:H1036–44. Article CAS Google Scholar * Coimbra R, Melbostad H,
Loomis W, et al. LPS-induced acute lung injury is attenuated by phosphodiesterase inhibition: effects on proinflammatory mediators, metalloproteinases, NF-kappaB, and ICAM-1 expression. _J
Trauma_ 2006;60:115–25. Article CAS Google Scholar * Kaye AD, Ibrahim IN, Kadowitz PJ, Nossaman BD . Analysis of responses to pentoxifylline in the pulmonary vascular bed of the cat.
_Crit Care Med_ 1996;24:263–7. Article CAS Google Scholar * McCord JM, Fridovich I . Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). _J Biol Chem_
1969;244:6049–55. CAS PubMed Google Scholar * Holmes RS, Masters CJ . Epigenetic interconversions of the multiple forms of mouse liver catalase. _FEBS Lett_ 1970;11:45–8. Article CAS
Google Scholar * Paglia DE, Valentine WN . Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. _J Lab Clin Med_ 1967;70:158–69. CAS PubMed
Google Scholar * Schmidt G . Determination of nucleic acids by phosphorus analysis. In: Colowick SP, Kaplan NO, eds. Methods in Enzymology, vol. 3. New York: Academic Press, 1957:671–9. *
Cooney TP, Thurlbeck WM . The radial alveolar count method of Emery and Mithal: a reappraisal 1–postnatal lung growth. _Thorax_ 1982;37:572–9. Article CAS Google Scholar * Chen S, Rong M,
Platteau A, et al. CTGF disrupts alveolarization and induces pulmonary hypertension in neonatal mice: implication in the pathogenesis of severe bronchopulmonary dysplasia. _Am J Physiol
Lung Cell Mol Physiol_ 2011;300:L330–40. Article CAS Google Scholar * Zheng W, Seftor EA, Meininger CJ, Hendrix MJ, Tomanek RJ . Mechanisms of coronary angiogenesis in response to
stretch: role of VEGF and TGF-β. _Am J Physiol Heart Circ Physiol_ 2001;280:H909–17. Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Pediatrics, Batchelor Children’s Research Institute, University of Miami Miller School of Medicine, Miami, Florida Beatriz Almario, Shu Wu, Jinghong Peng, Deepthi Alapati,
Shaoyi Chen & Ilene R.S. Sosenko Authors * Beatriz Almario View author publications You can also search for this author inPubMed Google Scholar * Shu Wu View author publications You can
also search for this author inPubMed Google Scholar * Jinghong Peng View author publications You can also search for this author inPubMed Google Scholar * Deepthi Alapati View author
publications You can also search for this author inPubMed Google Scholar * Shaoyi Chen View author publications You can also search for this author inPubMed Google Scholar * Ilene R.S.
Sosenko View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Ilene R.S. Sosenko. RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Almario, B., Wu, S., Peng, J. _et al._ Pentoxifylline and prevention of hyperoxia-induced lung injury in neonatal rats. _Pediatr Res_ 71,
583–589 (2012). https://doi.org/10.1038/pr.2012.14 Download citation * Received: 10 August 2011 * Accepted: 05 January 2012 * Published: 09 February 2012 * Issue Date: May 2012 * DOI:
https://doi.org/10.1038/pr.2012.14 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative