Identifying two ancient enzymes in archaea using predicted secondary structure alignment
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT It is now possible to compare life forms at high levels of detail and completeness due to the increasing availability of whole genomes from all three domains. However, exploration
of interesting hypotheses requires the ability to recognize a correspondence between proteins that may since have diverged beyond the threshold of detection by sequence-based methods. Since
protein structure is far better conserved than protein sequence, structural information can enhance detection sensitivity, and this is the basis for the field of structural genomics.
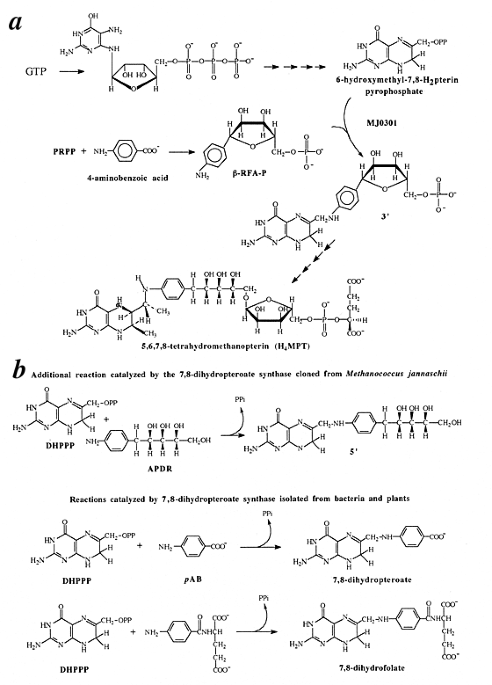
Demonstrating the effectiveness of this approach, we identify two important but previously elusive Archaeal enzymes: a homolog of dihydropteroate synthase and a thymidylate synthase. The
former is especially noteworthy in that no Archaeal homolog of a bacterial folate biosynthetic enzyme has been found to date. Experimental confirmation of the deduced activity of both
enzymes is described. Identification of two different proteins was attempted deliberately to help allay concern that predictive success is merely a lucky accident. Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print
issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS EXPANDED DIVERSITY OF ASGARD ARCHAEA AND THEIR RELATIONSHIPS WITH EUKARYOTES Article 28 April 2021 PROTEOME-WIDE 3D STRUCTURE PREDICTION PROVIDES INSIGHTS INTO THE
ANCESTRAL METABOLISM OF ANCIENT ARCHAEA AND BACTERIA Article Open access 21 December 2022 THE ORIGIN AND RADIATION OF THE PHOSPHOPROTEIN PHOSPHATASE (PPP) ENZYMES OF EUKARYOTES Article Open
access 01 July 2021 REFERENCES * Aurora, R. & Rose, G.D. _Proc. Natl. Acad. Sci. USA_ 95, 2818–2823 (1998). Article CAS Google Scholar * Bult, C.J. et al. _Science_ 273, 1058–1073
(1997). Article Google Scholar * Smith, D.R. et al. _J. Bacteriol._ 179,7135–7155 (1997). Article CAS Google Scholar * Klenk, H.P. et al. _Nature_ 390, 364–370 (1997). Article CAS
Google Scholar * Garnier, J. & Robson, B. In _The Development of the prediction of protein structure_ (ed. Fasman, G.) 417–465 (Plenum, New York; 1989). Google Scholar * Aurora, R.
& Rose, G.D. _Protein Sci._ 7, 21–38 (1998). Article CAS Google Scholar * Needleman, S.B. & Wunsch, C.D. _J. Mol. Biol._ 48, 443–453 (1970). Article CAS Google Scholar * White,
R.H. _Biochemistry_ 32, 745–753 (1993). Article CAS Google Scholar * Keltjens, J.T., Huberts, M.J., Laarhoven, W.H. & Vogels, G.D. _Eur. J. Biochem._ 130, 537–544 (1983). Article
CAS Google Scholar * DiMarco, A.A., Bobik, T.A. & Wolfe, R.S. _Annu. Rev. Biochem._ 59, 355–394 (1990). Article CAS Google Scholar * Keltjens, J.T., Raemakers-Franken, P.C. &
Vogels, G.D. In _Microb. Growth C1 Compd._ [7th Int. Sym.] 135–150 (Intercept, Andover, UK; 1993). Google Scholar * Leigh, J.A. _Appl. Environ. Microbiol._ 45, 800–803 (1983). CAS PubMed
PubMed Central Google Scholar * Green, J.M., Nichols, B.P. & Matthews, R.G. In _Escherichia coli and Salmonella, Cellular and Molecular biology._ (ed. Neidhardt, F.C.) 665–673
(American Society for Microbiology; 1996). Google Scholar * White, R.H. _Biochemistry_ 35, 3447–3456 (1996). Article CAS Google Scholar * White, R.H. _Biochemistry_ 29, 5397–5404 (1990).
Article CAS Google Scholar * Howell, D.M. & White, R.H. _J. Bacteriol._ 179, 5165–5170 (1997). Article CAS Google Scholar * Myers, E.W. & Miller, W. _Bull. Math. Biol._ 51,
5–37 (1989). Article CAS Google Scholar * Achari, A. et al. _Nature Struct. Biol._ 4, 490–497 (1997). Article CAS Google Scholar * Hampele, I.C. et al. _J. Mol. Biol._ 268, 21–30
(1997). Article CAS Google Scholar * Bernstein, F.C. et al. _J. Mol. Biol._ 112, 535–542 (1977). Article CAS Google Scholar * Altschul, S.F. et al. _Nucleic Acids Res._ 25, 3389–3402
(1997). Article CAS Google Scholar * White, R.H. _Methods Enzymol._ 44, 391–401 (1997). Article Google Scholar * Shiota, T. In _Folates and pterins, Vol. 1, chemistry and biochemistry
of folates_ (eds Blakley, R.L. & Benkovic, S.J.) 121 (John Wiley & Sons, New York; 1984). Google Scholar * White, R.H. _J. Bacteriol._ 162, 516–520 (1985). CAS PubMed PubMed
Central Google Scholar * Perry, K.M. et al. _Proteins_, 8, 315–333 (1990). Article CAS Google Scholar * Nyce, G.W. & White, R.H. _J. Bacteriol._ 178, 914–916 (1996). Article CAS
Google Scholar * Krone, U.E., McFarlan, S.C. & Hognekamp, H.P.C. _Eur. J. Biochem._ 220, 789–794 (1994). Article CAS Google Scholar * Vaupel, M., Dietz, H., Linder, D. & Thauer,
R.K. _Eur. J. Biochem._ 236, 294–300 (1996). Article CAS Google Scholar * Priest, D.G. & Doig, M.T. _Methods Enzymol._ 122, 313–319 (1986). Article CAS Google Scholar * Holm, L.
& Sander, C. _Science_ 273, 595–603 (1996). Article CAS Google Scholar * Doolittle, R.F. _Annu. Rev. Biochem._ 64, 287–314 (1995). Article CAS Google Scholar * Gibrat, J., Madej,
T. & Bryant, S.H. _Curr. Opin. Struct. Biol._ 6, 377–385 (1996). Article CAS Google Scholar * Zhou, D. & White, R.H. _J. Bacteriol._ 174, 4576–4582 (1992). Article CAS Google
Scholar * Myers, E and Miller W. _Comput. Appl. Biosci._ 4, 11–17 (1988). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS Supported by a National Science Foundation grant
to R.H.W. and a National Institutes of Health grant to G.D.R. We thank M.E. Rasche for running the experiments to trap the covalent complex between FdUMP and thymidylate synthase, K. Harich
for preforming the GC-MS analyses, and D. Grahame for supplying the [_methyl_-3H]-methyl-tetrahydrosarcinapterin. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biochemistry,
Virginia Polytechnic Institute and State University, Blacksburg, 24061-0308, Virginia, USA Huimin Xu & Robert H. White * Monsanto Company, Mail Zone AA3G, 700 Chesterfield Parkway North,
St. Louis, 63198, Missouri, USA Rajeev Aurora * Department of Biophysics and Biophysical Chemistry, Johns Hopkins University, School of Medicine, 725 N. Wolfe Street, Baltimore, 21205,
Maryland, USA George D. Rose Authors * Huimin Xu View author publications You can also search for this author inPubMed Google Scholar * Rajeev Aurora View author publications You can also
search for this author inPubMed Google Scholar * George D. Rose View author publications You can also search for this author inPubMed Google Scholar * Robert H. White View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Robert H. White. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Xu, H., Aurora, R., Rose, G. _et al._ Identifying two ancient enzymes in Archaea using predicted secondary structure alignment. _Nat Struct Mol Biol_ 6, 750–754
(1999). https://doi.org/10.1038/11525 Download citation * Received: 18 November 1998 * Accepted: 13 May 1999 * Issue Date: August 1999 * DOI: https://doi.org/10.1038/11525 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative