A novel pro-apoptotic function of rack1: suppression of src activity in the intrinsic and akt pathways
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
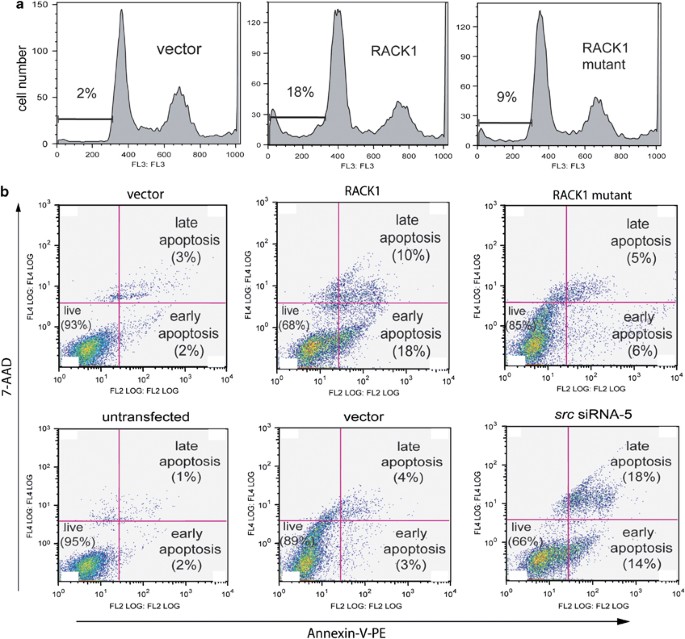
ABSTRACT Earlier we showed that RACK1 regulates growth of human colon cells by suppressing Src activity at G1 and mitotic checkpoints. Here, we show that RACK1 also induces apoptosis of the
cells, partly by inhibiting Src. In the intrinsic pathway, RACK1 inhibits expression of anti-apoptotic Bcl-2 and Bcl-XL, induces expression of pro-apoptotic Bim, targets Bim and Bax to the
mitochondria, induces oligomerization of Bax (which requires Bim and inhibition of Src), depolarizes mitochondria membranes, releases cytochrome _c_, and activates caspases-9 and -3 and
death substrates. Bax and Bim are required for RACK1-mediated mitochondrial cell death. RACK1-induced oligomerization of Bax is required for staurosporine-mediated cell death. RACK1 also
induces apoptosis by blocking Src activation of the Akt cell survival pathway. This leads to activation of the transcription factor FOXO3, a potent inducer of apoptosis and G1 arrest.
Collectively, our results show that RACK1, partly by inhibiting Src, promotes mitochondrial cell death and blocks Akt-mediated cell survival. Thus, RACK1 inhibits growth and induces death of
colon cells. Exploitation of these dual functions could lead to novel colon cancer therapies that mimic RACK1 function. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 50 print issues and online access $259.00 per year only
$5.18 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS CASPASE-8 AS A NOVEL MEDIATOR
LINKING SRC KINASE SIGNALING TO ENHANCED GLIOBLASTOMA MALIGNANCY Article 02 December 2022 MITOCHONDRIAL E3 UBIQUITIN LIGASE MARCHF5 CONTROLS BAK APOPTOTIC ACTIVITY INDEPENDENTLY OF BH3-ONLY
PROTEINS Article 28 September 2022 THE MULTIPLE MECHANISMS OF MCL1 IN THE REGULATION OF CELL FATE Article Open access 02 September 2021 REFERENCES * Antonsson B, Conti F, Ciavatta FA,
Montessuit S, Lewis S, Martinou I _et al_. (1997). Inhibition of Bax channel-forming activity by Bcl-2. _Science_ 277: 370–372. Article CAS Google Scholar * Arimoto K, Fukuda H,
Imajoh-Ohmi S, Saito H, Takekawa M . (2008). Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. _Nature Cell Biol_ 10: 1324–1332. Article CAS
Google Scholar * Buensuceso CS, Woodside D, Huff JL, Plopper GE, O’Toole TE . (2001). The WD Rack1 protein mediates protein kinase C and integrin-dependent cell migration. _J Cell Sci_ 114:
1691–1698. CAS PubMed Google Scholar * Chandra D, Choy G, Deng X, Bhatia B, Daniel P, Tang DG . (2004). Association of active caspase 8 with the mitochondrial membrane during apoptosis:
potential roles in cleaving BAP31 and caspase 3 and mediating mitochondrion-endoplasmic reticulum cross talk in etoposide-induced cell death. _Mol Cell Biol_ 24: 6592–6607. Article CAS
Google Scholar * Chang BY, Chiang M, Cartwright CA . (2001). The interaction of Src and RACK1 is enhanced by activation of protein kinase C and tyrosine phosphorylation of RACK1. _J Biol
Chem_ 276: 20346–20356. Article CAS Google Scholar * Chang BY, Conroy KB, Machleder EM, Cartwright CA . (1998). RACK1, a receptor for activated C kinase and a homolog of the beta subunit
of G proteins, inhibits activity of src tyrosine kinases and growth of NIH 3T3 cells. _Mol Cell Biol_ 18: 3245–3256. Article CAS Google Scholar * Chang BY, Harte R, Cartwright CA .
(2002). RACK1: a novel substrate for the Src protein-tyrosine kinase. _Oncogene_ 21: 7619–7629. Article CAS Google Scholar * Chen R, Kim O, Yang J, Sato K, Eisenmann KM, McCarthy J _et
al_. (2001). Regulation of Akt/PKB activation by tyrosine phosphorylation. _J Biol Chem_ 34: 31858–31862. Article Google Scholar * Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV .
(2005). The Akt/PKB pathway: molecular target for cancer drug discovery. _Oncogene_ 24: 7482–7492. Article CAS Google Scholar * Chipuk JE, Green DR . (2008). How do BCL-2 proteins induce
mitochondrial outer membrane permeabilization? _Trends Cell Biol_ 18: 157–164. Article CAS Google Scholar * Cox EA, Bennin D, Doan AT, O’Toole T, Huttenlocher A . (2003). RACK1 regulates
integrin-mediated adhesion, protrusion, and chemotactic cell migration via its Src-binding site. _Mol Biol Cell_ 14: 658–669. Article CAS Google Scholar * Danial NN, Korsmeyer SJ .
(2004). Cell death: critical control points. _Cell_ 116: 205–219. Article CAS Google Scholar * Dorn GW, Mochly-Rosen D . (2002). Intracellular transport mechanisms of signal transducers.
_Annu Rev Physiol_ 64: 407–429. Article CAS Google Scholar * Galluzzi L, Joza N, Tasdemir E, Maiuri MC, Hengartner M, Abrams JM _et al_. (2008). No death without life: vital functions of
apoptotic effectors. _Cell Death Diff_ 15: 1113–1123. Article CAS Google Scholar * Gilley J, Coffer PJ, Ham J . (2003). FOXO transcription factors directly activate bim gene expression
and promote apoptosis in sympathetic neurons. _J Cell Biol_ 162: 613–622. Article CAS Google Scholar * Golubovskaya VM, Gross S, Kaur AS, Wilson RI, Xu L, Yang XH _et al_. (2003).
Simultaneous inhibition of focal adhesion kinase and Src enhance detachment and apoptosis in colon cancer cell lines. _Mol Cancer Res_ 1: 755–764. CAS PubMed Google Scholar * Gottlob K,
Fulco M, Levrero M, Graessmann A . (1998). The hepatitis B virus HBx protein inhibits caspase 3 activity. _J Biol Chem_ 273: 33347–33353. Article CAS Google Scholar * Green DR . (2005).
Apoptotic pathways: ten minutes to dead. _Cell_ 121: 671–674. Article CAS Google Scholar * Griffiths GJ, Koh MY, Brunton VG, Cawthorne C, Reeves NA, Greaves M _et al_. (2004). Expression
of kinase defective mutants of c-Src in human metastatic colon cancer cells decreases Bcl-XL and increases Oxaliplatin and Fas-induced apoptosis. _J Biol Chem_ 279: 46113–46121. Article CAS
Google Scholar * Hermanto U, Zong CS, Li W, Wang L-H . (2002). RACK1, an insulin-like growth factor I (IGF-I) receptor-interacting protein, modulates IGF-I-dependent integrin signaling
and promotes cell spreading and contact with extracellular matrix. _Mol Cell Biol_ 22: 2345–2365. Article CAS Google Scholar * Inagaki K, Churchill E, Mochly-Rosen D . (2006). Epsilon
protein kinase C as a potential therapeutic target for the ischemic heart. _Cardiovasc Res_ 70: 222–230. Article CAS Google Scholar * Jiang T, Qiu Y . (2003). Interaction between Src and
a C-terminal proline-rich motif of Akt is required for Akt activation. _J Biol Chem_ 278: 15789–15793. Article CAS Google Scholar * Kang BP, Urbonas A, Baddoo A, Baskin S, Malhotra A,
Meggs LG . (2003). IGF-1 inhibits the mitochondrial apoptosis program in mesangial cells exposed to high glucose. _Am J Physiol Renal Physiol_ 285: F1013–F1024. Article CAS Google Scholar
* Kiely PA, Leahy M, O’Gorman D, O’Connor R . (2005). RACK1-mediated integration of adhesion and insulin-like growth factor I (IGF-I) signaling and cell migration are defective in cells
expressing an IGF-I receptor mutated at tyrosines 1250 and 1251. _J Biol Chem_ 280: 7624–7633. Article CAS Google Scholar * Kiely PA, Sant A, O’Connor R . (2002). RACK1 is an insulin-like
growth factor 1 (IGF-1) receptor-interacting protein that can regulate IGF-1-mediated Akt activation and protection from cell death. _J Biol Chem_ 277: 22581–22589. Article CAS Google
Scholar * Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S _et al_. (2006). Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid
leukemia. _Cancer Cell_ 5: 375–388. Article Google Scholar * Liou J-Y, Aleksic N, Chen S-F, Han T-J, Shyue S-K, Wu KK . (2005). Mitochondrial localization of cyclooxygenase-2 and
calcium-independent phospholipase A2 in human cancer cells: implication in apoptosis resistance. _Exp Cell Res_ 306: 75–84. Article CAS Google Scholar * Lipsich LA, Lewis AJ, Brugge JS .
(1983). Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. _J Virol_ 48: 352–360. CAS PubMed PubMed Central Google Scholar * Makin G,
Dive C . (2003). Recent advances in understanding apoptosis: new therapeutic opportunities in cancer chemotherapy. _Trends Mol Med_ 9: 251–255. Article CAS Google Scholar * Mamidipudi V,
Chang BY, Harte RA, Lee KC, Cartwright CA . (2004b). RACK1 inhibits the serum- and anchorage-independent growth of v-Src transformed cells. _FEBS Lett_ 567: 321–326. Article CAS Google
Scholar * Mamidipudi V, Dhillon NK, Parman T, Miller LD, Lee KC, Cartwright CA . (2007). RACK1 inhibits colonic cell growth by regulating Src activity at cell cycle checkpoints. _Oncogene_
26: 2914–2924. Article CAS Google Scholar * Mamidipudi V, Zhang J, Lee KC, Cartwright CA . (2004a). RACK1 regulates G1/S progression by suppressing Src kinase activity. _Mol Cell Biol_
24: 6788–6798. Article CAS Google Scholar * Meggio F, Donella-Deana A, Ruzzene M, Brunati AM, Cesaro L, Guerra B _et al_. (1995). Different susceptibility of protein kinases to
staurosporine inhibition - kinetic studies and molecular bases for the resistance of protein kinase CK2. _Eur J Biochem_ 234: 317–322. Article CAS Google Scholar * Miyazaki T, Neff L,
Tanaka S, Horne WC, Baron R . (2003). Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. _J Cell Biol_ 160: 709–718. Article CAS Google Scholar * Muslin AJ, Xing H .
(2000). 14-3-3 proteins: regulation of subcellular localization by molecular interference. _Cell Signal_ 12: 703–709. Article CAS Google Scholar * Nakamura N, Ramaswamy S, Vazquez F,
Signoretti S, Loda M, Sellers WR . (2000). Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. _Mol Cell Biol_ 20: 8969–8982.
Article CAS Google Scholar * Oberst A, Bender C, Green DR . (2008). Living with death: the evolution of the mitochondrial pathway of apoptosis in animals. _Cell Death Differ_ 15:
1139–1146. Article CAS Google Scholar * Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR . (2002). A novel mechanism of gene regulation and tumor suppression by the transcription
factor FKHR. _Cancer Cell_ 2: 81–91. Article CAS Google Scholar * Reginato MJ, Mills KR, Becker EB, Lynch DK, Bonni A, Muthuswamy SK _et al_. (2005). Bim regulation of lumen formation in
cultured mammary epithelial acini is targeted by oncogenes. _Mol Cell Biol_ 25: 4591–4601. Article CAS Google Scholar * Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J
_et al_. (2003). Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. _Nat Cell Biol_ 5: 733–740. Article CAS Google Scholar * Saito M, Korsmeyer SJ,
Schlesinger PH . (2000). BAX-dependent transport of cytochrome c reconstituted in pure liposomes. _Nat Cell Biol_ 2: 553–555. Article CAS Google Scholar * Sharma SV, Gajowniczek P, Way
IP, Lee DY, Jiang J, Yuza Y _et al_. (2006). A common signaling cascade may underlie ‘addiction’ to the Src, BCR-ABL, and EGF receptor oncogenes. _Cancer Cell_ 5: 425–435. Article Google
Scholar * Shi Y . (2004). Caspase activation: revisiting the induced proximity model. _Cell_ 117: 855–858. Article CAS Google Scholar * Souroujon MC, Mochly-Rosen D . (1998). Peptide
modulators of protein-protein interactions in intracellular signaling. _Nat Biotechnol_ 16: 919–924. Article CAS Google Scholar * Sun Y, Tang XM, Half E, Kuo T, Sinicrope FA . (2002).
Cyclooxygenase-2 overexpression reduces apoptotic susceptibility by inhibiting the cytochrome c-dependent apoptotic pathway in human colon cancer cells. _Cancer Res_ 21: 6323–6328. Google
Scholar * Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP . (2006). Identification of a tumour suppressor network opposing nuclear Akt function. _Nature_ 441:
523–527. Article CAS Google Scholar * van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE _et al_. 2006. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and
efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. _Cancer Cell_ 10: 389–399. Article CAS Google Scholar * Vomastek T, Iwanicki MP, Schaeffer H-J, Tarcsafalvi A, Parsons
JT, Weber MJ . (2007). RACK1 targets the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway to link integrin engagement with focal adhesion disassembly and cell
motility. _Mol Cell Biol_ 27: 8296–8305. Article CAS Google Scholar * Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE _et al_. (2007). Apoptosis initiated when BH3
ligands engage multiple Bcl-2 homologs, not Bax or Bak. _Science_ 315: 856–859. Article CAS Google Scholar * Windham TC, Parikh NU, Siwak DR, Summy JM, McConkey DJ, Kraker AJ _et al_.
(2002). Src activation regulates anoikis in human colon tumor cell lines. _Oncogene_ 21: 7797–7807. Article CAS Google Scholar * Yethon JA, Epand RF, Leber B, Epand RM . (2003).
Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. _J Biol Chem_ 278: 48935–48941. Article CAS Google
Scholar * Yip KW, Reed JC . (2008). Bcl-2 family proteins and cancer. _Oncogene_ 27: 6398–6406. Article CAS Google Scholar * Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, Wang CY .
(2005). Clusterin inhibits apoptosis by interacting with activated Bax. _Nat Cell Biol_ 7: 909–915. Article CAS Google Scholar * Zhou H, Li XM, Meinkoth J, Pittman RN . (2000). Akt
regulates cell survival and apoptosis at a postmitochondrial level. _J Cell Biol_ 151: 483–494. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Jenny Cheng and
Gayathri Swaminathan for many helpful discussions and critical review of the data and manuscript. This work was supported by a grant from the National Institutes of Health to CAC (DK43743).
Preliminary studies were supported by a pilot grant from the Broad Medical Research Program (IBD-0068). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Medicine, Stanford
University, Stanford, CA, USA V Mamidipudi & C A Cartwright Authors * V Mamidipudi View author publications You can also search for this author inPubMed Google Scholar * C A Cartwright
View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to C A Cartwright. ADDITIONAL INFORMATION Supplementary Information
accompanies the paper on the Oncogene website (http://www.nature.com/onc) SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 (PDF 160 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Mamidipudi, V., Cartwright, C. A novel pro-apoptotic function of RACK1: suppression of Src activity in the intrinsic and Akt pathways. _Oncogene_ 28, 4421–4433
(2009). https://doi.org/10.1038/onc.2009.293 Download citation * Received: 28 May 2009 * Revised: 12 August 2009 * Accepted: 23 August 2009 * Published: 21 September 2009 * Issue Date: 17
December 2009 * DOI: https://doi.org/10.1038/onc.2009.293 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * RACK1 * Src * apoptosis * mitochondrial
cell death * intrinsic pathway * Akt cell survival pathway