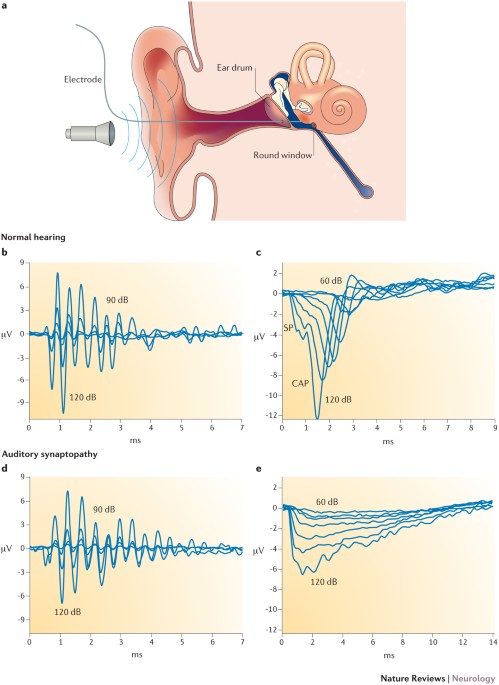
Auditory neuropathy — neural and synaptic mechanisms
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
KEY POINTS * Auditory neuropathy impairs speech comprehension severely, beyond the extent that would be expected on the basis of increased threshold of audibility * Auditory neuropathy
encompasses a range of disease mechanisms that typically disrupt the synaptic encoding and/or neural transmission of auditory information in the cochlea and auditory nerve * Auditory
synaptopathy, impaired sound encoding at the synapses between inner hair cells and spiral ganglion neurons, results from genetic defects or insults such as exposure to loud noise * Advanced
physiological and psychophysical testing combined with molecular genetic analysis facilitate diagnostics of auditory synaptopathy and neuropathy * Although traditional hearing aids often do
not provide substantial benefit for patients with auditory synaptopathy or neuropathy, cochlear implants can provide effective hearing rehabilitation depending on the site(s) of disorder
ABSTRACT Sensorineural hearing impairment is the most common form of hearing loss, and encompasses pathologies of the cochlea and the auditory nerve. Hearing impairment caused by abnormal
neural encoding of sound stimuli despite preservation of sensory transduction and amplification by outer hair cells is known as 'auditory neuropathy'. This term was originally
coined for a specific type of hearing impairment affecting speech comprehension beyond changes in audibility: patients with this condition report that they “can hear but cannot understand”.
This type of hearing impairment can be caused by damage to the sensory inner hair cells (IHCs), IHC ribbon synapses or spiral ganglion neurons. Human genetic and physiological studies, as
well as research on animal models, have recently shown that disrupted IHC ribbon synapse function — resulting from genetic alterations that affect presynaptic glutamate loading of synaptic
vesicles, Ca2+ influx, or synaptic vesicle exocytosis — leads to hearing impairment termed 'auditory synaptopathy'. Moreover, animal studies have demonstrated that sound
overexposure causes excitotoxic loss of IHC ribbon synapses. This mechanism probably contributes to hearing disorders caused by noise exposure or age-related hearing loss. This Review
provides an update on recently elucidated sensory, synaptic and neural mechanisms of hearing impairment, their corresponding clinical findings, and discusses current rehabilitation
strategies as well as future therapies. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through
your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant
access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions *
Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS CONDUCTIVE HEARING LOSS DURING DEVELOPMENT DOES NOT APPRECIABLY ALTER THE SHARPNESS OF COCHLEAR TUNING Article
Open access 17 February 2021 DEAFNESS: FROM GENETIC ARCHITECTURE TO GENE THERAPY Article 12 May 2023 AN INTEGRATIVE APPROACH FOR PEDIATRIC AUDITORY NEUROPATHY SPECTRUM DISORDERS: REVISITING
ETIOLOGIES AND EXPLORING THE PROGNOSTIC UTILITY OF AUDITORY STEADY-STATE RESPONSE Article Open access 17 June 2020 REFERENCES * World Health Organization. Primary ear and hearing care
training resource. Advanced level. [online], (2006). * Starr, A., Picton, T. W., Sininger, Y., Hood, L. J. & Berlin, C. I. Auditory neuropathy. _Brain_ 119, 741–754 (1996). STARR AND
COLLEAGUES FIRST COINED THE TERM 'AUDITORY NEUROPATHY' AND PROVIDED A DETAILED AUDITORY PHENOTYPE FOR HEREDITARY SENSORY AND MOTOR NEUROPATHY. Article PubMed Google Scholar *
Zeng, F.-G., Kong, Y.-Y., Michalewski, H. J. & Starr, A. Perceptual consequences of disrupted auditory nerve activity. _J. Neurophysiol._ 93, 3050–3063 (2005). Article PubMed Google
Scholar * Moser, T. _ et al_. Diagnostik und therapie der auditorischen synaptopathie/neuropathie. _HNO_ 54, 833–841 (in German) (2006). Article CAS PubMed Google Scholar * Sutton, G.
J. _ et al_. Assessment and management of auditory neuropathy/auditory dys-synchrony: a recommended protocol. _Newborn Hearing Screening Programme England)_ [online], (2004). Google Scholar
* Starr, A., Zeng, F. G., Michalewski, H. J. & Moser, T. in _The Senses: A Comprehensive Reference_ Vol 3. Ch. 23 (eds Basbaum, A. I. _ et al_.) 397–412 (Academic Press, 2008). Book
Google Scholar * Penido, R. C. & Isaac, M. L. Prevalence of auditory neuropathy spectrum disorder in an auditory health care service. _Braz. J. Otorhinolaryngol._ 79, 429–433 (2013).
Article PubMed Google Scholar * Rance, G. _ et al_. Clinical findings for a group of infants and young children with auditory neuropathy. _Ear Hear._ 20, 238–252 (1999). Article CAS
PubMed Google Scholar * Foerst, A. _ et al_. Prevalence of auditory neuropathy/synaptopathy in a population of children with profound hearing loss. _Int. J. Pediatr. Otorhinolaryngol._ 70,
1415–1422 (2006). Article PubMed Google Scholar * Rodríguez-Ballesteros, M. _ et al_. A multicenter study on the prevalence and spectrum of mutations in the otoferlin gene (_OTOF_) in
subjects with nonsyndromic hearing impairment and auditory neuropathy. _Hum. Mutat._ 29, 823–831 (2008). Article CAS PubMed Google Scholar * Thirlwall, A. S., Brown, D. J., McMillan, P.
M., Barker, S. E. & Lesperance, M. M. Phenotypic characterization of hereditary hearing impairment linked to DFNA25. _Arch. Otolaryngol. Head Neck Surg._ 129, 830–835 (2003). Article
PubMed Google Scholar * Matthews, G. & Fuchs, P. The diverse roles of ribbon synapses in sensory neurotransmission. _Nat. Rev. Neurosci._ 11, 812–822 (2010). Article CAS PubMed
PubMed Central Google Scholar * Bech-Hansen, N. T. _ et al_. Loss-of-function mutations in a calcium-channel α1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary
night blindness. _Nat. Genet._ 19, 264–267 (1998). Article CAS PubMed Google Scholar * Strom, T. M. _ et al_. An L-type calcium-channel gene mutated in incomplete X-linked congenital
stationary night blindness. _Nat. Genet._ 19, 260–263 (1998). Article CAS PubMed Google Scholar * Zeitz, C. _ et al_. Mutations in _CABP4_, the gene encoding the Ca2+-binding protein 4,
cause autosomal recessive night blindness. _Am. J. Hum. Genet._ 79, 657–667 (2006). Article CAS PubMed PubMed Central Google Scholar * Khimich, D. _ et al_. Hair cell synaptic ribbons
are essential for synchronous auditory signalling. _Nature_ 434, 889–894 (2005). USING MOUSE MUTANTS WITH INNER HAIR CELL SYNAPSES DEFICIENT IN THE SCAFFOLD PROTEIN BASSOON AND THE SYNAPTIC
RIBBON, KHIMICH _ ET AL_ . DEMONSTRATED HOW HIGH RATES OF PRESYNAPTIC VESICLE EXOCYTOSIS ARE REQUIRED FOR SYNCHRONOUS ACTIVATION OF THE SPIRAL GANGLION NEURONS, REFLECTED BY THE COMPOUND
ACTION POTENTIAL. Article CAS PubMed Google Scholar * Buran, B. N. _ et al_. Onset coding is degraded in auditory nerve fibers from mutant mice lacking synaptic ribbons. _J. Neurosci._
30, 7587–7597 (2010). Article CAS PubMed PubMed Central Google Scholar * Jung, S. _ et al_. Disruption of adaptor protein 2 (AP-2) in cochlear hair cells impairs vesicle reloading of
synaptic release sites and hearing. _EMBO J._ 34, 2686–2702 (2015). NEAR-COMPLETE RESTORATION OF HEARING IN A KNOCKOUT MOUSE MODEL OF AUDITORY SYNAPTOPATHY BY A POSTNATAL GENE TRANSFER VIA
INJECTION OF A VIRAL VECTOR INTO THE COCHLEA. Article CAS PubMed PubMed Central Google Scholar * Pangrsic, T. _ et al_. Hearing requires otoferlin-dependent efficient replenishment of
synaptic vesicles in hair cells. _Nat. Neurosci._ 13, 869–876 (2010). THIS STUDY DEMONSTRATED THAT REDUCED OTOFERLIN LEVELS DISRUPT VESICLE REPLENISHMENT AND, THEREFORE, IMPAIR INDEFATIGABLE
TRANSMITTER RELEASE FROM INNER HAIR CELLS. Article CAS PubMed Google Scholar * Parkinson, N. J. _ et al_. Mutant β-spectrin 4 causes auditory and motor neuropathies in quivering mice.
_Nat. Genet._ 29, 61–65 (2001). Article CAS PubMed Google Scholar * Lacas-Gervais, S. _ et al_. βIVΣ1 spectrin stabilizes the nodes of Ranvier and axon initial segments. _J. Cell Biol._
166, 983–990 (2004). Article CAS PubMed PubMed Central Google Scholar * Wichmann, C. & Moser, T. Relating structure and function of inner hair cell ribbon synapses. _Cell Tissue
Res._ 361, 95–114 (2015). Article CAS PubMed PubMed Central Google Scholar * Fuchs, P. A. Time and intensity coding at the hair cell's ribbon synapse. _J. Physiol._ 566, 7–12
(2005). Article CAS PubMed PubMed Central Google Scholar * Moser, T., Neef, A. & Khimich, D. Mechanisms underlying the temporal precision of sound coding at the inner hair cell
ribbon synapse. _J. Physiol._ 576, 55–62 (2006). Article CAS PubMed PubMed Central Google Scholar * Pangrs˘ic˘, T., Reisinger, E. & Moser, T. Otoferlin: a multi-C2 domain protein
essential for hearing. _Trends Neurosci._ 35, 671–680 (2012). A REVIEW OF THE MOLECULAR PHYSIOLOGY OF INNER HAIR CELL RIBBON SYNAPSES, WITH A FOCUS ON OTOFERLIN. Article CAS Google Scholar
* Nouvian, R. _ et al_. Exocytosis at the hair cell ribbon synapse apparently operates without neuronal SNARE proteins. _Nat. Neurosci._ 14, 411–413 (2011). Article CAS PubMed Google
Scholar * Strenzke, N. _ et al_. Complexin-I is required for high-fidelity transmission at the endbulb of held auditory synapse. _J. Neurosci._ 29, 7991–8004 (2009). Article CAS PubMed
PubMed Central Google Scholar * Uthaiah, R. C. & Hudspeth, A. J. Molecular anatomy of the hair cell's ribbon synapse. _J. Neurosci._ 30, 12387–12399 (2010). Article CAS PubMed
PubMed Central Google Scholar * Vogl, C. _ et al_. Unconventional molecular regulation of synaptic vesicle replenishment in cochlear inner hair cells. _J. Cell. Sci._ 128, 638–644 (2015).
Article CAS PubMed Google Scholar * Roux, I. _ et al_. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. _Cell_ 127, 277–289
(2006). THIS STUDY DESCRIBES THE AUDITORY PHENOTYPE OF OTOFERLIN KNOCKOUT MICE AND DEMONSTRATES THAT OTOFERLIN HAS AN ESSENTIAL ROLE IN INNER HAIR CELL EXOCYTOSIS. Article CAS PubMed
Google Scholar * Seal, R. P. _ et al_. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. _Neuron_ 57, 263–275 (2008). THIS _SLC17A8_ -KNOCKOUT MOUSE
STUDY DEMONSTRATED THAT VGLUT3 HAS AN ESSENTIAL ROLE IN SOUND ENCODING AT THE INNER HAIR CELL RIBBON SYNAPSE. Article CAS PubMed PubMed Central Google Scholar * Ruel, J. _ et al_.
Impairment of _SLC17A8_ encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. _Am. J. Hum. Genet._ 83,
278–292 (2008). THIS STUDY REVEALED THAT A MUTATION IN _VGLUT3_ UNDERLIES AUTOSOMAL DOMINANT DEAFNESS-25 AND SHOWED THE REQUIREMENT OF VGLUT3 IN SOUND ENCODING AT THE INNER HAIR CELL RIBBON
SYNAPSE. Article CAS PubMed PubMed Central Google Scholar * Platzer, J. _ et al_. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels.
_Cell_ 102, 89–97 (2000). Article CAS PubMed Google Scholar * Brandt, A., Striessnig, J. & Moser, T. Cav1. 3 channels are essential for development and presynaptic activity of
cochlear inner hair cells. _J. Neurosci._ 23, 10832–10840 (2003). Article CAS PubMed PubMed Central Google Scholar * Geisler, C. D. _From Sound to Synapse: Physiology of the Mammalian
Ear_ (Oxford Univ. Press, 1998). Google Scholar * Rutherford, M. A. & Moser, T. in _The Primary Auditory Neurons of the Mammalian Cochlea_ (eds Dabdoub, A. _ et al_.) 117–156
(Springer-Verlag, 2016). THIS BOOK CHAPTER IS PART OF A RECENTLY PUBLISHED TEXTBOOK ON SPIRAL GANGLION NEURONS, AND PROVIDES A COMPREHENSIVE OVERVIEW ON THE INNER HAIR CELL–SPIRAL GANGLION
NEURON SYNAPSE. Book Google Scholar * Mo, Z. L., Adamson, C. L. & Davis, R. L. Dendrotoxin-sensitive K+ currents contribute to accommodation in murine spiral ganglion neurons. _J.
Physiol._ 542, 763 (2002). Article CAS PubMed PubMed Central Google Scholar * Rutherford, M. A., Chapochnikov, N. M. & Moser, T. Spike encoding of neurotransmitter release timing by
spiral ganglion neurons of the cochlea. _J. Neurosci._ 32, 4773–4789 (2012). Article CAS PubMed PubMed Central Google Scholar * Glowatzki, E. & Fuchs, P. A. Transmitter release at
the hair cell ribbon synapse. _Nat. Neurosci._ 5, 147–154 (2002). THE FIRST POSTSYNAPTIC PATCH-CLAMP RECORDING FROM THE INNER HAIR CELL RIBBON SYNAPSE OF THE RAT DEMONSTRATES MASSIVE
HETEROGENEITY IN SIZE AND SHAPE OF THE EXCITATORY POSTSYNAPTIC CURRENTS, INTERPRETED TO REFLECT SYNCHRONOUS RELEASE OF MULTIPLE VESICLES DESPITE THE ABSENCE OF A PRESYNAPTIC ACTION
POTENTIAL. Article CAS PubMed Google Scholar * Chapochnikov, N. M. _ et al_. Uniquantal release through a dynamic fusion pore is a candidate mechanism of hair cell exocytosis. _Neuron_
17, 1389–1403 (2014). Article CAS Google Scholar * Hossain, W. A., Antic, S. D., Yang, Y., Rasband, M. N. & Morest, D. K. Where is the spike generator of the cochlear nerve?
Voltage-gated sodium channels in the mouse cochlea. _J. Neurosci._ 25, 6857–6868 (2005). Article CAS PubMed PubMed Central Google Scholar * Yasunaga, S. _ et al_. A mutation in _OTOF_,
encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. _Nat. Genet._ 21, 363–369 (1999). Article CAS PubMed Google Scholar * Varga, R. _ et al_. OTOF
mutations revealed by genetic analysis of hearing loss families including a potential temperature sensitive auditory neuropathy allele. _J. Med. Genet._ 43, 576–581 (2006). Article CAS
PubMed Google Scholar * Marlin, S. _ et al_. Temperature-sensitive auditory neuropathy associated with an otoferlin mutation: deafening fever! _Biochem. Biophys. Res. Commun._ 394, 737–742
(2010). Article CAS PubMed Google Scholar * Wang, D.-Y. _ et al_. Screening mutations of _OTOF_ gene in Chinese patients with auditory neuropathy, including a familial case of
temperature-sensitive auditory neuropathy. _BMC Med. Genet._ 11, 79 (2010). Article CAS PubMed PubMed Central Google Scholar * Romanos, J. _ et al_. Novel _OTOF_ mutations in Brazilian
patients with auditory neuropathy. _J. Hum. Genet._ 54, 382–385 (2009). Article CAS PubMed Google Scholar * Matsunaga, T. _ et al_. A prevalent founder mutation and genotype–phenotype
correlations of _OTOF_ in Japanese patients with auditory neuropathy. _Clin. Genet._ 82, 425–432 (2012). Article CAS PubMed Google Scholar * McNeil, P. L. & Kirchhausen, T. An
emergency response team for membrane repair. _Nat. Rev. Mol. Cell. Biol._ 6, 499–505 (2005). Article CAS PubMed Google Scholar * Liu, J. _ et al_. Dysferlin, a novel skeletal muscle
gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. _Nat. Genet._ 20, 31–36 (1998). Article CAS PubMed Google Scholar * Bansal, D. _ et al_. Defective membrane
repair in dysferlin-deficient muscular dystrophy. _Nature_ 423, 168–172 (2003). Article CAS PubMed Google Scholar * Jiménez, J. L. & Bashir, R. _In silico_ functional and structural
characterisation of ferlin proteins by mapping disease-causing mutations and evolutionary information onto three-dimensional models of their C2 domains. _J. Neurol. Sci._ 260, 114–123
(2007). Article CAS PubMed Google Scholar * Johnson, C. P. & Chapman, E. R. Otoferlin is a calcium sensor that directly regulates SNARE-mediated membrane fusion. _J. Cell Biol._ 191,
187–197 (2010). Article CAS PubMed PubMed Central Google Scholar * Padmanarayana, M. _ et al_. Characterization of the lipid binding properties of Otoferlin reveals specific
interactions between PI(4,5)P2 and the C2C and C2F domains. _Biochemistry_ 53, 5023–5033 (2014). Article CAS PubMed Google Scholar * Ramakrishnan, N. A., Drescher, M. J. & Drescher,
D. G. Direct interaction of otoferlin with syntaxin 1A, SNAP-25, and the L-type voltage-gated calcium channel Cav1.3. _J. Biol. Chem._ 284, 1364–1372 (2009). Article CAS PubMed PubMed
Central Google Scholar * Helfmann, S. _ et al_. The crystal structure of the C2A domain of otoferlin reveals an unconventional top loop region. _J. Mol. Biol._ 406, 479–490 (2011). Article
CAS PubMed Google Scholar * Fuson, K. _ et al_. Alternate splicing of dysferlin C2A confers Ca2+-dependent and Ca2+-independent binding for membrane repair. _Structure_ 22, 104–115
(2014). Article CAS PubMed Google Scholar * Santarelli, R., del Castillo, I., Cama, E., Scimemi, P. & Starr, A. Audibility, speech perception and processing of temporal cues in
ribbon synaptic disorders due to _OTOF_ mutations. _Hear. Res._ 330 (Pt B), 200–212 (2015). Article PubMed Google Scholar * Reisinger, E. _ et al_. Probing the functional equivalence of
otoferlin and synaptotagmin 1 in exocytosis. _J. Neurosci._ 31, 4886–4895 (2011). Article CAS PubMed PubMed Central Google Scholar * Dulon, D., Safieddine, S., Jones, S. M. & Petit,
C. Otoferlin is critical for a highly sensitive and linear calcium-dependent exocytosis at vestibular hair cell ribbon synapses. _J. Neurosci._ 29, 10474–10487 (2009). Article CAS PubMed
PubMed Central Google Scholar * Varga, R. _ et al_. Non-syndromic recessive auditory neuropathy is the result of mutations in the otoferlin (_OTOF_) gene. _J. Med. Genet._ 40, 45–50
(2003). Article CAS PubMed PubMed Central Google Scholar * Schwander, M. _ et al_. A forward genetics screen in mice identifies recessive deafness traits and reveals that pejvakin is
essential for outer hair cell function. _J. Neurosci._ 27, 2163–2175 (2007). Article CAS PubMed PubMed Central Google Scholar * Wynne, D. P. _ et al_. Loudness adaptation accompanying
ribbon synapse and auditory nerve disorders. _Brain_ 136, 1626–1638 (2013). Article PubMed PubMed Central Google Scholar * Duncker, S. V. _ et al_. Otoferlin couples to clathrin-mediated
endocytosis in mature cochlear inner hair cells. _J. Neurosci._ 33, 9508–9519 (2013). Article CAS PubMed PubMed Central Google Scholar * Greene, C. C. _ et al_. DFNA25, a novel locus
for dominant nonsyndromic hereditary hearing impairment, maps to 12q21-24. _Am. J. Hum. Genet._ 68, 254–260 (2001). Article CAS PubMed Google Scholar * Obholzer, N. _ et al_. Vesicular
glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. _J. Neurosci._ 28, 2110–2118 (2008). Article CAS PubMed PubMed Central Google Scholar * Petek, E.
_ et al_. Molecular characterization of a 12q22-q24 deletion associated with congenital deafness: confirmation and refinement of the DFNA25 locus. _Am. J. Med. Genet. A_ 117A, 122–126
(2003). Article PubMed Google Scholar * Baig, S. M. _ et al_. Loss of Cav1.3 (_CACNA1D_) function in a human channelopathy with bradycardia and congenital deafness. _Nat. Neurosci._ 14,
77–84 (2011). THE FIRST REPORT OF A HUMAN DEAFNESS SYNDROME ATTRIBUTED TO A LOSS-OF-FUNCTION MUTATION IN _CACNA1D_. Article CAS PubMed Google Scholar * Surmeier, D. J. Calcium, ageing,
and neuronal vulnerability in Parkinson's disease. _Lancet Neurol._ 6, 933–938 (2007). Article CAS PubMed Google Scholar * McKinney, B. C. & Murphy, G. G. The L-Type
voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. _Learn. Mem._ 13, 584–589 (2006). Article CAS PubMed PubMed
Central Google Scholar * Neef, J. _ et al_. The Ca2+ channel subunit β2 regulates Ca2+ channel abundance and function in inner hair cells and is required for hearing. _J. Neurosci._ 29,
10730 (2009). Article CAS PubMed PubMed Central Google Scholar * Wycisk, K. A. _ et al_. Mutation in the auxiliary calcium-channel subunit _CACNA2D4_ causes autosomal recessive cone
dystrophy. _Am. J. Hum. Genet._ 79, 973–977 (2006). Article CAS PubMed PubMed Central Google Scholar * Schrauwen, I. _ et al_. A mutation in _CABP2_, expressed in cochlear hair cells,
causes autosomal-recessive hearing impairment. _Am. J. Hum. Genet._ 91, 636–645 (2012). A REPORT OF A HEARING IMPAIRMENT IN A FAMILY WITH A _CABP2_ MUTATION, SUGGESTING THAT INNER HAIR CELL
SYNAPTIC DYSFUNCTION IS CAUSED BY IMPAIRED PRESYNAPTIC CA2+ INFLUX. Article CAS PubMed PubMed Central Google Scholar * Haeseleer, F. _ et al_. Essential role of Ca2+-binding protein 4,
a Cav1.4 channel regulator, in photoreceptor synaptic function. _Nat. Neurosci._ 7, 1079–1087 (2004). Article CAS PubMed PubMed Central Google Scholar * Santarelli, R. _ et al_.
_OPA1_-related auditory neuropathy: site of lesion and outcome of cochlear implantation. _Brain_ 138, 563–576 (2015). Article PubMed PubMed Central Google Scholar * Yu-Wai-Man, P. _ et
al_. Multi-system neurological disease is common in patients with _OPA1_ mutations. _Brain_ 133, 771–786 (2010). Article CAS PubMed PubMed Central Google Scholar * La Morgia, C.,
Carbonelli, M., Barboni, P., Sadun, A. A. & Carelli, V. Medical management of hereditary optic neuropathies. _Front. Neurol._ 5, 141 (2014). Article PubMed PubMed Central Google
Scholar * Alexander, C. _ et al_. _OPA1_, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. _Nat. Genet._ 26, 211–215 (2000).
Article CAS PubMed Google Scholar * Delettre, C. _ et al_. Nuclear gene _OPA1_, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. _Nat. Genet._ 26,
207–210 (2000). Article CAS PubMed Google Scholar * Kasahara, A. & Scorrano, L. Mitochondria: from cell death executioners to regulators of cell differentiation. _Trends Cell Biol._
24, 761–770 (2014). Article CAS PubMed Google Scholar * Ferré, M. _ et al_. Molecular screening of 980 cases of suspected hereditary optic neuropathy with a report on 77 novel _OPA1_
mutations. _Hum. Mutat._ 30, E692–E705 (2009). Article PubMed Google Scholar * Alavi, M. V. _ et al_. A splice site mutation in the murine _Opa1_ gene features pathology of autosomal
dominant optic atrophy. _Brain_ 130, 1029–1042 (2006). Article Google Scholar * Starr, A., Dong, C. J. & Michalewski, H. J. Brain potentials before and during memory scanning.
_Electroencephalogr. Clin. Neurophysiol._ 99, 28–37 (1996). Article CAS PubMed Google Scholar * Starr, A. Pathology and physiology of auditory neuropathy with a novel mutation in the
_MPZ_ gene (Tyr145→Ser). _Brain_ 126, 1604–1619 (2003). Article PubMed Google Scholar * Kabzin´ska, D. _ et al_. Late-onset Charcot−Marie−Tooth type 2 disease with hearing impairment
associated with a novel Pro105Thr mutation in the _MPZ_ gene. _Am. J. Med. Genet. A_ 143A, 2196–2199 (2007). Article CAS Google Scholar * Verhagen, W. I. M. _ et al_. Sensorineural
hearing impairment in patients with Pmp22 duplication, deletion, and frameshift mutations. _Otol. Neurotol._ 26, 405–414 (2005). Article CAS PubMed Google Scholar * Kovach, M. J. _ et
al_. Anticipation in a unique family with Charcot−Marie−Tooth syndrome and deafness: delineation of the clinical features and review of the literature. _Am. J. Med. Genet._ 108, 295–303
(2002). Article CAS PubMed Google Scholar * Rance, G. & Starr, A. Pathophysiological mechanisms and functional hearing consequences of auditory neuropathy. _Brain_ 138, 3141–3158
(2015). IN THIS REVIEW, RANCE AND STARR PROVIDE A COMPREHENSIVE OVERVIEW OF SPIRAL GANGLION DISORDERS AND THEIR CLINICAL MANIFESTATIONS. Article PubMed Google Scholar * Delmaghani, S. _
et al_. Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. _Nat. Genet._ 38, 770–778 (2006). Article CAS
PubMed Google Scholar * Borck, G. _ et al_. High frequency of autosomal-recessive DFNB59 hearing loss in an isolated Arab population in Israel. _Clin. Genet._ 82, 271–276 (2012). Article
CAS PubMed Google Scholar * Ebermann, I. _ et al_. Truncating mutation of the _DFNB59_ gene causes cochlear hearing impairment and central vestibular dysfunction. _Hum. Mutat._ 28,
571–577 (2007). Article CAS PubMed Google Scholar * Hashemzadeh Chaleshtori, M. _ et al_. Novel mutations in the pejvakin gene are associated with autosomal recessive non-syndromic
hearing loss in Iranian families. _Clin. Genet._ 72, 261–263 (2007). Article CAS PubMed Google Scholar * Collin, R. W. J. _ et al_. Involvement of _DFNB59_ mutations in autosomal
recessive nonsyndromic hearing impairment. _Hum. Mutat._ 28, 718–723 (2007). Article CAS PubMed Google Scholar * Delmaghani, S. _ et al_. Hypervulnerability to sound exposure through
impaired adaptive proliferation of peroxisomes. _Cell_ 163, 894–906 (2015). THIS STUDY DEMONSTRATED THAT GENETIC DISRUPTION OF PEVJAKIN INCREASES VULNERABILITY OF HAIR CELLS AND NEURONS TO
NOISE EXPOSURE, PROVIDING AN INTERESTING EXPERIMENTAL MODEL FOR HEARING IMPAIRMENT AS A MULTIFACTORIAL DISEASE. Article CAS PubMed Google Scholar * Kim, T. B. _ et al_. A gene
responsible for autosomal dominant auditory neuropathy (AUNA1) maps to 13q14–21. _J. Med. Genet._ 41, 872 (2004). Article CAS PubMed PubMed Central Google Scholar * Starr, A. _ et al_.
A dominantly inherited progressive deafness affecting distal auditory nerve and hair cells. _J. Assoc. Res. Otolaryngol._ 5, 411–426 (2004). Article PubMed PubMed Central Google Scholar
* Schoen, C. J. _ et al_. Increased activity of _Diaphanous homolog 3 (DIAPH3)/diaphanous_ causes hearing defects in humans with auditory neuropathy and in _Drosophila_. _Proc. Natl Acad.
Sci. USA_ 107, 13396–13401 (2010). Article CAS PubMed PubMed Central Google Scholar * Schoen, C. J., Burmeister, M. & Lesperance, M. M. _Diaphanous homolog 3 (Diap3)_ overexpression
causes progressive hearing loss and inner hair cell defects in a transgenic mouse model of human deafness. _PLoS ONE_ 8, e56520 (2013). Article CAS PubMed PubMed Central Google Scholar
* Rance, G. _ et al_. Speech perception ability in individuals with Friedreich ataxia. _Brain_ 131, 2002–2012 (2008). Article PubMed Google Scholar * Kirkim, G., Serbetcioglu, B.,
Erdag, T. K. & Ceryan, K. The frequency of auditory neuropathy detected by universal newborn hearing screening program. _Int. J. Pediatr. Otorhinolaryngol._ 72, 1461–1469 (2008). Article
PubMed Google Scholar * Schulman-Galambos, C. & Galambos, R. Brain stem evoked response audiometry in newborn hearing screening. _Arch. Otolaryngol._ 105, 86–90 (1979). Article CAS
PubMed Google Scholar * Oh, W. _ et al_. Association between peak serum bilirubin and neurodevelopmental outcomes in extremely low birth weight infants. _Pediatrics_ 112, 773–779 (2003).
Article PubMed Google Scholar * Olds, C. & Oghalai, J. S. Audiologic impairment associated with bilirubin-induced neurologic damage. _Semin. Fetal Neonatal Med._ 20, 42–46 (2015).
Article PubMed PubMed Central Google Scholar * Smith, C. M., Barnes, G. P., Jacobson, C. A. & Oelberg, D. G. Auditory brainstem response detects early bilirubin neurotoxicity at low
indirect bilirubin values. _J. Perinatol._ 24, 730–732 (2004). Article PubMed Google Scholar * Amatuzzi, M. G. _ et al_. Selective inner hair cell loss in premature infants and cochlea
pathological patterns from neonatal intensive care unit autopsies. _Arch. Otolaryngol. Head Neck Surg._ 127, 629–636 (2001). Article CAS PubMed Google Scholar * Uziel, A., Marot, M.
& Pujol, R. The Gunn rat: an experimental model for central deafness. _Acta Otolaryngol._ 95, 651–656 (1983). Article CAS PubMed Google Scholar * Haustein, M. D. _ et al_. Acute
hyperbilirubinaemia induces presynaptic neurodegeneration at a central glutamatergic synapse. _J. Physiol._ 588, 4683–4693 (2010). Article CAS PubMed PubMed Central Google Scholar *
Spencer, R. F., Shaia, W. T., Gleason, A. T., Sismanis, A. & Shapiro, S. M. Changes in calcium-binding protein expression in the auditory brainstem nuclei of the jaundiced Gunn rat.
_Hear. Res._ 171, 129–141 (2002). Article CAS PubMed Google Scholar * Attias, J., Raveh, E., Aizer-Dannon, A., Bloch-Mimouni, A. & Fattal-Valevski, A. Auditory system dysfunction due
to infantile thiamine deficiency: long-term auditory sequelae. _Audiol. Neurootol._ 17, 309–320 (2012). Article CAS PubMed Google Scholar * Oishi, K. _ et al_. Targeted disruption of
_Slc19a2_, the gene encoding the high-affinity thiamin transporter Thtr-1, causes diabetes mellitus, sensorineural deafness and megaloblastosis in mice. _Hum. Mol. Genet._ 11, 2951–2960
(2002). Article CAS PubMed Google Scholar * Liberman, M. C., Tartaglini, E., Fleming, J. C. & Neufeld, E. J. Deletion of _SLC19A2_, the high affinity thiamine transporter, causes
selective inner hair cell loss and an auditory neuropathy phenotype. _J. Assoc. Res. Otolaryngol._ 7, 211–217 (2006). Article CAS PubMed PubMed Central Google Scholar * Yang, C.-H.,
Schrepfer, T. & Schacht, J. Age-related hearing impairment and the triad of acquired hearing loss. _Front. Cell. Neurosci._ 9, 276 (2015). THIS ARTICLE, PART OF A SPECIAL REVIEW ISSUE OF
_FRONTIERS IN CELLULAR NEUROSCIENCE_ , PROVIDES A DETAILED UPDATE ON SENSORY HAIR CELL DEATH AND STUDIES AIMING AT INDUCING HAIR CELL REGENERATION. PubMed PubMed Central Google Scholar *
Henry, W. R. & Mulroy, M. J. Afferent synaptic changes in auditory hair cells during noise-induced temporary threshold shift. _Hear. Res._ 84, 81–90 (1995). Article CAS PubMed Google
Scholar * Puel, J. L., Pujol, R., Ladrech, S. & Eybalin, M. α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid electrophysiological and neurotoxic effects in the guinea-pig cochlea.
_Neuroscience_ 45, 63–72 (1991). Article CAS PubMed Google Scholar * Puel, J. L., Ruel, J., Gervais d'Aldin, C. & Pujol, R. Excitotoxicity and repair of cochlear synapses after
noise-trauma induced hearing loss. _Neuroreport_ 9, 2109–2114 (1998). Article CAS PubMed Google Scholar * Stamataki, S., Francis, H. W., Lehar, M., May, B. J. & Ryugo, D. K.
Synaptic alterations at inner hair cells precede spiral ganglion cell loss in aging C57BL/6J mice. _Hear. Res._ 221, 104–118 (2006). Article PubMed Google Scholar * Kujawa, S. G. &
Liberman, M. C. Adding insult to injury: cochlear nerve degeneration after 'temporary' noise-induced hearing loss. _J. Neurosci._ 29, 14077 (2009). THIS STUDY SHOWS THAT IN MICE,
SOUND OVEREXPOSURE THAT ONLY TEMPORARILY REDUCES AUDIBILITY CAN CAUSE A PERMANENT LOSS OF INNER HAIR CELL RIBBON SYNAPSES AND SUBSEQUENT LOSS OF SPIRAL GANGLION NEURONS. Article CAS PubMed
PubMed Central Google Scholar * Sergeyenko, Y., Lall, K., Liberman, M. C. & Kujawa, S. G. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional
decline. _J. Neurosci._ 33, 13686–13694 (2013). Article CAS PubMed PubMed Central Google Scholar * Kujawa, S. G. & Liberman, M. C. Synaptopathy in the noise-exposed and aging
cochlea: primary neural degeneration in acquired sensorineural hearing loss. _Hear. Res._ 330(Pt B), 191–199 (2015). THIS REVIEW IS A PART OF A REVIEW SERIES ON AUDITORY SYNAPSES AND
SYNAPTOPATHIES AND SUMMARIZES A LARGE BODY OF ANIMAL WORK ON SYNAPTIC ALTERATIONS IN NOISE-INDUCED AND AGE-RELATED HEARING LOSS. Article PubMed PubMed Central Google Scholar * Hakuba,
N., Koga, K., Gyo, K., Usami, S. I. & Tanaka, K. Exacerbation of noise-induced hearing loss in mice lacking the glutamate transporter GLAST. _J. Neurosci._ 20, 8750–8753 (2000). Article
CAS PubMed PubMed Central Google Scholar * Meyer, A. C. _ et al_. Tuning of synapse number, structure and function in the cochlea. _Nat. Neurosci._ 12, 444–453 (2009). Article CAS
PubMed Google Scholar * Furman, A. C., Kujawa, S. G. & Liberman, M. C. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. _J. Neurophysiol._ 110,
577–586 (2013). Article PubMed PubMed Central Google Scholar * Bourien, J. _ et al_. Contribution of auditory nerve fibers to compound action potential of the auditory nerve. _J.
Neurophysiol._ 112, 1025–1039 (2014). Article CAS PubMed Google Scholar * Wan, G., Gómez-Casati, M. E., Gigliello, A. R., Liberman, M. C. & Corfas, G. Neurotrophin-3 regulates ribbon
synapse density in the cochlea and induces synapse regeneration after acoustic trauma. _eLIFE_ 3, e03564 (2014). Article CAS PubMed Central Google Scholar * Schaette, R. & McAlpine,
D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. _J. Neurosci._ 31, 13452–13457 (2011). Article CAS PubMed PubMed Central
Google Scholar * Starr, A. _ et al_. Absence of both auditory evoked potentials and auditory percepts dependent on timing cues. _Brain_ 114, 1157–1180 (1991). Article PubMed Google
Scholar * Starr, A. _ et al_. Cochlear receptor (microphonic and summating potentials, otoacoustic emissions) and auditory pathway (auditory brain stem potentials) activity in auditory
neuropathy. _Ear Hear._ 22, 91 (2001). Article CAS PubMed Google Scholar * Santarelli, R. _ et al_. Abnormal cochlear potentials from deaf patients with mutations in the otoferlin gene.
_J. Assoc. Res. Otolaryngol._ 10, 545–556 (2009). Article PubMed PubMed Central Google Scholar * Pauli-Magnus, D. _ et al_. Detection and differentiation of sensorineural hearing loss in
mice using auditory steady-state responses and transient auditory brainstem responses. _Neuroscience_ 149, 673–684 (2007). Article CAS PubMed Google Scholar * Shearer, A. E. &
Smith, R. J. H. Massively parallel sequencing for genetic diagnosis of hearing loss: the new standard of care. _Otolaryngol. Head Neck Surg._ 153, 175–182 (2015). Article PubMed PubMed
Central Google Scholar * Dean, C., Felder, G. & Kim, A. H. Analysis of speech perception outcomes among patients receiving cochlear implants with auditory neuropathy spectrum disorder.
_Otol. Neurotol._ 34, 1610–1614 (2013). Article PubMed Google Scholar * Humphriss, R. _ et al_. Does cochlear implantation improve speech recognition in children with auditory neuropathy
spectrum disorder? A systematic review. _Int. J. Audiol._ 52, 442–454 (2013). Article PubMed Google Scholar * Roush, P., Frymark, T., Venediktov, R. & Wang, B. Audiologic management
of auditory neuropathy spectrum disorder in children: a systematic review of the literature. _Am. J. Audiol._ 20, 159–170 (2011). Article PubMed Google Scholar * Giraudet, F. & Avan,
P. Auditory neuropathies: understanding their pathogenesis to illuminate intervention strategies. _Curr. Opin. Neurol._ 25, 50–56 (2012). Article PubMed Google Scholar * Rance, G. &
Barker, E. J. Speech and language outcomes in children with auditory neuropathy/dys-synchrony managed with either cochlear implants or hearing aids. _Int. J. Audiol._ 48, 313–320 (2009).
Article PubMed Google Scholar * Ching, T. Y. C. _ et al_. Impact of the presence of auditory neuropathy spectrum disorder (ANSD) on outcomes of children at three years of age. _Int. J.
Audiol._ 52, S55–S64 (2013). Article PubMed Google Scholar * Hall, R. D. Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked auditory brainstem
response. _Hear. Res._ 49, 155–168 (1990). Article CAS PubMed Google Scholar * Hall, R. D. Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked
auditory brainstem response. _Hear. Res._ 45, 123–136 (1990). Article CAS PubMed Google Scholar * Zhou, R., Abbas, P. J. & Assouline, J. G. Electrically evoked auditory brainstem
response in peripherally myelin-deficient mice. _Hear Res._ 88, 98–106 (1995). Article CAS PubMed Google Scholar * Rouillon, I. _ et al_. Results of cochlear implantation in two children
with mutations in the _OTOF_ gene. _Int. J. Pediatr. Otorhinolaryngol._ 70, 689–696 (2006). Article CAS PubMed Google Scholar * Hernandez, V. H. _ et al_. Optogenetic stimulation of the
auditory pathway. _J. Clin. Invest._ 124, 1114–1129 (2014). Article CAS PubMed PubMed Central Google Scholar * Jeschke, M. & Moser, T. Considering optogenetic stimulation for
cochlear implants. _Hear. Res._ 322, 224–234 (2015). Article PubMed Google Scholar * Akil, O. _ et al_. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene
therapy. _Neuron_ 75, 283–293 (2012). THIS MOUSE STUDY DEMONSTRATED A NEAR-COMPLETE RESTORATION OF HEARING IN A KNOCKOUT MOUSE MODEL OF AUDITORY SYNAPTOPATHY BY A POSTNATAL GENE TRANSFER VIA
INJECTION OF A VIRAL VECTOR INTO THE COCHLEA. Article CAS PubMed PubMed Central Google Scholar * US National Library of Medicine. _ClinicalTrials.gov_[online] (2015). Download
references ACKNOWLEDGEMENTS The authors would like to thank Drs Nicola Strenzke and Regis Nouvian for feedback on the manuscript, Dr Carolin Wichmann for the electron micrograph in Figure 3
and Dr Nouvian for providing artwork for Figures 3 and 4. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute for Auditory Neuroscience and InnerEarLab, University Medical Center
Göttingen, Göttingen, 37099, Germany Tobias Moser * Center for Hearing Research, University of California, Irvine, 92697, California, USA Arnold Starr Authors * Tobias Moser View author
publications You can also search for this author inPubMed Google Scholar * Arnold Starr View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
Both authors researched the literature, assembled the Figures, and wrote, edited and revised the manuscript. CORRESPONDING AUTHOR Correspondence to Tobias Moser. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing financial interests. RELATED LINKS FURTHER INFORMATION MITOchondrial DYNamics variation pages is a portal specializing in genes involved
in disorders of mitochondrial dynamics, and describes the known human OPA1 mutations POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3
POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 POWERPOINT SLIDE FOR FIG. 6 POWERPOINT SLIDE FOR TABLE 1 GLOSSARY * Auditory neuropathy A hearing impairment found in individuals with
hereditary motor and sensory neuropathy; impairs speech comprehension beyond what would be expected on the basis of pure tone audiograms. * Ribbon synapses Highly specialized synapses
between the inner hair cells and spiral ganglion neurons, with an electron-dense structure — the synaptic ribbon — at the presynaptic active zone that mediates neurotransmitter release. *
Cochlear microphone potentials Outer hair cells generate local cochlear potentials that follow the sound stimulus so precisely that they are called 'microphone potentials'. *
Otoacoustic emission Sound generated from within the inner ear that can be measured with a sensitive microphone in the external ear canal to assess outer hair cell function. * Auditory
brainstem responses Evoked potentials in response to repetitive acoustic stimulation that are recorded from scalp EEG electrodes and typically have five peaks, referred to as waves I–V. *
Spiral ganglion compound action potential The first auditory brainstem response peak, wave I, reflects the spiral ganglion compound action potential; this potential can be recorded with
better resolution using electrocochleography. * Auditory synaptopathy Hearing impairment caused by dysfunction or loss of ribbon synapses in the inner hair cells; has been termed auditory
synaptopathy and can show clinical findings similar to those described above for auditory neuropathy. * Organ of Corti The organ of Corti is the end organ of the sense of hearing that
harbours the sensory inner and outer hair cells, as well as afferent and efferent nerve fibres and various types of supporting cells. * Compound action potential Reflects the synchronized
firing of spiral ganglion neurons; assessed by intrameatal or transtympanic electrocochleography. * Glutamate excitotoxicity Excessive presynaptic glutamate release leading to massive
depolarization and subsequent synapse loss. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Moser, T., Starr, A. Auditory neuropathy — neural and
synaptic mechanisms. _Nat Rev Neurol_ 12, 135–149 (2016). https://doi.org/10.1038/nrneurol.2016.10 Download citation * Published: 19 February 2016 * Issue Date: March 2016 * DOI:
https://doi.org/10.1038/nrneurol.2016.10 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative