Inhibition of jnk enhances tgf-β1-activated smad2 signaling in mouse embryonic lung
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The Smad2/3 pathway plays a key role in mediating TGF-β1 inhibition of branching morphogenesis and induction of connective tissue growth factor (CTGF) expression in embryonic lungs.
Because a number of cell-specific interactions have been described between TGF-β1-driven Smad signaling and the c-Jun N-terminal kinase (JNK) pathway, we have investigated the effects of
JNK inhibition on TGF-β1 activation of Smad2, inhibition of branching, induction of CTGF expression, and apoptosis in mouse embryonic lung explants. Mouse embryonic day 12.5 (E12.5) lung
explants were treated with TGF-β1 in the presence or absence of a specific pharmacologic JNK inhibitor (SP600125) and a specific JNK peptide inhibitor (JNKI). We found that TGF-β1 activated
the JNK pathway by stimulating c-Jun phosphorylation, which was blocked by JNK inhibitors. Treatment with SP600125 stimulated Smad2 phosphorylation and enhanced TGF-β1-induced Smad2
phosphorylation. Treatment with JNK inhibitors also decreased normal branching morphogenesis and induced CTGF expression as well as augmented TGF-β1 inhibition of branching and induction of
CTGF expression. Furthermore, JNK inhibition-induced apoptosis. Our results demonstrate that inhibition of the JNK pathway promotes TGF-β1-driven Smad2 responses in lung branching
morphogenesis. These data suggest that the JNK pathway may antagonize TGF-β1 dependent Smad2 signaling during mouse embryonic lung development. SIMILAR CONTENT BEING VIEWED BY OTHERS EFFECT
OF SLIT/ROBO SIGNALING ON REGENERATION IN LUNG EMPHYSEMA Article Open access 25 May 2021 SPRED2-DEFICIENCY ENHANCES THE PROLIFERATION OF LUNG EPITHELIAL CELLS AND ALLEVIATES PULMONARY
FIBROSIS INDUCED BY BLEOMYCIN Article Open access 05 October 2020 PHOSPHORYLATION OF FOXN3 BY NEK6 PROMOTES PULMONARY FIBROSIS THROUGH SMAD SIGNALING Article Open access 21 February 2025
MAIN TGF-β1 is a multifunctional cytokine that regulates diverse biologic processes, including cell proliferation, differentiation, and apoptosis during development and tissue injury repair
(1,2). Both _in vivo_ studies in transgenic mice and _in vitro_ studies in embryonic lung organ culture have demonstrated that TGF-β1 is a key negative regulator for embryonic lung branching
morphogenesis (3–7). However, the molecular mechanisms mediating TGF-β1 cellular responses in lung development are not well understood. TGF-β1 initiates its cellular action by inducing
heterodimerization of type I (TβRI) and type II (TβRII) transmembrane TGF-β receptors (8,9). On ligand-induced dimerization, constitutively active TβRII kinase phosphorylates TβRI that, in
turn, activates the downstream signal transduction cascades. Smad2 and Smad3 phosphorylation is the most prominent pathway (10,11). Smad7 is an inducible intracellular inhibitor that
decreases Smad2/3 phosphorylation by blocking their access to TGF-β receptors (12). Once activated, Smad2/3 associates with Smad4 and translocates to the nucleus, where the complex
transcriptionally regulates expression of target genes. Besides the Smad2/3 pathway, TGF-β can also activate the c-Jun NH2-terminal kinase (JNK) signaling pathway (13–16). The activation of
the c-Jun N-terminal kinase (JNK) pathway by TGF-β1 is mediated through sequential phosphorylation and activation of mitogen-activated protein kinase (MAPK) kinase 1, then MAPK kinase (MKK)
4 or MKK 7 and finally JNK. JNK then translocates to the nucleus where it phosphorylates c-Jun. Activated c-Jun homodimerizes with the members of the Jun family or heterodimerizes with the
members of the Fos family. Such complexes named activating-protein-1 (AP-1) bind to AP-1 sites and can control the expression of a number of genes (17–19). There is growing evidence that the
JNK pathway can cross talk with the TGF-β-dependent Smad signaling at multiple sites and both positively and negatively modulate TGF-β induced gene expression and cellular responses
(20,21). We and others have previously demonstrated the critical role of the Smad2/3 pathway in mediating TGF-β1 inhibition of branching morphogenesis in mouse embryonic lung explants
(22,23). Connective tissue growth factor (CTGF) is a member of a family of immediate-early gene products that coordinate complex biologic processes during differentiation and tissue repair
(24,25). TGF-β1 is a major inducer of CTGF expression in mesenchymal type cells and many of the TGF-β1 effects on mesenchymal cells, including stimulation of fibroblast proliferation; ECM
production and myofibroblast differentiation are mediated by CTGF (26–29). We have provided evidence that endogenous and TGF-β1-induced CTGF expression in embryonic lung explants is mediated
by the Smad2 pathway (22). Moreover, like TGF-β, CTGF inhibits branching morphogenesis (22). Because the JNK pathway cross talks with the Smad2/3 pathway and the Smad2/3 pathway is crucial
in mediating TGF-β inhibition of branching and induction of CTGF expression, we postulated that the JNK pathway would play a pivotal role in branching morphogenesis and CTGF expression in
embryonic lung explants. Hence, we investigated the effect of blocking the JNK pathway with SP600125, a specific pharmacologic inhibitor (30) and with a specific peptide inhibitor of JNK
(JNKI) (31), on Smad2 phosphorylation, CTGF induction, branching inhibition, and apoptosis. Our results demonstrate that inactivation of the JNK pathway enhances TGF-β-dependent Smad2
signaling and induces apoptosis in embryonic lung explants. MATERIALS AND METHODS CD1 mice were purchased from Harlan (Indianapolis, IN). Trizol reagents and first strand cDNA synthesis kits
were obtained from Invitrogen (Carlsbad, CA). Recombinant TGF-β1 and a pan TGF-β neutralizing antibody (clone 1D11) were purchased from R&D Systems (Minneapolis, MN). SP600125, a
chemical inhibitor of JNK with IC50 of 0.04 (30) was obtained from EMD Chemicals (San Diego, CA). JNKI and JNKC (control peptide for JNKI) were obtained from Alexis Biochemicals (San Diego,
CA). Primers and reagents for quantitative real-time RT-PCR were purchased from Applied Biosystems (Foster City, CA). A rabbit polyclonal antibody for phosphorylated smad2 (p-Smad2) was from
Chemicon (Temecula, CA). Rabbit polyclonal antibodies for phosphorylated c-Jun (p-c-Jun) and total c-Jun (t-c-Jun), and a MAb for cleaved caspase 3 were obtained from Cell Signaling
Technology (Denver, MA). Goat polyclonal antibodies for CTGF and total Smad2/3 (t-Smad2/3) were obtained from Santa Cruz Biotechnology Inc (Santa Cruz, CA). A mouse MAb for β-actin was
obtained from Sigma Chemical Co. (St. Louis, MO). Horseradish peroxidase conjugated anti-mouse, anti-rabbit and anti-goat IgGs and enhanced chemiluminescence reagents were obtained from
Amersham (Piscataway, NJ). EMBRYONIC LUNG EXPLANT CULTURE. The study protocol was reviewed and approved by the Animal Care and Use Committee of the University of Miami. Eight-week-old female
mice were mated, and noon on the day of vaginal plug formation was set as E0.5. On E12.5, pregnant mice were killed by cervical dislocation after receiving CO2 narcosis. Embryos were
obtained by hysterectomy. Lungs were dissected under a dissection microscope and placed on 8-μm nucleopore membranes floating on 0.5 mL of chemical defined serum-free medium in each well of
a 24-well plate as previously described (22). The lung explants were preincubated with inhibitors, JNKC, or TGF-β neutralizing antibodies for 1 h and then treated with TGF-β1. Cultures were
maintained at 37°C in a humidified 5% CO2 incubator for 2–48 h. The lung explants exposed to medium alone served as controls. QUANTIFICATION OF BRANCHING MORPHOGENESIS. Branching
morphogenesis was measured as the number of the terminal sacs around the circumference of the lung explants as previously described (22). Lung explants in culture on the nucleopore membranes
were photographed at 48 h using an Olympus digital camera attached to an inverted microscope for permanent imaging analysis. RNA ISOLATION AND QUANTITATIVE REAL-TIME RT-PCR. Total RNA was
isolated from pooled lung explants as described (22). Two μg of total RNA was reverse-transcribed in a 20 μL reaction by using a first-strand cDNA synthesis kit according to
manufacturer's protocol (Invitrogen). The Real-time RT-PCR was performed on an ABI Fast 7500 System (Applied Biosystems). Each reaction included diluted first-strand cDNA, mouse CTGF,
TGF-β1, TGF-β2, TGF-β3, TβRI, TβRII, or GAPDH primers, and master mix containing TaqMan probes according to the manufacturer's instruction (Applied Biosystems). Real-time RT-PCR
conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 30 s. RNase-free water was used as a negative control. For each target gene, a standard curve was
established by performing a series of dilutions of the first-strand cDNA. The mRNA expression levels of target genes were determined from the standard curve and normalized to GAPDH. WESTERN
BLOT ANALYSIS. Total protein was extracted from pooled lung explants with a lysis buffer from Active Motif (Carlsbad, CA), according to manufacturer's protocol as previously described
(22). The protein concentrations were measured by BCA protein assay using a commercial kit from Pierce Biotechnology (Rockford, IL). Seventy-five microgram samples of total protein were
fractionated by SDS–PAGE on 4–12% Tris-glycine precast gradient gels (Invitrogen) and then transferred to nitrocellulose membranes (Amersham, Piscataway, NJ). The membranes were incubated
overnight at 4°C with primary antibodies specific to proteins of interest and then incubated for 1 h at room temperature with HRP-conjugated secondary antibodies. Antibody-bound proteins
were detected using ECL chemiluminescence methodology. Membranes were then stripped with 0.2 N NaOH and reincubated with primary antibodies reactive with normalization proteins. The
intensities of protein bands were quantified by Quantity One Imaging Analysis Program (Bio-Rad, Hercules, CA). The relative protein levels of CTGF and cleaved caspase 3 were normalized to
β-actin, p-Smad2 levels were normalized to t-Smad2 and p-c-Jun levels were normalized to t-c-Jun. DNA FRAGMENTATION ASSAY. DNA was isolated from pooled lung explants as described (32). The
DNA (1 μg/lane) was electrophoresed on a 1.5% agarose gel. The gel was stained with ethidium bromide and photographed under transmitted UV light. DATA PRESENTATION AND STATISTICAL ANALYSIS.
Results are expressed as means ± SD. Comparisons were performed by one-way ANOVA followed by Student-Newman-Keuls test. A _p_ < 0.05 was considered significant. RESULTS JNK INHIBITORS
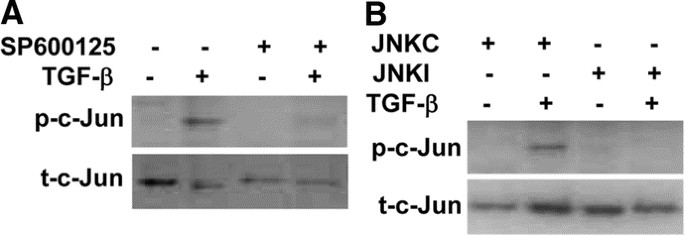
EFFECTIVELY BLOCK TGF-Β1 STIMULATION OF C-JUN PHOSPHORYLATION. We first examined c-Jun phosphorylation to determine if TGF-β1 can activate the JNK pathway and whether SP600125 and JNKI can
block the JNK pathway. As demonstrated in Figure 1, treatment with TGF-β1 resulted in c-Jun phosphorylation. However, preincubation with SP600125 or JNKI abolished TGF-β1 induced c-Jun
phosphorylation. These results demonstrate that TGF-β1 activates the JNK pathway, which can be effectively blocked by JNK inhibitors. BLOCKING THE JNK PATHWAY AUGMENTS ENDOGENOUS AND
TGF-Β1-INDUCED SMAD2 PHOSPHORYLATION. To determine whether cross talk occurs between the JNK and Smad2 pathways in response to TGF-β1, we examined the effect of JNK inactivation on Smad2
phosphorylation. As we have demonstrated previously, treatment with TGF-β1 induces sustained Smad2 phosphorylation (Fig. 2). Interestingly, treatment with SP600125 alone induced Smad2
phosphorylation and treatment with SP600125 plus TGF-β1 induced a greater Smad2 phosphorylation (Fig. 2). Treatment with JNKI or JNKC did not affect TGF-β1 phosphorylation of Smad2 (data not
shown). BLOCKING THE JNK PATHWAY INHIBITS BRANCHING MORPHOGENESIS AND ENHANCES TGF-Β1 INHIBITION OF BRANCHING. We have previously shown that inactivation of the Smad2 pathway increases
normal branching morphogenesis and blocks TGF-β1 inhibition of branching in E12.5 lung explants (22). We hypothesized that the JNK pathway antagonizes the Smad2 pathway. Therefore, blocking
the JNK pathway would enhance Smad2 signaling that results in branching inhibition. As demonstrated in Figure 3, treatments with a low-dose of SP600125 or TGF-β did not affect branching
morphogenesis; however, the combination of the two decreased branching by 30%. Treatment with 10 μM SP600125 or JNKI alone decreased normal branching morphogenesis by 55 and 35%. The
combination of a high-dose of TGF-β1 with SP600125 or JNKI resulted in further decrease of branching up to 75%. Treatment with low-dose of JNKI (1 μM) did not affect normal branching or
TGF-β1 inhibition of branching (data not shown). BLOCKING THE JNK PATHWAY INDUCES ENDOGENOUS CTGF GENE EXPRESSION AND ENHANCES TGF-Β1-INDUCED CTGF GENE EXPRESSION. In a previous study, we
showed that endogenous and TGF-β1-induced CTGF expression in embryonic lung explants is Smad2-dependent (22). We analyzed the effect of SP600125 and JNKI on endogenous and TGF-β1 induced
CTGF gene expression. As demonstrated in Figure 4, compared with the control, treatment with TGF-β1 increased CTGF mRNA expression by nearly 5-fold. Lung explants treated with SP600125 also
showed a 4-fold increase of CTGF mRNA expression. However, the combination of SP600125 and TGF-β1 treatment resulted in a 14-fold increase of CTGF mRNA expression (Fig. 4). Consistent with
CTGF mRNA expression, treatment with SP600125 or TGF-β1 alone induced 1.6- and 2-folds increase in CTGF protein expression, and treatment with SP600125 plus TGF-β1 induced more than a 3-fold
increase in CTGF protein expression (Fig. 4). Treatment with JNKI resulted in similar changes of CTGF mRNA expression (Fig. 4). These results demonstrate that blocking the JNK pathway
induces endogenous CTGF gene expression and also enhances TGF-β1-induced CTGF gene expression in embryonic lung explants. BLOCKING THE JNK PATHWAY DOES NOT AFFECT TGF-Β GENE EXPRESSION AND
TGF-Β BINDING TO ITS RECEPTORS. To determine whether blocking the JNK pathway in mouse embryonic lung explants triggers TGF-β autoregulation, we measured mRNA expression of TGF-β1, 2, 3,
TβRI, and TβRII. Treatment with either TGF-β1 or SP600125 or their combination did not significantly change mRNA expression of these genes (data not shown). To determine whether enhanced
branching inhibition and CTGF mRNA expression caused by JNK inhibition is mediated by increased TGF-β binding to its receptors, lung explants were pretreated with a TGF-β neutralizing
antibody and then treated with TGF-β1 or SP600125. As demonstrated in Figure 5, pretreatment with the antibody significantly blocked TGF-β1 inhibition of branching. However, the antibody had
no effect on branching inhibition caused by SP600125. Correlating with branching morphogenesis, TGF-β antibody abolished TGF-β1-induced CTGF mRNA expression, but had no effect on
SP600125-induced CTGF mRNA expression (Fig. 5). BLOCKING THE JNK PATHWAY INDUCES APOPTOSIS. To investigate the potential mechanisms of the decreased branching morphogenesis caused by JNK
inhibition and TGF-β1, apoptosis was examined by DNA fragmentation assay and expression of cleaved caspase 3, a crucial effector of apoptosis cascades (33). Treatment with SP600125 resulted
in DNA internucleosomal cleavage as demonstrated by ladder appearance of low molecular weight DNAs on agarose gel electrophoresis (Fig. 6). Treatment with SP600125 also induced expression of
cleaved caspase 3 (Fig. 6). In contrast, treatment with TGF-β did not induce apoptosis. DISCUSSION The Smad family of proteins is thought to be the primary intracellular mediators of TGF-β
signaling. However, increasing evidence indicates that TGF-β can also activate MAPK cascades and that cross talk between the Smad pathway and MAPKs determines the overall cellular responses
to TGF-β. Among the MAPKs, the best characterized is the JNK signaling pathway, but its role in embryonic lung development is largely unknown. Thus, we have investigated the effects of JNK
inhibition on TGF-β responsiveness during embryonic lung development. We focused our investigations on branching morphogenesis and CTGF gene expression because our previous study showed that
these two responses are Smad2-dependent (22). The data from this study demonstrate for the first time that TGF-β activates the JNK pathway, inhibition of this pathway enhances TGF-β1-Smad2
responses in mouse embryonic lung explants. In this study, we investigated whether TGF-β1 activates the JNK pathway and showed that TGF-β1 induces c-Jun phosphorylation in mouse embryonic
lung explants. Moreover, SP600125 and JNKI, two well-characterized JNK inhibitors (30,31), were found to effectively abolish TGF-β1-induced c-Jun phosphorylation, confirming that TGF-β1
activates the JNK pathway leading to c-Jun phosphorylation. Multiple mechanisms have been reported by which the JNK pathway interacts with the Smad pathway to provide a negative feed back
loop to control TGF-β responses. In human hepatoma cell line HepG2, activation of the JNK pathway negatively regulates TGF-β induced Smad signaling _via_ c-Jun interacting with Smad2/3
corepressors such as TGIF and Ski (34,35). In human dermal fibroblasts, 5-fluorouracil induces c-Jun phosphorylation and activation of AP-1 that inhibits Smad3/4 specific transcription and
formation of Smad/DNA complex induced by TGF-β (36). Furthermore, over-expression of JNK inhibits Smad2 phosphorylation, but lack of JNK induces Smad2 phosphorylation in fibroblasts (37). In
previous studies, we demonstrated that TGF-β1 induces Smad2 phosphorylation in mouse embryonic lung explants (22). In this study, we investigated whether JNK inhibition affects Smad2
phosphorylation. We showed that SP600125 induces endogenous Smad2 phosphorylation and enhances TGF-β1-induced Smad2 phosphorylation. However, we did not observe any effect of JNKI on Smad2
phosphorylation. One possible explanation for the differential effect of SP600125 and JNKI on Smad2 phosphorylation may be that SP600125 and JNKI act differently to inhibit the JNK pathway.
SP600125 inhibits JNK phosphorylation of c-Jun (IC 50 of 0.04), and to a lesser degree inhibits MKK4 phosphorylation of JNK (IC50 of 0.4) (30). In contrast, JNKI blocks the interaction
between JNK and c-Jun, but has no effect on MKK4 phosphorylation of JNK (31). These may explain why SP600125 and JNKI both inhibited c-Jun phosphorylation but only SP600125 induced Smad2
phosphorylation in this study. The biologic significance of the Smad2/3 pathway in regulating branching morphogenesis is well documented and multiple lines of evidence indicate that
activation of the Smad2/3 pathway inhibits branching morphogenesis (22,23). Accordingly, we also investigated whether JNK inhibition influences branching morphogenesis in embryonic lung
explants. The results showed that blocking the JNK pathway inhibited normal branching morphogenesis and enhanced TGF-β1 inhibition of branching. Extensive studies have demonstrated that the
JNK pathway plays a critical role in modulating cell death. A recent study has shown that activation of JNK promotes cell survival during short bursts of oxidative stress, and that JNK
inhibition accelerates caspase-3 and -9 cleavage, which triggers apoptosis in primary culture of rat neonatal cardiomyocytes (33). Data from this study demonstrated that JNK inhibition alone
or in the presence of TGF-β1 results in apoptosis in embryonic lung explants. In contrast, treatment with TGF-β1 alone did not cause apoptosis. The differential effects of JNK inhibition
and TGF-β on apoptosis observed in this study suggest that the Smad-independent mechanisms are also involved in JNK action during embryonic lung development. Future studies are needed to
explore the potential mechanisms by which the JNK pathway modulates cell proliferation and apoptosis in embryonic lung branching morphogenesis. CTGF is a TGF-β responsive gene that mediates
many of the TGF-β effects on mesenchymal cell types (26–29). The Smad2/3 pathway plays a key role in TGF-β induction of CTGF expression (38,39). A functional Smad element is found in the
CTGF promoter and it can be potently activated by Smad2/3 and suppressed by Smad7 (38). The JNK pathway is implicated to play both agonistic and antagonistic roles in TGF-β1 induction of
CTGF expression. It has been reported that induction of CTGF by TGF-β is antagonized by hyperactive JNK and AP-1. This involves c-Jun binding to Smad2/3, blocking its interaction with the
Smad element of the CTGF promoter (39). However, studies have also demonstrated that TGF-β activation of Smad2 and induction of CTGF in human lung fibroblasts is attenuated by JNK inhibition
(40). Our previous studies have demonstrated that induction of CTGF expression by TGF-β1 in embryonic lung explants is Smad-dependent (22). In this study, we investigated whether inhibition
of the JNK pathway induces CTGF gene expression in mouse embryonic lung explants and found this to be the case in both the basal and TGF-β1-induced states. Thus, the increased Smad2
activity and decreased c-Jun activity caused by JNK inhibition plays an agonistic role in TGF-β induction of CTGF expression in embryonic lung explants. This is different from what has been
reported in mature lung fibroblasts (40). Previous studies have indicated that JNK disruption results in TGF-β autoregulation (37). To elucidate additional potential mechanisms by which JNK
inhibition enhances Smad2 signaling in embryonic lung explants, we examined TGF-β autoregulation in this study. Our results demonstrated that JNK inhibition does not alter expression of
TGF-β ligands and receptors. Furthermore, JNK inhibition did not affect TGF-βs binding to their receptors. Therefore, the decreased branching and increased CTGF gene expression caused by JNK
inhibition are unlikely the results of TGF-β autoregulation. In conclusion, our results demonstrate that blocking the endogenous JNK pathway inhibits branching morphogenesis and induces
CTGF gene expression in embryonic lung explants. Furthermore, blocking the TGF-β 1-induced JNK pathway enhances Smad2 phosphorylation, decreases branching morphogenesis, and increases CTGF
gene expression. These data suggest that the JNK pathway antagonizes the Smad2/3 pathway to modulate TGF-β responses in lung branching morphogenesis. ABBREVIATIONS * AP-1:
activating-protein-1 * CTGF: connective tissue growth factor * JNK: c-Jun NH2-terminal kinase * JNKI: JNK peptide inhibitor * JNKC: JNK peptide control * MAPK: mitogen-activated protein
kinase * MKK: MAPK kinase * p-c-Jun: phosphorylated c-Jun * p-Smad2: phosphorylated smad2 * TβRI: TGF-β receptor I * TβRII: TGF-β receptor II * t-c-Jun: total c-Jun * t-Smad2: total Smad2
REFERENCES * Moses HL 1990 Growth Factors from Genes to Clinical Application. Raven Press, New York. pp 141–155 * Moses HL, Yang EY, Pietenpol JA 1990 TGF-beta stimulation and inhibition of
cell proliferation: new mechanistic insights. _Cell_ 63: 245–247 Article CAS Google Scholar * Zhou L, Dey CR, Wert SE, Whitsett JA 1996 Arrested lung morphogenesis in transgenic mice
bearing a SP-C-TGF-beta 1 chimeric gene. _Dev Biol_ 175: 227–238 Article CAS Google Scholar * Zeng X, Gray M, Stahlman MT, Whitsett JA 2001 TGF-beta1 perturbs vascular development and
inhibits epithelial differentiation in fetal lung in vivo. _Dev Dyn_ 221: 289–301 Article CAS Google Scholar * Serra R, Pelton RW, Moses HL 1994 TGF beta 1 inhibits branching
morphogenesis and n-myc expression in lung bud organ cultures. _Development_ 120: 2153–2161 CAS PubMed Google Scholar * Bragg AD, Moses HL, Serra R 2001 Signaling to the epithelium is not
sufficient to mediate all of the effects of transforming growth factor beta and bone morphogenetic protein 4 on murine embryonic lung development. _Mech Dev_ 109: 13–26 Article CAS Google
Scholar * Zhao J, Bu D, Lee M, Slavkin HC, Hall FL, Warburton D 1996 Abrogation of transforming growth factor-beta type II receptor stimulates embryonic mouse lung branching morphogenesis
in culture. _Dev Biol_ 180: 242–257 Article CAS Google Scholar * Massague J, Cheifetz S, Boyd FT, Andres LJ 1990 TGF-beta receptors and TGF-beta binding proteoglycans: recent progress in
identifying their functional properties. _Ann NY Acad Sci_ 593: 59–72 Article CAS Google Scholar * Franzen P, ten Dijke P, Ichijo H, Yamashita H, Schulz P, Heldin CH, Miyazono K 1993
Cloning of a TGF beta type I receptor that forms a heteromeric complex with the TGF beta type II receptor. _Cell_ 75: 681–692 Article CAS Google Scholar * Massague J 1998 TGF-beta signal
transduction. _Annu Rev Biochem_ 67: 753–791 Article CAS Google Scholar * Massague J, Chen YG 2000 Controlling TGF-beta signaling. _Genes Dev_ 14: 627–644 CAS PubMed Google Scholar *
Afrakhte M, Moren A, Jossan S, Itoh S, Sampath K, Westermark B, Heldin CH, Heldin NE, ten Dijke P 1998 Induction of inhibitory Smad6 and Smad7 mRNA by TGF-beta family members. _Biochem
Biophys Res Commun_ 249: 505–511 Article CAS Google Scholar * Derynck R, Zhang YE 2003 Smad-dependent and Smad-independent pathways in TGF-β signaling. _Nature_ 425: 577–584 Article CAS
Google Scholar * Moustakas A, Heldin CH 2005 Non-Smad TGF-β signals. _J Cell Sci_ 118: 3573–3584 Article CAS Google Scholar * Watanabe H, de Caestecker MP, Yamada Y 2001
Transcriptional cross-talk between Smad, erk1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic
atdc5 cells. _J Biol Chem_ 276: 14466–14473 Article CAS Google Scholar * Kale VP 2004 Differential activation of MAPK signaling pathways by TGF-beta1 forms the molecular mechanism behind
its dose-dependent bidirectional effects on hematopoiesis. _Stem Cells Dev_ 13: 27–38 Article CAS Google Scholar * Davis RJ 2000 Signal transduction by the JNK group of MAP kinases.
_Cell_ 103: 239–252 Article CAS Google Scholar * Angel P, Hattori K, Smeal T, Karin M 1988 The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. _Cell_ 55: 875–885
Article CAS Google Scholar * Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ 1994 JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and
phosphorylates the c-Jun activation domain. _Cell_ 76: 1025–1037 Article CAS Google Scholar * Zhang Y, Feng XH, Derynck R 1998 Smad3 and Smad4 cooperate with c-jun/c-fos to
mediateTGF-beta-induced transcription. _Nature_ 394: 909–913 Article CAS Google Scholar * Engel ME, McDonnell MA, Law BK, Moses HL 1999 Interdependent Smad and JNK signaling in
transforming growth factor-beta-mediated transcription. _J Biol Chem_ 274: 37413–37420 Article CAS Google Scholar * Wu S, Peng J, Duncan MR, Kasisomayajula K, Grotendorst G, Bancalari E
2007 Alk-5 mediates endogenous and tgf-beta1-induced expression of connective tissue growth factor in embryonic lung. _Am J Respir Cell Mol Biol_ 36: 552–561 Article CAS Google Scholar *
Zhao J, Lee M, Smith S, Warburton D 1998 Abrogation of Smad3 and Smad2 or of Smad4 gene expression positively regulates murine embryonic lung branching morphogenesis in culture. _Dev Biol_
194: 182–195 Article CAS Google Scholar * Bork P 1993 The modular architecture of a new family of growth regulators related to connective tissue growth factor. _FEBS Lett_ 327: 125–130
Article CAS Google Scholar * Leask A, Abraham DJ 2003 The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. _Biochem Cell Biol_ 81:
355–363 Article CAS Google Scholar * Grotendorst GR 1997 Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. _Cytokine Growth Factor Rev_ 8: 171–179 Article
CAS Google Scholar * Kothapalli D, Hayashi N, Grotendorst GR 1998 Inhibition of TGF-beta-stimulated CTGF gene expression and anchorage-independent growth by cAMP identifies a
CTGF-dependent restriction point in the cell cycle. _FASEB J_ 12: 1151–1161 Article CAS Google Scholar * Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR
1999 Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. _FASEB J_ 13: 1774–1786 Article CAS Google Scholar *
Grotendorst GR, Rahmanie H, Duncan MR 2004 Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. _FASEB J_ 18: 469–479 Article CAS Google
Scholar * Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y 2001 SP600125, an anthrapyrazolone inhibitor of jun n-terminal
kinase. _Proc Natl Acad Sci USA_ 98: 13681–13686 Article CAS Google Scholar * Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF 2001 Cell-permeable peptide inhibitors of JNK: novel
blockers of beta-cell death. _Diabetes_ 50: 77–82 Article CAS Google Scholar * Hamada M, Sumi T, Iwai S, Nakazawa M, Yura Y 2006 Induction of endonuclease G-medictaed apoptosis in human
oral sequamous cell carcinoma cells by protein kinase C inhibitor satingol. _Apoptosis_ 11: 47–56 Article CAS Google Scholar * Tran TH, Andreka P, Rodrigues CO, Webster KA, Bishopric NH
2007 Jun kinase delay caspase-9 activation by interaction with apoptosome. _J Biol Chem_ 282: 20340–20350 Article CAS Google Scholar * Pessah M, Prunier C, Marais J, Ferrand N, Mazars A,
Lallemand F, Gauthier JM, Atfi A 2001 C-jun interacts with the corepressor TG-interacting factor (TGIF) to suppress Smad2 transcriptional activity. _Proc Natl Acad Sci USA_ 98: 6198–6203
Article CAS Google Scholar * Pessah M, Marais J, Prunier C, Ferrand N, Lallemand F, Mauviel A, Atfi A 2002 C-jun associates with the oncoprotein ski and suppresses Smad2 transcriptional
activity. _J Biol Chem_ 277: 29094–29100 Article CAS Google Scholar * Wendling J, Marchand A, Mauviel A, Verrecchia F 2003 5-fluorouracil blocks transforming growth factor-beta-induced
alpha 2 type I collagen gene (COL1A2) expression in human fibroblasts via c-Jun NH2-terminal kinase/activator protein-1 activation. _Mol Pharmacol_ 64: 707–713 Article CAS Google Scholar
* Ventura JJ, Kennedy NJ, Flavell RA, Davis RJ 2004 JNK regulates autocrine expression of TGF-beta1. _Mol Cell_ 15: 269–278 Article CAS Google Scholar * Holmes A, Abraham DJ, Sa S, Shiwen
X, Black CM, Leask A 2001 CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. _J Biol Chem_ 276: 10594–10601 Article CAS Google Scholar * Leask A,
Holmes A, Black CM, Abraham DJ 2003 Connective tissue growth factor gene expression: requirments for its induction by transforming growth factor-β2 in fibroblasts. _J Biol Chem_ 278:
13008–13015 Article CAS Google Scholar * Utsugi M, Dobashi K, Ishizuka T, Masubuchic K, Shimizu Y, Nakazawa T, Mori M 2003 C-Jun-NH2-terminal kinase mediates expression of connective
tissue growth factor induced by transforming growth factor-beta1 in human lung fibroblasts. _Am J Respir Cell Mol Biol_ 28: 754–761 Article CAS Google Scholar Download references AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Pediatrics, University of Miami Miller School of Medicine, Miami, 33101, Florida Shu Wu, Kalyani Kasisomayajula, Jinghong Peng &
Eduardo Bancalari Authors * Shu Wu View author publications You can also search for this author inPubMed Google Scholar * Kalyani Kasisomayajula View author publications You can also search
for this author inPubMed Google Scholar * Jinghong Peng View author publications You can also search for this author inPubMed Google Scholar * Eduardo Bancalari View author publications You
can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Shu Wu. ADDITIONAL INFORMATION Supported, in part, by grant K08 HD046582 from NIH, by Project
NewBorn University of Miami, and by a grant from the Bank of American Charitable Foundation, Inc. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wu, S.,
Kasisomayajula, K., Peng, J. _et al._ Inhibition of JNK Enhances TGF-β1-Activated Smad2 Signaling in Mouse Embryonic Lung. _Pediatr Res_ 65, 381–386 (2009).
https://doi.org/10.1203/PDR.0b013e3181991c67 Download citation * Received: 18 July 2008 * Accepted: 09 November 2008 * Issue Date: April 2009 * DOI:
https://doi.org/10.1203/PDR.0b013e3181991c67 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative