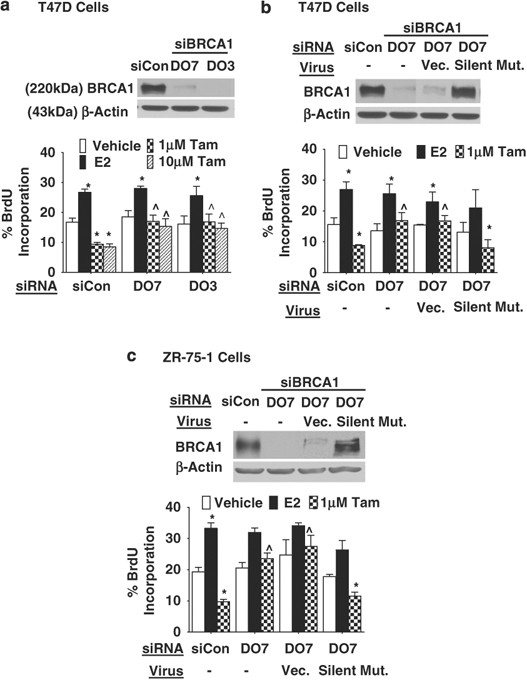
Decreased brca1 confers tamoxifen resistance in breast cancer cells by altering estrogen receptor–coregulator interactions
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The breast cancer susceptibility gene 1 (_BRCA1_) is mutated in approximately 50% of hereditary breast cancers, and its expression is decreased in 30–40% of sporadic breast cancers,
suggesting a general role in breast cancer development. BRCA1 physically and functionally interacts with estrogen receptor-α (ERα) and several transcriptional regulators. We investigated
the relationship between cellular BRCA1 levels and tamoxifen sensitivity. Decreasing BRCA1 expression in breast cancer cells by small interfering RNA alleviated tamoxifen-mediated growth
inhibition and abolished tamoxifen suppression of several endogenous ER-targeted genes. ER-stimulated transcription and cytoplasmic signaling was increased without detectable changes in ER
or ER coregulator expression. Co-immunoprecipitation studies showed that with BRCA1 knockdown, tamoxifen-bound ERα was inappropriately associated with coactivators, and not effectively with
corepressors. Chromatin immunoprecipitation studies demonstrated that with tamoxifen, BRCA1 knockdown did not change ERα promoter occupancy, but resulted in increased coactivator and
decreased corepressor recruitment onto the endogenous cyclin D1 promoter. Our results suggest that decreased BRCA1 levels modify ERα-mediated transcription and regulation of cell
proliferation in part by altering ERα–coregulator association. In the presence of tamoxifen, decreased BRCA1 expression results in increased coactivator and decreased corepressor recruitment
on ER-regulated gene promoters. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Subscribe to this journal Receive 50 print issues and online access $259.00 per year only $5.18 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access
to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our
FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS XAF1 DESTABILIZES ESTROGEN RECEPTOR Α THROUGH THE ASSEMBLY OF A BRCA1-MEDIATED DESTRUCTION COMPLEX AND PROMOTES
ESTROGEN-INDUCED APOPTOSIS Article 16 April 2022 CIRCRNA-SFMBT2 ORCHESTRATES ERΑ ACTIVATION TO DRIVE TAMOXIFEN RESISTANCE IN BREAST CANCER CELLS Article Open access 31 July 2023 ERΑ/PR
CROSSTALK IS ALTERED IN THE CONTEXT OF THE ERΑ Y537S MUTATION AND CONTRIBUTES TO ENDOCRINE THERAPY-RESISTANT TUMOR PROLIFERATION Article Open access 30 November 2023 REFERENCES * Basu A,
Rowan BG . (2005). Genes related to estrogen action in reproduction and breast cancer. _Front Biosci_ 10: 2346–2372. Article CAS Google Scholar * Birgisdottir V, Stefansson OA,
Bodvarsdottir SK, Hilmarsdottir H, Jonasson JG, Eyfjord JE . (2006). Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. _Breast Cancer Res_ 8: R38. Article
PubMed Central Google Scholar * Bissonauth V, Shatenstein B, Ghadirian P . (2008). Nutrition and breast cancer among sporadic cases and gene mutation carriers: an overview. _Cancer
Detection and Prevention_ 32: 52–64. Article Google Scholar * Chen YM, Farmer AA, Chen CF, Jones DC, Chen PL, Lee WH . (1996). BRCA1 is a 220-kDa nuclear phosphoprotein that is expressed
and phosphorylated in a cell cycle-dependent manner. _Cancer Res_ 56: 3168–3172. CAS PubMed Google Scholar * Clarke R, Liu MC, Bouker KB, Gu ZP, Lee RY, Zhu YL _et al_. (2003).
Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. _Oncogene_ 22: 7316–7339. Article CAS PubMed Central Google Scholar * Clarke R, Skaar TC, Bouker KB,
Davis N, Lee YR, Welch JN _et al_. (2001). Molecular and pharmacological aspects of antiestrogen resistance. _J Steroid Biochem Mol Biol_ 76: 71–84. Article CAS Google Scholar * Curtin
D, Ferris HA, Hakli M, Gibson M, Janne OA, Palvimo JJ _et al_. (2004). Small nuclear RING finger protein stimulates the rat luteinizing hormone-beta promoter by interacting with Sp1 and
steroidogenic factor-1 and protects from androgen suppression. _Mol Endocrinol_ 18: 1263–1276. Article CAS Google Scholar * de Mora JF, Brown M . (2000). AIB1 is a conduit for
kinase-mediated growth factor signaling to the estrogen receptor. _Mol Cell Biol_ 20: 5041–5047. Article CAS Google Scholar * Doisneau-Sixou SF, Sergio CM, Carroll JS, Hui R, Musgrove EA,
Sutherland RL . (2003). Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. _Endocr Relat Cancer_ 10: 179–186. Article CAS Google Scholar * Dowsett M,
Houghton J, Iden C, Salter J, Farndon J, A'Hern R _et al_. (2006). Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone
receptor, EGF receptor and HER2 status. _Annu Oncol_ 17: 818–826. Article CAS Google Scholar * Dubik D, Shiu RPC . (1992). Mechanism of estrogen activation of C-Myc oncogene expression.
_Oncogene_ 7: 1587–1594. CAS PubMed Google Scholar * Eakin CM, MacCoss MJ, Finney GL, Klevit RE . (2007). Estrogen receptor alpha is a putative substrate for the BRCA1 ubiquitin ligase.
_Proc Natl Acad Sci USA_ 104: 5794–5799. Article CAS Google Scholar * Fan S, Wang JA, Yuan R, Ma Y, Meng Q, Erdos MR _et al_. (1999). BRCA1 inhibition of estrogen receptor signaling in
transfected cells. _Science_ 284: 1354–1356. Article CAS Google Scholar * Fan SJ, Ma YX, Wang CG, Yuan RQ, Meng QH, Wang JA _et al_. (2001). Role of direct interaction in BRCA1 inhibition
of estrogen receptor activity. _Oncogene_ 20: 77–87. Article CAS Google Scholar * Fan SJ, Ma YX, Wang CG, Yuan RQ, Meng QH, Wang JA _et al_. (2002). p300 modulates the BRCA1 inhibition
of estrogen receptor activity. _Cancer Res_ 62: 141–151. CAS PubMed Google Scholar * Ferris HA, Walsh HE, Stevens J, Fallest PC, Shupnik MA . (2007). Luteinizing hormone beta promoter
stimulation by adenylyl cyclase and cooperation with gonadotropin-releasing hormone 1 in transgenic mice and LBetaT2 cells. _Biol Reprod_ 77: 1073–1080. Article CAS Google Scholar * Fink
M . (2006). Effects of chemotherapy and hormonal therapy for early breast cancer recurrence and 15-year survival. _Strahlenther Onkol_ 182: 53–55. Google Scholar * Fleming FJ, Hill ADK,
McDermott EW, O'Higgins NJ, Young LS . (2004). Differential recruitment of coregulator proteins steroid receptor coactivator-1 and silencing mediator for retinoid and thyroid receptors
to the estrogen receptor-estrogen response element by beta-estradiol and 4-hydroxytamoxifen in human breast cancer. _J Clin Endocrinol Metab_ 89: 375–383. Article CAS Google Scholar * Fox
EM, Bernaciak TM, Wen J, Weaver AM, Shupnik MA, Silva CM . (2008). Signal transducer and activator of transcription 5b, c-Src, and epidermal growth factor receptor signaling play integral
roles in estrogen-stimulated proliferation of estrogen receptor-positive breast cancer cells. _Mol Endocrinol_ 22: 1781–1796. Article CAS PubMed Central Google Scholar * Girault I,
Lerebours F, Amarir S, Tozlu S, Tubiana-Hulin M, Lidereau R _et al_. (2003). Expression analysis of estrogen receptor alpha coregulators in breast carcinoma: evidence that NCOR1 expression
is predictive of the response to tamoxifen. _Clin Cancer Res_ 9: 1259–1266. CAS PubMed Google Scholar * Glover JNM, Williams RS, Lee MS . (2004). Interactions between BRCT repeats and
phosphoproteins: tangled up in two. _Trends in Biochemical Sciences_ 29: 579–585. Article CAS Google Scholar * Graham JD, Roman SD, McGowan E, Sutherland RL, Clarke CL . (1995).
Preferential stimulation of human progesterone receptor B expression by estrogen in T-47D human breast cancer cells. _J Biol Chem_ 270: 30693–30700. Article CAS Google Scholar * Hall JM,
Couse JF, Korach KS . (2001). The multifaceted mechanisms of estradiol and estrogen receptor signaling. _J Biol Chem_ 276: 36869–36872. Article CAS Google Scholar * Hashizume R, Fukuda M,
Maeda I, Nishikawa H, Oyake D, Yabuki Y _et al_. (2001). The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. _J Biol Chem_ 276:
14537–14540. Article CAS Google Scholar * Horner-Glister E, Maleki-Dizaji M, Guerin CJ, Johnson SM, Styles J, White INH . (2005). Influence of oestradiol and tamoxifen on oestrogen
receptors-alpha and -beta protein degradation and non-genomic signalling pathways in uterine and breast carcinoma cells. _J Mol Endocrinol_ 35: 421–432. Article CAS Google Scholar * Hu
YF, Ghosh S, Amleh A, Yue W, Lu YZ, Katz A _et al_. (2005). Modulation of aromatase expression by BRCA1: a possible link to tissue-specific tumor suppression. _Oncogene_ 24: 8343–8348.
Article CAS Google Scholar * Jones LP, Li ML, Halama ED, Ma YX, Lubet R, Grubbs CJ _et al_. (2005). Promotion of mammary cancer development by tamoxifen in a mouse model of
Brca1-mutation-related breast cancer. _Oncogene_ 24: 3554–3562. Article CAS Google Scholar * Kenny FS, Hui R, Musgrove EA, Gee JMW, Blamey RW, Nicholson RI _et al_. (1999). Overexpression
of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. _Clin Cancer Res_ 5: 2069–2076. CAS PubMed Google Scholar * Kilker RL, Planas-Silva MD
. (2006). Cyclin D1 is necessary for tamoxifen-induced cell cycle progression in human breast cancer cells. _Cancer Res_ 66: 11478–11484. Article CAS Google Scholar * Krum SA, Miranda
GA, Lin CW, Lane TF . (2003). BRCA1 associates with processive RNA polymerase II. _J Biol Chem_ 278: 52012–52020. Article CAS Google Scholar * Kurtev V, Margueron R, Kroboth K, Ogris E,
Cavailles V, Seiser C . (2004). Transcriptional regulation by the repressor of estrogen receptor activity via recruitment of histone deacetylases. _J Biol Chem_ 279: 24834–24843. Article
CAS Google Scholar * Lin VY, Resnick EM, Shupnik MA . (2003). Truncated estrogen receptor product-1 stimulates estrogen receptor alpha transcriptional activity by titration of repressor
proteins. _J Biol Chem_ 278: 38125–38131. Article CAS Google Scholar * Liu XF, Bagchi MK . (2004). Recruitment of distinct chromatin-modifying complexes by tamoxifen-complexed estrogen
receptor at natural target gene promoters _in vivo_. _J Biol Chem_ 279: 15050–15058. Article CAS Google Scholar * Ma YX, Hu CY, Riegel AT, Fan SJ, Rosen EM . (2007). Growth factor
signaling pathways modulate BRCA1 repression of estrogen receptor-alpha activity. _Mol Endocrinol_ 21: 1905–1923. Article CAS Google Scholar * Ma YX, Tomita Y, Fan SJ, Wu KM, Tong YZ,
Zhao ZG _et al_. (2005). Structural determinants of the BRCA1: estrogen receptor interaction. _Oncogene_ 24: 1831–1846. Article CAS Google Scholar * Magdinier F, Ribieras S, Lenoir GM,
Frappart L, Dante R . (1998). Down-regulation of BRCA1 in human sporadic breast cancer; analysis of DNA methylation patterns of the putative promoter region. _Oncogene_ 17: 3169–3176.
Article CAS Google Scholar * Murphy L, Cherlet T, Adeyinka A, Niu YL, Snell L, Watson P . (2004). Phospho-serine-118 estrogen receptor-alpha detection in human breast tumors _in vivo_.
_Clin Cancer Res_ 10: 1354–1359. Article CAS PubMed Central Google Scholar * Murphy LC, Simon SLR, Parkes A, Leygue E, Dotzlaw H, Snell L _et al_. (2000). Altered expression of estrogen
receptor coregulators during human breast tumorigenesis. _Cancer Res_ 60: 6266–6271. CAS PubMed Google Scholar * Narod SA, Foulkes WD . (2004). BRCA1 and BRCA2: 1994 and beyond. _Nat Rev
Cancer_ 4: 665–676. Article CAS Google Scholar * Petz LN, Ziegler YS, Schultz JR, Kim H, Kemper JK, Nardulli AM . (2004). Differential regulation of the human progesterone receptor gene
through an estrogen response element half site and Sp1 sites. _J Steroid Biochem Mol Biol_ 88: 113–122. Article CAS PubMed Central Google Scholar * Razandi M, Pedram A, Rosen EM, Levin
ER . (2004). BRCA1 inhibits membrane estrogen and growth factor receptor signaling to cell proliferation in breast cancer. _Mol Cell Biol_ 24: 5900–5913. Article CAS PubMed Central Google
Scholar * Reid G, Hubner MR, Metivier R, Brand H, Denger S, Manu D _et al_. (2003). Cyclic, proteasome-mediated turnover of unliganded and liganded ER alpha on responsive promoters is an
integral feature of estrogen signaling. _Mol Cell_ 11: 695–707. Article CAS Google Scholar * Sabbah M, Courilleau D, Mester J, Redeuilh G . (1999). Estrogen induction of the cyclin D1
promoter: Involvement of a cAMP response-like element. _Proc Natl Acad Sci USA_ 96: 11217–11222. Article CAS Google Scholar * Schreihofer DA, Resnick EM, Lin VY, Shupnik MA . (2001).
Ligand-independent activation of pituitary ER: dependence on PKA-stimulated pathways. _Endocrinology_ 142: 3361–3368. Article CAS Google Scholar * Shang YF, Brown M . (2002). Molecular
determinants for the tissue specificity of SERMs. _Science_ 295: 2465–2468. Article CAS Google Scholar * Shang YF, Hu X, DiRenzo J, Lazar MA, Brown M . (2000). Cofactor dynamics and
sufficiency in estrogen receptor-regulated transcription. _Cell_ 103: 843–852. Article CAS PubMed Central Google Scholar * Shupnik MA . (2004). Crosstalk between steroid receptors and
the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. _Oncogene_ 23: 7979–7989. Article CAS Google Scholar * Smith CL, O'Malley BW . (2004). Coregulator
function: a key to understanding tissue specificity of selective receptor modulators. _Endocr Rev_ 25: 45–71. Article CAS Google Scholar * Srinivas G, Annab LA, Gopinath G, Banerji A,
Srinivas P . (2004). Antisense blocking of BRCA1 enhances sensitivity to plumbagin but not tamoxifen in BG-1 ovarian cancer cells. _Mol Carcinog_ 39: 15–25. Article CAS Google Scholar *
Starita LM, Horwitz AA, Keogh MC, Ishioka C, Parvin JD, Chiba N . (2005). BRCA1/BARD1 ubiquitinate phosphorylated RNA polymerase II. _J Biol Chem_ 280: 24498–24505. Article CAS Google
Scholar * Thasni KA, Rakesh S, Rojini G, Ratheeshkumar T, Srinivas G, Priya S . (2008). Estrogen-dependent cell signaling and apoptosis in BRCA1-blocked BG1 ovarian cancer cells in response
to plumbagin and other chemotherapeutic agents. _Annu Oncol_ 19: 696–705. Article CAS Google Scholar * Tsai HW, Katzenellenbogen JA, Katzenellenbogen BS, Shupnik MA . (2004). Protein
kinase A activation of estrogen receptor a transcription does not require proteasome activity and protects the receptor from ligand-mediated degradation. _Endocrinology_ 145: 2730–2738.
Article CAS Google Scholar * Wu LJC, Wang ZW, Tsan JT, Spillman MA, Phung A, Xu XL _et al_. (1996). Identification of a RING protein that can interact _in vivo_ with the BRCA1 gene
product. _Nat Genet_ 14: 430–440. Article CAS Google Scholar * Xu JW, Fan SJ, Rosen EM . (2005). Regulation of the estrogen-inducible gene expression profile by the breast cancer
susceptibility gene BRCA1. _Endocrinology_ 146: 2031–2047. Article CAS Google Scholar * Yarden RI, Brody LC . (1999). BRCA1 interacts with components of the histone deacetylase complex.
_Proc Natl Acad Sci USA_ 96: 4983–4988. Article CAS Google Scholar * Zheng L, Annab LA, Afshari CA, Lee WH, Boyer TG . (2001). BRCA1 mediates ligand-independent transcriptional repression
of the estrogen receptor. _Proc Natl Acad Sci USA_ 98: 9587–9592. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Dr Sarah E Aiyar for technical advice and Dr
Sarah Parsons for HER2 antibodies. The authors acknowledge support of the National Institutes of Health (DK57082 to MA Shupnik and CA93506 to R Li), the University of Virginia Cancer Center
(P30-CA44579) and Women's Oncology Fund (MA Shupnik). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Molecular Physiology and Biological Physics, University of Virginia,
Charlottesville, VA, USA J Wen & M A Shupnik * Department of Molecular Medicine, Institute of Biotechnology, University of Texas, Health Science Center at San Antonio, San Antonio, TX,
USA R Li & Y Lu * Department of Medicine, School of Medicine, University of Virginia, Charlottesville, VA, USA M A Shupnik Authors * J Wen View author publications You can also search
for this author inPubMed Google Scholar * R Li View author publications You can also search for this author inPubMed Google Scholar * Y Lu View author publications You can also search for
this author inPubMed Google Scholar * M A Shupnik View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to M A Shupnik.
RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wen, J., Li, R., Lu, Y. _et al._ Decreased BRCA1 confers tamoxifen resistance in breast cancer cells by
altering estrogen receptor–coregulator interactions. _Oncogene_ 28, 575–586 (2009). https://doi.org/10.1038/onc.2008.405 Download citation * Received: 02 April 2008 * Revised: 25 September
2008 * Accepted: 01 October 2008 * Published: 10 November 2008 * Issue Date: 29 January 2009 * DOI: https://doi.org/10.1038/onc.2008.405 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative KEYWORDS * tamoxifen resistance * BRCA1, ERα * coactivators * corepressors