The rho pathway mediates transition to an alveolar type i cell phenotype during static stretch of alveolar type ii cells
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Stretch is an essential mechanism for lung growth and development. Animal models in which fetal lungs have been chronically over or underdistended demonstrate a disrupted mix of
type II and type I cells, with static overdistention typically promoting a type I cell phenotype. The Rho GTPase family, key regulators of cytoskeletal signaling, are known to mediate
cellular differentiation in response to stretch in other organs. Using a well-described model of alveolar epithelial cell differentiation and a validated stretch device, we investigated the
effects of supraphysiologic stretch on human fetal lung alveolar epithelial cell phenotype. Static stretch applied to epithelial cells suppressed type II cell markers (SP-B and Pepsinogen C,
PGC), and induced type I cell markers (Caveolin-1, Claudin 7 and Plasminogen Activator Inhibitor-1, PAI-1) as predicted. Static stretch was also associated with Rho A activation.
Furthermore, the Rho kinase inhibitor Y27632 decreased Rho A activation and blunted the stretch-induced changes in alveolar epithelial cell marker expression. Together these data provide
further evidence that mechanical stimulation of the cytoskeleton and Rho activation are key upstream events in mechanotransduction-associated alveolar epithelial cell differentiation.
SIMILAR CONTENT BEING VIEWED BY OTHERS HEPARAN SULFATE REGULATES MYOFIBROBLAST HETEROGENEITY AND FUNCTION TO MEDIATE NICHE HOMEOSTASIS DURING ALVEOLAR MORPHOGENESIS Article Open access 21
February 2025 ALVEOLAR CELL FATE SELECTION AND LIFELONG MAINTENANCE OF AT2 CELLS BY FGF SIGNALING Article Open access 21 November 2022 RELEASE OF NOTCH ACTIVITY COORDINATED BY IL-1Β
SIGNALLING CONFERS DIFFERENTIATION PLASTICITY OF AIRWAY PROGENITORS VIA FOSL2 DURING ALVEOLAR REGENERATION Article 02 September 2021 MAIN The alveolar epithelium is composed of two cell
types: type II and type I cells (1). Type II cells are responsible for surfactant production and play a role in lung host defense. Type I cells, although less numerous, cover the majority of
the gas exchange surface area of the lung. Development and maintenance of this mixed population of alveolar epithelial cells depend on both the biochemical milieu of growth factors,
hormones, and extracellular matrix, and the interplay of physical forces mediated intrinsically by the cytoskeleton and extrinsically by cell-cell and cell-matrix interactions. Stretch plays
a critical role in lung development (2). Static stretch provided by fetal lung fluid provides a constant distending force of approximately 2.5 mm Hg (3). Fetal breathing movements provide
intermittent cyclic stretch (4) resulting in 3–5% change in alveolar surface area (3). By comparison, changes in surface area with tidal breathing in adults are minimal (5), whereas
expansion to total lung capacity changes surface area by 40–45% (6). The importance of stretch as a mechanism for lung development has been shown in human pregnancy complicated by premature
membrane rupture (7), in neonatal neuromuscular disorders (8), and in animal models (9). By extension, enhanced stretch, generally from tracheal obstruction, promotes lung growth (10,11),
providing the rationale for the use of tracheal occlusion to reverse pulmonary hypoplasia in congenital diaphragmatic hernia. Although tracheal occlusion increases lung growth through the
retention of fetal lung fluid, the effects of this supraphysiologic stretch on differentiation of the alveolar epithelium are less clear (12). Animal studies suggest that static stretch
favors the formation of type I cells (13), whereas cyclic stretch favors type II cells (14), but the mechanisms by which stretch is translated into molecular signals to modify gene
expression in the alveolar epithelium are poorly understood. The Rho-GTPase family of small messengers is an attractive candidate for mediating stretch-induced cell signaling because of its
tight coupling to the cytoskeleton. As the cytoskeleton is a global receiver and transmitter of mechanical forces (15), Rho-GTP activation could be an early, upstream intracellular event in
response to stretch. Rho GTPases have been implicated in lung branching morphogenesis (16), alveolar epithelial permeability (17), migration (18), and recently, maturation of alveolar type
II cells (19). We now show, using a validated, equibiaxial stretch device and human fetal lung (HFL) epithelial cells, that changes in epithelial cell phenotype between type I and type II
cells with static stretch are associated with activation of the Rho GTPase pathway. METHODS REAGENTS. Dexamethasone, isobutyl methylxanthine (DCI), and 8-bromo-cAMP were purchased from Sigma
Chemical Co. (St. Louis, MO). All other supplies were purchased from Fisher (Fair Lawn, NJ), Pierce (Rockford, IL) or Invitrogen (Carlsbad, CA). Antisera included SP-B (Chemicon, Temecula,
CA), Pepsinogen C (Abcam, Cambridge, UK), Claudin 7 (Zymed, South San Francisco, CA), Plasminogen Activator Inhibitor-1 (BD Transduction Laboratories, Lexington, KY), Caveolin-1α (Santa Cruz
Biotechnologies, Santa Cruz, CA), and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Chemicon). CELL CULTURE. HFLs from 14- to 18-wk therapeutic abortions were obtained from Advance
Bioscience Resources, Inc. (Alameda, CA) and used in protocols approved by the Committee for Human Research at The Children's Hospital of Philadelphia. A stable population of alveolar
type II cells (with an average 10% contaminating fibroblasts, <5% endothelial cells, and no inflammatory cells) were prepared as described previously (20) and plated at a density of 7 ×
105 cells/cm2 on deformable silastic membranes (Specialty Manufacturing, Saginaw, MI) coated with 50 μg/mL of fibronectin (BD Biosciences, Medford, MA) and mounted into custom-made wells.
Waymouth's media containing 10 nM dexamethasone, 0.1 mM 8-bromo-cAMP, and 0.1 mM DCI was used to maintain the type II cell phenotype. EQUIBIAXIAL STRETCH. Cells on silastic membranes
were mounted onto individual cell-stretching devices capable of applying static equibiaxial strain, as described previously (6). Seventy-two hours after plating, cells were stretched
continuously for 24 h at either 10 or 37% change in surface area. These equibiaxial deformations correspond to static stretches in isolated rat lungs at 55 and 100% of total lung capacity,
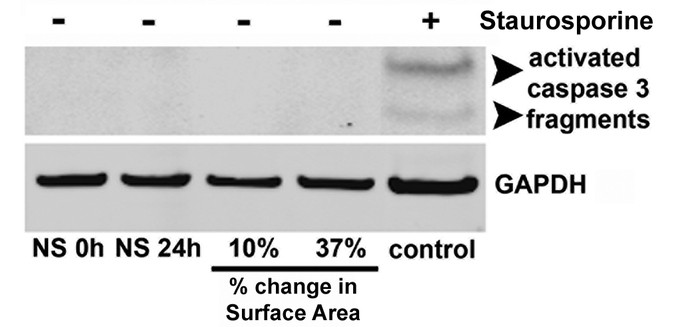
respectively. CELL VIABILITY AND APOPTOSIS. Ethidium homodimer-1 and calcein AM were added to the wells to assess cell viability (LIVE/DEAD, Molecular Probes, Eugene, OR). Apoptosis was
assessed by immunoblotting for activated caspase 3 (R and D Systems, Minneapolis, MN). Cells treated with 1 μM Staurosporine (Sigma Chemical Co.) served as a positive control. WESTERN
IMMUNOBLOTTING. Cells were harvested in lysis buffer (1% Triton X-100, 150 mM NaCl, 50 mM Tris-HCl, 5 mM ethylenediamine tetraacetic acid, 5% glycerol, pH 8.0) with 1× Protease inhibitor
(Roche, Indianapolis, IN), and samples immunoblotted using NuPAGE Bis-Tris gels (Invitrogen). Primary antibody concentrations were SP-B 1:4000; PGC 1:5000; Claudin 7, PAI-1, and Caveolin 1
at 1:1000; GAPDH at 1:20,000. Secondary antibodies conjugated to Alexa Fluor 680 (Molecular Probes) or IRdye 800 (Rockland, Gilbertsville, PA) were used at a dilution of 1:10,000. Membranes
were analyzed using the Odyssey infrared imaging system (Li-Cor, Lincoln, NE). REAL-TIME REVERSE TRANSCRIPTASE PCR. Total cellular RNA was isolated using RNA STAT-60 Reagent (Tel-Test,
Friarswood, TX). Purity was verified by the OD 260:280 ratio and integrity assessed using the Agilent 2100 bioanalyzer system (Agilent, Palo Alto, CA). Real-time (RT) PCR assays using a
singleplex strategy were done using an ABI Prism 7900 system (ABI, Foster City, CA). Details of the two-step protocol have been described previously (21). The primer/probe sets (listed on
the ABI website; available at: http://www.allgenes.com) were SP-B Hs00167036, PGC Hs00160052, claudin 7 Hs00600772, PAI-1 Hs00167155, Cav-1 Hs00184697, and 18s Hs99999901_S1. Standards for
comparison of RT-PCR results were derived from RNA isolated from alveolar type II cells cultured from HFL or from banked frozen adult human lung tissue. IMMUNOFLUORESCENCE IMAGING.
Experimental membranes with adherent cells were mounted on glass slides using Fluoromount (Sigma Chemical Co.). Cells were fixed with 1% paraformaldehyde in phosphate-buffered saline, and
immunostained with Claudin 7 antibody (1:100). Nuclei were counterstained with DAPI. Fluorescence was examined at 20× with an Olympus IX81 microscope and Slidebook 4.2.0 digital microscopy
software (Olympus, San Diego, CA). STRESS FIBER ANALYSIS. Alexa Fluor 549 Phalloidin (Molecular Probes, Eugene, OR) was used to stain the actin cytoskeleton at the end of the stretching
period. Stress fibers were counted in seven random high power fields (60×) per treatment group using Image Pro-Plus software (Version 6.0, MediaCybernetics Inc, Bethesda, MD) to determine
mean stress fiber intensity per cell. RHO INHIBITION. Y27632, a selective Rho kinase (ROCK) inhibitor (Tocris, Ellisville, MO) was added to cells at a concentration of 20 μM/mL, 1 h before
stretch and maintained throughout the experiment. Direct activation of Rho was assessed using the G-Lisa Rho A Activation Assay (Cytoskeleton, Denver, CO), which measures activated Rho-GTP,
per the manufacturer's instructions. STATISTICAL ANALYSIS. Results are expressed as mean ± SE. Analysis of variance (for LIVE/DEAD) and _t_ tests (all other experiments) were performed
with GraphPad Prism 5.0 for Macintosh (GraphPad, San Diego, CA). All protein and RNA studies were normalized with GAPDH and 18S, respectively. RESULTS STATIC STRETCH MODIFIED ALVEOLAR
EPITHELIAL CELL PHENOTYPE WITHOUT CELL TOXICITY. Random field counting (_n_ = 4 experiments) revealed no changes in cell viability because of culture or stretch (before stretch 92.8 ± 2.6%
live cells; no stretch 24 h 91.6 ± 1.2%; 10% stretch 24 h 94.0 ± 1.1%; 37% stretch 24 h 93.8 ± 2.4%; _p_ > 0.05), and there was no evidence of apoptosis after static stretch (Fig. 1).
RT-PCR revealed decreased expression of the type II cell markers SP-B and PGC (Fig. 2_A_) at 10% (SP-B: 61 ± 8% and PGC: 50 ± 5% of unstretched control) and 37% change in surface area (SP-B:
50 ± 5% and PGC: 51 ± 7%; _n_ = 5–7, _p_ < 0.01). These changes were not evident at the protein level (Fig. 2_B_ and _C_). Induction of RNA for the type I cell markers Claudin 7 and
PAI-1 (Fig. 3_A_) occurred at both 10% (Claudin 7: 1.5 ± 0.09-fold and PAI-1: 2.0 ± 0.04-fold _versus_ unstretched) and 37% change in surface area (Claudin 7: 1.8 ± 0.1-fold and PAI-1: 3.4 ±
0.4-fold; _n_ = 3, _p_ < 0.05). There was a modest induction of Caveolin-1 RNA with 10% stretch that did not reach statistical significance (_n_ = 3, _p_ = 0.08). Immunoblotting
demonstrated induction of PAI-1 and Caveolin-1 protein with 37% stretch (Fig. 3_B_ and C; _n_ = 4–5, _p_ < 0.05), and a modest increase in Claudin 7 protein expression with 10% stretch.
We characterized Claudin 7 localization to the plasma membrane as a proxy for alveolar epithelial barrier changes because the silastic membranes precluded traditional permeability testing.
Claudin 7 was distributed throughout the cytoplasm before stretch (Fig. 4_A_) and in unstretched cells (not shown). With static stretch (Fig. 4_B_ and _C_), Claudin 7 immunostaining was more
prominent at the plasma membrane. RHO-GTP FUNCTION IN RESPONSE TO STATIC STRETCH. Stress fibers are longitudinal bundles of contractile actin-myosin filaments resulting from activation of
the Rho-GTP/ROCK pathway (22). Stress fibers (as shown in Fig. 5_A_) are present in most cultured cells but are markedly increased by stretch (23). There was a 27% increase in the mean
intensity of phalloidin-positive stress fibers per cell at 37% stretch compared with unstretched controls (Fig. 5_B_; _n_ = 3, _p_ < 0.05), whereas no significant change was observed at
10% stretch. Because stress fibers are a late endpoint in Rho pathway activation, we measured direct Rho/ROCK pathway activation by ELISA in response to 37% stretch (Fig. 6). Activated
Rho-GTP increased by 21.4 ± 1.5% 15 min after initiation of 37% stretch (_n_ = 3–4, _p_ < 0.01), followed by a decrease at 1 and 4 h (at 4 h: 46.7 ± 5.4%, _n_ = 4, _p_ < 0.01), with
variable rebound to baseline by 24 h. INHIBITION OF RHO DIMINISHES STRETCH PHENOTYPE CHANGES. To determine whether the stretch induced changes in gene expression could be due to activation
of Rho, we used a selective Rho inhibitor, Y27632, during epithelial cell stretch (Fig. 7). Y27632 partially restored SP-B RNA expression at 10% stretch but not at 37% stretch (_n_ = 4, _p_
< 0.05 _versus_ 10% stretch without inhibitor). By comparison, expression of PAI-1 was blunted at both 10% and 37% stretch in the presence of Y27632 (_n_ = 4–5, _p_ < 0.05 _versus_ no
inhibitor). DISCUSSION Mechanical forces are important regulators of organogenesis and differentiation during fetal development. Although reduced stretch results in lung hypoplasia while
overdistention stimulates lung growth (3), the effects of stretch on differentiation have been less clear. Animal models suggest that static stretch promotes type I cell phenotype (13), and
cyclic stretch promotes type II cell phenotype (24). More recent studies of animal models of congenital diaphragmatic hernia and postmortem human studies suggest that despite improved lung
growth, supraphysiologic stretch _in utero_ does not result in a mature alveolar epithelium with an appropriate mixed population of type I and type II cells (25,26). This is the first study
to assess the impact of supraphysiologic stretch on alveolar epithelial cell differentiation using a well-characterized model of type II cells derived from HFL in which both the local
effects of static stretch and alterations in intracellular pathways can be easily monitored. Mechanistic studies of stretch-induced epithelial differentiation have been hampered by
controversy surrounding alveolar epithelial cell lineage and alveolar epithelial marker expression. The classic dogma—alveolar type II cells as the progenitor for terminally differentiated
alveolar type I cells—came from interpretations of older studies using electron microscopy to understand the resolution of lung injury (27). These early studies were supported by later
evidence that type II cells served as progenitors for injured type I cells in mature lung (28) and that isolated type II cells in culture for over 24 h to lose characteristics of type II
cells and adopt features of type I cells in the absence of serum (29). Another obstacle to understanding the impact of stretch on differentiation is the paucity of markers that clearly
differentiate type I from type II cells across species. Markers such as T1α (30) and RTI40 (31), now recognized as podoplanin, have not been useful in human lung. Markers that clearly
distinguish type I cells from type II cells, such as Caveolin 1, are also found in other cells locally in the lung (32). Antibodies to markers in rodent models have poor crossreactivity to
human cells. This study attempts to solve these problems by using a relevant human model and choosing markers that have been reproducible in this and other human models. Our
well-characterized cell culture model has been used to examine the cell biology and biochemistry of alveolar type I and II cells (20,33–35). Cells are derived from HFL making this model
particularly relevant to lung development. The primary cultures have been consistently ∼90% pure, with contamination chiefly from fibroblasts. However, the use of a human cell culture model
restricts the choice of available type I cell markers. We have previously shown that PAI-1, Caveolin 1, and Claudin 7 are robust, reproducible markers of type I cells in this system (34).
Although not exclusively expressed by type I cells in the lung, they are clearly not expressed by human type II cells or by contaminating fibroblasts. Therefore, our choice of cell culture
system and markers provide a robust model to study the effects of stretch on human fetal alveolar epithelial cell phenotype. Equibiaxial stretch of type II cells had very predictable effects
on the epithelial cell markers. We demonstrated a decline in SP-B and PGC RNA within 24 h of initiating both 10% and 37% stretch, concomitant with an increase in PAI-1 and Caveolin 1 RNA.
Compared with our prior report of epithelial cell marker expression in transdifferentiation experiments (34), the decline in type II cell markers with stretch despite the presence of
hormones was blunted yet significant. In the prior study, DCI withdrawal effectively eliminated SP-B and PGC RNA expression by 96 h, with persistence of some SP-B protein through 120 h and
PGC protein through 96 h. Persistence of type II cell proteins after cessation of RNA expression has been commonly reported in rodent models of alveolar epithelial cell transdifferentiation
(36,37). Importantly, despite culture conditions that should sustain type II cell marker expression, specifically the presence of glucocorticoid and cAMP, stretch significantly impaired SP-B
and PGC expression. Few studies of the effects of stretch on alveolar epithelial cell differentiation have examined type I cell markers (31). In this study, type I cell markers behaved as
predicted, increasing in response to 24 h of static stretch as type II marker expression waned. The magnitude of changes in type I markers was less than we observed by transdifferentiation
previously. Claudin 7 was helpful in this study because of its type I cell specificity specific in HFL (33,34) and its role in alveolar epithelial barrier function (38). Although Claudin 7
behaved similarly to our prior study, demonstrating only a modest induction of RNA and no significant change in protein content over 24 h, it was remarkable that localization of Claudin 7
became directed to the plasma membrane with stretch. Studies of rat type I cell transdifferentiation also observed an increase in Claudin 7 that was maximal after 7d in culture, with a more
plasma membrane distribution in type I cells that correlated with increased barrier function (39). The silastic membranes we used for the static stretch in our system precluded a more
functional approach to assessing barrier function. However, redistribution of Claudin 7 is indirect but consistent evidence of enhanced barrier function with stretch. Taken together, changes
in type I and II cell marker expression clearly show that despite culture conditions that support maintenance of type II cell phenotype, stretch fosters change toward a type I cell
phenotype in HFL epithelial cells. Several signal transduction pathways have been implicated in stretch-mediated lung maturation, including extracellular signal related kinases (24), protein
kinase A and C (2), heparin-binding epidermal growth-like factor, and transforming growth factor alpha (40). The Rho/ROCK pathway is an excellent candidate for transmitting alveolar
epithelial cell stretch into gene expression due to its pivotal role in regulating the actin cytoskeleton, and in mechanotransduction-mediated differentiation, most notably in smooth muscle
cells (41). We provide compelling evidence that Rho pathway activation plays an important role in static stretch-induced differentiation of HFL epithelium: indirectly by the increase in
stress fibers with static stretch and directly by detecting GTP-bound Rho within 15 min of applying static stretch. More importantly, stretch-induced changes in epithelial cell marker
expression were blunted in the presence of a specific Rho inhibitor, Y27632. Our data echo similar observations in rodent type II cells, with the ROCK inhibitor H-1152 blunting the effect of
cyclic stretch on expression of the type II cell marker SP-C (19). Similar to their studies, our data show that Rho A is a negative regulator of type II cell markers, especially at reduced
levels of stretch—5% in their study, and 10% in ours. What is more impressive is that Rho A is a positive regulator of type I cell markers at both levels of stretch in this study, placing
the Rho pathway as a central regulator of alveolar epithelial differentiation in response to stretch. The differences in response between type I and type II markers and between 10 and 37%
stretch may reflect events downstream of Rho A. Y27632 is a ROCK inhibitor, which will account for only one limb of the pathway after Rho A activation leading to inhibition of cofilin and
promoting actin polymerization (23). Because an intact cytoskeleton is a critical factor in mediating mechanotransduction, the role of profilin actions could be important but are not
regulated by ROCK and thus not susceptible to Y27632. The inability of ROCK inhibition to restore type II cell marker expression to unstretched levels illustrates that there are additional
mechanotransduction pathways involved, such as the mitogen-activated protein kinase pathway (24). The resultant intermediate type I/II cell phenotype may simply reflect the short duration of
these experiments. However, they suggest a reasonable explanation for the presence of such intermediate cells observed by others in response to lung injury as repopulating type II cells
begin to transdifferentiate (26,42,43). Although there is increasing evidence of type I to type II cell plasticity (44), it remains unclear whether this is stretch-responsive _in vivo_ and
which pathways might be involved. In summary, we have shown that static stretch is an important determinant of alveolar epithelial cell plasticity and that mechanotransduction is partially
mediated by the Rho pathway. The Rho GTPase pathway may provide an important early indicator of alveolar epithelial cell well being in studies designed to evaluate lung-protective
ventilation strategies and may offer a targeted pathway for the design of novel pharmacologic interventions, due to the accessibility of the alveolar epithelium, to prevent lung injury
during mechanical ventilation. ABBREVIATIONS * DCI: Dexamethasone, isobutyl methylxanthine, and 8-bromo-cAMP * GAPDH: Glyceraldehyde-3-phosphate dehydrogenase * PGC: Pepsinogen C * PAI-1:
Plasminogen Activator Inhibitor-1 * ROCK: Rho kinase * SP-B: Surfactant Protein B * HFL: human fetal lung REFERENCES * Guttentag SH, Ballard PL 2005 Lung development: embryology, growth,
maturation, and developmental biology. In: Taeusch HW, Ballard RA, Gleason CA (eds) _Avery's Diseases of the Newborn_. Elsevier Saunders, Philadelphia, PA, pp 601–615 Chapter Google
Scholar * Liu M, Post M 2000 Invited review: mechanochemical signal transduction in the fetal lung. _J Appl Physiol_ 89: 2078–2084 Article CAS PubMed Google Scholar * Kitterman JA 1996
The effects of mechanical forces on fetal lung growth. _Clin Perinatol_ 23: 727–740 Article CAS PubMed Google Scholar * Harding R, Liggins GC 1996 Changes in thoracic dimensions induced
by breathing movements in fetal sheep. _Reprod Fertil Dev_ 8: 117–124 Article CAS PubMed Google Scholar * Martin TR 2008 Interactions between mechanical and biological processes in acute
lung injury. _Proc Am Thorac Soc_ 5: 291–296 Article PubMed PubMed Central Google Scholar * Tschumperlin DJ, Margulies SS 1999 Alveolar epithelial surface area-volume relationship in
isolated rat lungs. _J Appl Physiol_ 86: 2026–2033 Article CAS PubMed Google Scholar * Rotschild A, Ling EW, Puterman ML, Farquharson D 1990 Neonatal outcome after prolonged preterm
rupture of the membranes. _Am J Obstet Gynecol_ 162: 46–52 Article CAS PubMed Google Scholar * Page DV, Stocker JT 1982 Anomalies associated with pulmonary hypoplasia. _Am Rev Respir
Dis_ 125: 216–221 CAS PubMed Google Scholar * Yoshizawa J, Chapin CJ, Sbragia L, Ertsey R, Gutierrez JA, Albanese CT, Kitterman JA 2003 Tracheal occlusion stimulates cell cycle
progression and type I cell differentiation in lungs of fetal rats. _Am J Physiol Lung Cell Mol Physiol_ 285: L344–L353 Article CAS PubMed Google Scholar * Adzick NS, Harrison MR, Glick
PL, Villa RL, Finkbeiner W 1984 Experimental pulmonary hypoplasia and oligohydramnios: relative contributions of lung fluid and fetal breathing movements. _J Pediatr Surg_ 19: 658–665
Article CAS PubMed Google Scholar * Kitterman JA, Chapin CJ, Vanderbilt JN, Porta NF, Scavo LM, Dobbs LG, Ertsey R, Goerke J 2002 Effects of oligohydramnios on lung growth and maturation
in the fetal rat. _Am J Physiol Lung Cell Mol Physiol_ 282: L431–L439 Article CAS PubMed Google Scholar * Khan PA, Cloutier M, Piedboeuf B 2007 Tracheal occlusion: a review of
obstructing fetal lungs to make them grow and mature. _Am J Med Genet C Semin Med Genet_ 145C: 125–138 Article PubMed Google Scholar * Dobbs LG, Gutierrez JA 2001 Mechanical forces
modulate alveolar epithelial phenotypic expression. _Comp Biochem Physiol A Mol Integr Physiol_ 129: 261–266 Article CAS PubMed Google Scholar * Sanchez-Esteban J, Wang Y, Filardo EJ,
Rubin LP, Ingber DE 2006 Integrins beta1, alpha6, and alpha3 contribute to mechanical strain-induced differentiation of fetal lung type II epithelial cells via distinct mechanisms. _Am J
Physiol Lung Cell Mol Physiol_ 290: L343–L350 Article CAS PubMed Google Scholar * Ingber DE 2003 Tensegrity II. How structural networks influence cellular information processing
networks. _J Cell Sci_ 116( Pt 8): 1397–1408 Article CAS PubMed Google Scholar * Moore KA, Polte T, Huang S, Shi B, Alsberg E, Sunday ME, Ingber DE 2005 Control of basement membrane
remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. _Dev Dyn_ 232: 268–281 Article CAS PubMed Google Scholar * Olivera DS, Boggs SE,
Beenhouwer C, Aden J, Knall C 2007 Cellular mechanisms of mainstream cigarette smoke-induced lung epithelial tight junction permeability changes in vitro. _Inhal Toxicol_ 19: 13–22 Article
CAS PubMed Google Scholar * Desai LP, Chapman KE, Waters CM 2008 Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1. _Am J
Physiol Lung Cell Mol Physiol_ 295: L958–L965 Article CAS PubMed PubMed Central Google Scholar * Silbert O, Wang Y, Maciejewski BS, Lee HS, Shaw SK, Sanchez-Esteban J 2008 Roles of RhoA
and Rac1 on actin remodeling and cell alignment and differentiation in fetal type II epithelial cells exposed to cyclic mechanical stretch. _Exp Lung Res_ 34: 663–680 Article CAS PubMed
Google Scholar * Gonzales LW, Angampalli S, Guttentag SH, Beers MF, Feinstein SI, Matlapudi A, Ballard PL 2001 Maintenance of differentiated function of the surfactant system in human fetal
lung type II epithelial cells cultured on plastic. _Pediatr Pathol Mol Med_ 20: 387–412 Article CAS PubMed Google Scholar * Foster C, Aktar A, Kopf D, Zhang P, Guttentag S 2004
Pepsinogen C: a type 2 cell-specific protease. _Am J Physiol Lung Cell Mol Physiol_ 286: L382–L387 Article CAS PubMed Google Scholar * Bishop AL, Hall A 2000 Rho GTPases and their
effector proteins. _Biochem J_ 348( Pt 2): 241–255 Article CAS PubMed PubMed Central Google Scholar * Hellstrand P, Albinsson S 2005 Stretch-dependent growth and differentiation in
vascular smooth muscle: role of the actin cytoskeleton. _Can J Physiol Pharmacol_ 83: 869–875 Article CAS PubMed Google Scholar * Sanchez-Esteban J, Wang Y, Gruppuso PA, Rubin LP 2004
Mechanical stretch induces fetal type II cell differentiation via an epidermal growth factor receptor-extracellular-regulated protein kinase signaling pathway. _Am J Respir Cell Mol Biol_
30: 76–83 Article CAS PubMed Google Scholar * Danzer E, Davey MG, Kreiger PA, Ruchelli ED, Johnson MP, Adzick NS, Flake AW, Hedrick HL 2008 Fetal tracheal occlusion for severe congenital
diaphragmatic hernia in humans: a morphometric study of lung parenchyma and muscularization of pulmonary arterioles. _J Pediatr Surg_ 43: 1767–1775 Article PubMed Google Scholar *
Flecknoe S, Harding R, Maritz G, Hooper SB 2000 Increased lung expansion alters the proportions of type I and type II alveolar epithelial cells in fetal sheep. _Am J Physiol Lung Cell Mol
Physiol_ 278: L1180–L1185 Article CAS PubMed Google Scholar * Adamson IY, Bowden DH 1975 Derivation of type 1 epithelium from type 2 cells in the developing rat lung. _Lab Invest_ 32:
736–745 CAS PubMed Google Scholar * Evans MJ, Cabral LJ, Stephens RJ, Freeman G 1975 Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. _Exp Mol Pathol_
22: 142–150 Article CAS PubMed Google Scholar * Borok Z, Hami A, Danto SI, Zabski SM, Crandall ED 1995 Rat serum inhibits progression of alveolar epithelial cells toward the type I cell
phenotype in vitro. _Am J Respir Cell Mol Biol_ 12: 50–55 Article CAS PubMed Google Scholar * Williams MC, Cao Y, Hinds A, Rishi AK, Wetterwald A 1996 T1a protein is developmentally
regulated and expressed by alveolar type I cells, choroid plexus, and ciliary epithelia of adult rats. _Am J Respir Cell Mol Biol_ 14: 577–585 Article CAS PubMed Google Scholar *
Gutierrez JA, Gonzalez RF, Dobbs LG 1998 Mechanical distension modulates pulmonary alveolar epithelial phenotypic expression in vitro. _Am J Physiol_ 274: L196–L202 CAS PubMed Google
Scholar * Williams MC 2003 Alveolar type I cells: molecular phenotype and development. _Annu Rev Physiol_ 65: 669–695 Article CAS PubMed Google Scholar * Daugherty BL, Mateescu M, Patel
AS, Wade K, Kimura S, Gonzales LW, Guttentag S, Ballard PL, Koval M 2004 Developmental regulation of claudin localization by fetal alveolar epithelial cells. _Am J Physiol Lung Cell Mol
Physiol_ 287: L1266–L1273 Article CAS PubMed Google Scholar * Foster CD, Varghese LS, Skalina RB, Gonzales LW, Guttentag SH 2007 In vitro transdifferentiation of human fetal type II
cells toward a type I-like cell. _Pediatr Res_ 61: 404–409 Article PubMed PubMed Central Google Scholar * Gonzales LW, Guttentag SH, Wade KC, Postle AD, Ballard PL 2002 Differentiation
of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. _Am J Physiol Lung Cell Mol Physiol_ 283: L940–L951 Article CAS PubMed Google Scholar * Bates SR, Gonzales LW, Tao
JQ, Rueckert P, Ballard PL, Fisher AB 2002 Recovery of rat type II cell surfactant components during primary cell culture. _Am J Physiol Lung Cell Mol Physiol_ 282: L267–L276 Article CAS
PubMed Google Scholar * Dobbs LG, Pian MS, Maglio M, Dumars S, Allen L 1997 Maintenance of the differentiated type II cell phenotype by culture with an apical air surface. _Am J Physiol_
273: L347–L354 Article CAS PubMed Google Scholar * Van Itallie CM, Anderson JM 2004 The molecular physiology of tight junction pores. _Physiology (Bethesda)_ 19: 331–338 CAS Google
Scholar * Chen SP, Zhou B, Willis BC, Sandoval AJ, Liebler JM, Kim KJ, Ann DK, Crandall ED, Borok Z 2005 Effects of transdifferentiation and EGF on claudin isoform expression in alveolar
epithelial cells. _J Appl Physiol_ 98: 322–328 Article CAS PubMed Google Scholar * Wang Y, Maciejewski BS, Soto-Reyes D, Lee HS, Warburton D, Sanchez-Esteban J 2009 Mechanical stretch
promotes fetal type II epithelial cell differentiation via shedding of HB-EGF and TGF-alpha. _J Physiol_ 587( Pt 8): 1739–1753 Article CAS PubMed PubMed Central Google Scholar * Owens
GK, Kumar MS, Wamhoff BR 2004 Molecular regulation of vascular smooth muscle cell differentiation in development and disease. _Physiol Rev_ 84: 767–801 Article CAS PubMed Google Scholar
* Clegg GR, Tyrrell C, McKechnie SR, Beers MF, Harrison D, McElroy MC 2005 Coexpression of RTI40 with alveolar epithelial type II cell proteins in lungs following injury: identification of
alveolar intermediate cell types. _Am J Physiol Lung Cell Mol Physiol_ 289: L382–L390 Article CAS PubMed Google Scholar * Olsen CO, Isakson BE, Seedorf GJ, Lubman RL, Boitano S 2005
Extracellular matrix-driven alveolar epithelial cell differentiation in vitro. _Exp Lung Res_ 31: 461–482 Article CAS PubMed Google Scholar * Gonzalez RF, Allen L, Dobbs LG 2009 Rat
alveolar type I cells proliferate, express OCT-4, and exhibit phenotypic plasticity in vitro. _Am J Physiol Lung Cell Mol Physiol_ 297: L1045–L1055 Article CAS PubMed PubMed Central
Google Scholar Download references ACKNOWLEDGEMENTS We thank Ping Wang for cell preparation and James Hayden and Frederick Keeney, from the Wistar Institute Microscopy Facility for
assistance with the stress fiber imaging and image quantitation studies. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pediatrics, The Children's Hospital of Philadelphia,
University of Pennsylvania School of Medicine, Philadelphia, 19104, PA Cherie D Foster, Linda S Varghese, Linda W Gonzales & Susan H Guttentag * Department of Bioengineering, University
of Pennsylvania School of Engineering and Applied Science, Philadelphia, 19104, PA Susan S Margulies Authors * Cherie D Foster View author publications You can also search for this author
inPubMed Google Scholar * Linda S Varghese View author publications You can also search for this author inPubMed Google Scholar * Linda W Gonzales View author publications You can also
search for this author inPubMed Google Scholar * Susan S Margulies View author publications You can also search for this author inPubMed Google Scholar * Susan H Guttentag View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Susan H Guttentag. ADDITIONAL INFORMATION Supported by grants HL-077266
(C.D.F.) and HL059959 (S.H.G.) from the National Institutes of Health. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Foster, C., Varghese, L.,
Gonzales, L. _et al._ The Rho Pathway Mediates Transition to an Alveolar Type I Cell Phenotype During Static Stretch of Alveolar Type II Cells. _Pediatr Res_ 67, 585–590 (2010).
https://doi.org/10.1203/PDR.0b013e3181dbc708 Download citation * Received: 17 September 2009 * Accepted: 17 February 2010 * Issue Date: June 2010 * DOI:
https://doi.org/10.1203/PDR.0b013e3181dbc708 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative