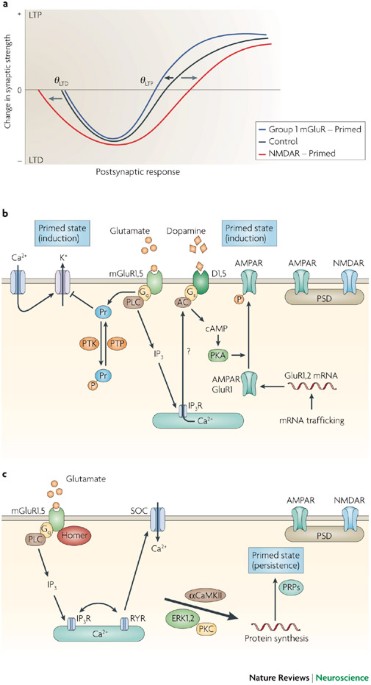
Metaplasticity: tuning synapses and networks for plasticity
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
KEY POINTS * Synaptic plasticity, such as long-term potentiation (LTP) and long-term depression (LTD), must be tightly regulated to prevent saturation, which would impair learning.
Metaplasticity mechanisms have evolved to help implement this essential computational constraint. Metaplasticity refers to neural changes that are induced by activity at one point in time
and that persist and affect subsequently induced LTP or LTD. * The activation of NMDA (_N_-methyl-D-aspartate) receptors can cause a persistent reduction in LTP induction and an enhancement
of LTD. These effects are synapse-specific, last tens of minutes and contribute to LTD induction during conventional low-frequency stimulation protocols. The mechanisms of this regulation
are poorly understood, but activation of protein phosphatases and alteration of calcium/calmodulin-dependent protein kinase II function are clear candidates. * Prior activation of group 1
metabotropic glutamate receptors (group 1 mGluRs) facilitates both the induction and the persistence of LTP in the hippocampus. The facilitated induction probably involves depression of
afterhyperpolarizations (AHPs) and trafficking of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors to the extrasynaptic membrane, whereas the facilitated persistence
entails _de novo_ local protein synthesis. * Heterosynaptic metaplasticity that crosses between synapses can also occur. Stimulation of protein synthesis by activity in one set of synapses
can facilitate LTP persistence through a synaptic tag-and-capture process operating at a second set of weakly activated synapses. Heterosynaptic metaplasticity can also be mediated by
altered postsynaptic ion-channel function and retrograde endocannabinoid signalling that reduces transmission at nearby inhibitory GABAergic terminals. * Behaviourally, stress can inhibit
LTP and facilitate LTD through NMDA-receptor-dependent mechanisms. Sensory stimulation or deprivation alters plasticity thresholds in cortical regions, especially during developmental
periods. Reductions in the slow AHP in piriform and hippocampal neurons support the learning of behavioural tasks, suggesting a metaplastic role for this mechanism in controlling
learning-related plasticity thresholds. * The ability to harness metaplasticity mechanisms might contribute to strategies for treating adult amblyopia or the development of therapies aimed
at improving cognition in individuals with neurological disorders. Metaplasticity paradigms also share commonalities with ischaemic preconditioning, so its mechanisms might present targets
for preventing stroke in at-risk individuals. * In conclusion, metaplasticity is a major regulator of plasticity thresholds and therefore has a key role in keeping synapses working in a
range that permits the full expression of plasticity. In turn, this helps to keep networks operating at an appropriate level for information processing and storage. Considerable research is
still needed to clarify the mechanisms that underpin different forms of metaplasticity and their contribution to network dynamics and behavioural learning. ABSTRACT Synaptic plasticity is a
key component of the learning machinery in the brain. It is vital that such plasticity be tightly regulated so that it occurs to the proper extent at the proper time. Activity-dependent
mechanisms that have been collectively termed metaplasticity have evolved to help implement these essential computational constraints. Various intercellular signalling molecules can trigger
lasting changes in the ability of synapses to express plasticity; their mechanisms of action are reviewed here, along with a consideration of how metaplasticity might affect learning and
clinical conditions. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution
Subscribe to this journal Receive 12 print issues and online access $189.00 per year only $15.75 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full
article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs *
Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ADAPTIVE CONTROL OF SYNAPTIC PLASTICITY INTEGRATES MICRO- AND MACROSCOPIC NETWORK FUNCTION Article 29 August 2022 THE
PLASTICITOME OF CORTICAL INTERNEURONS Article 30 December 2022 CO-DEPENDENT EXCITATORY AND INHIBITORY PLASTICITY ACCOUNTS FOR QUICK, STABLE AND LONG-LASTING MEMORIES IN BIOLOGICAL NETWORKS
Article Open access 20 March 2024 REFERENCES * Martin, S. J., Grimwood, P. D. & Morris, R. G. M. Synaptic plasticity and memory: an evaluation of the hypothesis. _Annu. Rev. Neurosci._
23, 649–711 (2000). Article CAS PubMed Google Scholar * Neves, G., Cooke, S. F. & Bliss, T. V. P. Synaptic plasticity, memory and the hippocampus: a neural network approach to
causality. _Nature Rev. Neurosci._ 9, 65–75 (2008). Article CAS Google Scholar * Abraham, W. C. & Bear, M. F. Metaplasticity: the plasticity of synaptic plasticity. _Trends Neurosci._
19, 126–130 (1996). THIS PAPER PROVIDED THE FIRST FORMAL DESCRIPTION AND REVIEW OF METAPLASTICITY PHENOMENA. Article CAS PubMed Google Scholar * Abraham, W. C. & Tate, W. P.
Metaplasticity: a new vista across the field of synaptic plasticity. _Prog. Neurobiol._ 52, 303–323 (1997). Article CAS PubMed Google Scholar * Huang, Y. Y., Colino, A., Selig, D. K.
& Malenka, R. C. The influence of prior synaptic activity on the induction of long-term potentiation. _Science_ 255, 730–733 (1992). THIS KEY PAPER PROVIDED THE FIRST CLEAR DEMONSTRATION
THAT PRIOR ACTIVATION OF NMDARS CAN INHIBIT THE INDUCTION OF SUBSEQUENT LTP. Article CAS PubMed Google Scholar * Coan, E. J., Irving, A. J. & Collingridge, G. L. Low-frequency
activation of the NMDA receptor system can prevent the induction of LTP. _Neurosci. Lett._ 105, 205–210 (1989). Article CAS PubMed Google Scholar * Youssef, F. F., Addae, J. I. &
Stone, T. W. NMDA-induced preconditioning attenuates synaptic plasticity in the rat hippocampus. _Brain Res._ 1073, 183–189 (2006). Article PubMed CAS Google Scholar * Fujii, S. et al.
The long-term suppressive effect of prior activation of synaptic inputs by low-frequency stimulation on induction of long-term potentiation in CA1 neurons of guinea pig hippocampal slices.
_Exp. Brain Res._ 111, 305–312 (1996). CAS PubMed Google Scholar * Woo, N. H. & Nguyen, P. V. “Silent” metaplasticity of the late phase of long-term potentiation requires protein
phosphatases. _Learn. Mem._ 9, 202–213 (2002). Article PubMed PubMed Central Google Scholar * Fujii, S. et al. Endogenous adenosine regulates the effects of low-frequency stimulation on
the induction of long-term potentiation in CA1 neurons of guinea pig hippocampal slices. _Neurosci. Lett._ 279, 121–124 (2000). Article CAS PubMed Google Scholar * Izumi, Y., Tokuda, K.
& Zorumski, C. F. Long-term potentiation inhibition by low-level _N_-methyl-D-aspartate receptor activation involves calcineurin, nitric oxide, and p38 mitogen-activated protein kinase.
_Hippocampus_ 18, 258–265 (2008). Article CAS PubMed Google Scholar * O'Connor, D. H., Wittenberg, G. M. & Wang, S. S. H. Dissection of bidirectional synaptic plasticity into
saturable unidirectional processes. _J. Neurophysiol._ 94, 1565–1573 (2005). Article PubMed Google Scholar * Abraham, W. C. & Huggett, A. Induction and reversal of long-term
potentiation by repeated high-frequency stimulation in rat hippocampal slices. _Hippocampus_ 7, 137–145 (1997). Article CAS PubMed Google Scholar * Christie, B. R. & Abraham, W. C.
Priming of associative long-term depression by θ frequency synaptic activity. _Neuron_ 8, 79–84 (1992). Article Google Scholar * Wang, Y., Wu, J., Rowan, M. J. & Anwyl, R. Role of
protein kinase C in the induction of homosynaptic long-term depression by brief low frequency stimulation in the dentate gyrus of the rat hippocampus _in vitro_. _J. Physiol._ 513, 467–475
(1998). Article CAS PubMed PubMed Central Google Scholar * Mockett, B., Coussens, C. & Abraham, W. C. NMDA receptor-mediated metaplasticity during the induction of long-term
depression by low-frequency stimulation. _Eur. J. Neurosci._ 15, 1819–1826 (2002). THIS PAPER PROVIDED THE FIRST DEMONSTRATION THAT THE INDUCTION OF LTD BY LFS INVOLVES AN INITIAL
NMDAR-INDUCED REDUCTION IN THE THRESHOLD FOR LTD BY PULSES EARLY IN THE LFS, SO THAT PULSES LATER IN THE LFS CAN INDUCE THE LTD. Article PubMed Google Scholar * Ngezahayo, A., Schachner,
M. & Artola, A. Synaptic activity modulates the induction of bidirectional synaptic changes in adult mouse hippocampus. _J. Neurosci._ 20, 2451–2458 (2000). Article CAS PubMed PubMed
Central Google Scholar * Lau, C. G. & Zukin, R. S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. _Nature Rev. Neurosci._ 8, 413–426 (2007). Article
CAS Google Scholar * Perez-Otano, I. & Ehlers, M. D. Homeostatic plasticity and NMDA receptor trafficking. _Trends Neurosci._ 28, 229–238 (2005). Article CAS PubMed Google Scholar
* Selig, D. K., Hjelmstad, G. O., Herron, C., Nicoll, R. A. & Malenka, R. C. Independent mechanisms for long-term depression of AMPA and NMDA responses. _Neuron_ 15, 417–426 (1995).
Article CAS PubMed Google Scholar * Morishita, W., Marie, H. & Malenka, R. C. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. _Nature
Neurosci._ 8, 1043–1050 (2005). Article CAS PubMed Google Scholar * Sobczyk, A. & Svoboda, K. Activity-dependent plasticity of the NMDA-receptor fractional Ca2+ current. _Neuron_ 53,
17–24 (2007). THIS PAPER PROVIDED A CLEAR DEMONSTRATION, USING 2-PHOTON RELEASE OF CAGED GLUTAMATE, THAT SYNAPTIC ACTIVATION OF NMDARS CAN LEAD TO DOWN-REGULATION OF NMDAR FUNCTION AND THUS
SERVE AS A POSSIBLE MECHANISM OF METAPLASTICITY. Article CAS PubMed Google Scholar * Kato, K. & Zorumski, C. F. Nitric oxide inhibitors facilitate the induction of hippocampal
long-term potentiation by modulating NMDA responses. _J. Neurophysiol._ 70, 1260–1263 (1993). Article CAS PubMed Google Scholar * Murphy, K. P. S. J., Williams, J. H., Bettache, N. &
Bliss, T. V. P. Photolytic release of nitric oxide modulates NMDA receptor-mediated transmission but does not induce long-term potentiation at hippocampal synapses. _Neuropharmacology_ 33,
1375–1385 (1994). Article CAS PubMed Google Scholar * Fong, D. K., Rao, A., Crump, F. T. & Craig, A. M. Rapid synaptic remodeling by protein kinase C: reciprocal translocation of
NMDA receptors and calcium/calmodulin-dependent kinase II. _J. Neurosci._ 22, 2153–2164 (2002). Article CAS PubMed PubMed Central Google Scholar * Groc, L. et al. Differential
activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. _Nature Neurosci._ 7, 695–696 (2004). Article CAS PubMed Google Scholar * Montgomery, J., Selcher, J.,
Hanson, J. & Madison, D. Dynamin-dependent NMDAR endocytosis during LTD and its dependence on synaptic state. _BMC Neurosci._ 6, 48 (2005). Article PubMed PubMed Central CAS Google
Scholar * Stanton, P. K. Transient protein kinase C activation primes long-term depression and suppresses long-term potentiation of synaptic transmission in hippocampus. _Proc. Natl Acad.
Sci. USA_ 92, 1724–1728 (1995). Article CAS PubMed PubMed Central Google Scholar * Izumi, Y., Clifford, D. B. & Zorumski, C. F. Inhibition of long-term potentiation by NMDA-mediated
nitric oxide release. _Science_ 257, 1273–1276 (1992). Article CAS PubMed Google Scholar * Hsu, K. S., Ho, W. C., Huang, C. C. & Tsai, J. J. Transient removal of extracellular Mg2+
elicits persistent suppression of LTP at hippocampal CA1 synapses via PKC activation. _J. Neurophysiol._ 84, 1279–1288 (2000). Article CAS PubMed Google Scholar * Gisabella, B., Rowan,
M. J. & Anwyl, R. Mechanisms underlying the inhibition of long-term potentiation by preconditioning stimulation in the hippocampus _in vitro_. _Neuroscience_ 121, 297–305 (2003). Article
CAS PubMed Google Scholar * Waxman, E. A. & Lynch, D. R. _N_-methyl-D-aspartate receptor subtype mediated bidirectional control of p38 mitogen-activated protein kinase. _J. Biol.
Chem._ 280, 29322–29333 (2005). Article CAS PubMed Google Scholar * McNaughton, B. L., Douglas, R. M. & Goddard, G. V. Synaptic enhancement in fascia dentate: cooperativity among
coactive afferents. _Brain Res._ 157, 277–293 (1978). Article CAS PubMed Google Scholar * Frey, U., Schollmeier, K., Reymann, K. G. & Seidenbecher, T. Asymptotic hippocampal
long-term potentiation in rats does not preclude additional potentiation at later phases. _Neuroscience_ 67, 799–807 (1995). THIS IMPORTANT PAPER DEMONSTRATED THAT THE APPARENT SATURATION OF
LTP BY REPEATED HFS CAN, IN SOME SITUATIONS AT LEAST, SIMPLY REFLECT A TRANSIENT METAPLASTIC INHIBITION OF FURTHER LTP. THUS, ADDITIONAL LTP CAN BE OBTAINED BY AN HFS GIVEN AFTER A REST
PERIOD. Article CAS PubMed Google Scholar * Moody, T. D., Carlisle, H. J. & O'Dell, T. J. A nitric oxide-independent and β-adrenergic receptor-sensitive form of metaplasticity
limits θ-frequency stimulation-induced LTP in the hippocampal CA1 region. _Learn. Mem._ 6, 619–633 (1999). Article CAS PubMed PubMed Central Google Scholar * Gold, J. I. & Bear, M.
F. A model of dendritic spine Ca2+ concentration exploring possible bases for a sliding synaptic modification threshold. _Proc. Natl Acad. Sci. USA_ 91, 3941–3945 (1994). Article CAS
PubMed PubMed Central Google Scholar * Alkon, D. L. et al. Learning and memory. FENS Study Group. _Brain Res. Brain Res. Rev._ 16, 193–220 (1991). Article CAS PubMed Google Scholar *
Bear, M. F. Mechanism for a sliding synaptic modification threshold. _Neuron_ 15, 1–4 (1995). Article CAS PubMed Google Scholar * Deisseroth, K., Bito, H., Schulman, H. & Tsien, R.
W. A molecular mechanism for metaplasticity. _Curr. Biol._ 5, 1334–1338 (1995). Article CAS PubMed Google Scholar * Mauceri, D., Cattabeni, F., Di Luca, M. & Gardoni, F.
Calcium/calmodulin-dependent protein kinase II phosphorylation drives synapse-associated protein 97 into spines. _J. Biol. Chem._ 279, 23813–23821 (2004). Article CAS PubMed Google
Scholar * Mayford, M., Wang, J., Kandel, E. R. & O'Dell, T. J. CaMKII regulates the frequency-response function of hippocampal synapses for the production of both LTD and LTP.
_Cell_ 81, 891–904 (1995). Article CAS PubMed Google Scholar * Fukunaga, K., Stoppini, L., Miyamoto, E. & Muller, D. Long-term potentiation is associated with an increased activity
of Ca2+/calmodulin-dependent protein kinase II. _J. Biol. Chem._ 268, 7863–7867 (1993). Article CAS PubMed Google Scholar * Krucker, T. et al. Targeted disruption of RC3 reveals a
calmodulin-based mechanism for regulating metaplasticity in the hippocampus. _J. Neurosci._ 22, 5525–5535 (2002). Article CAS PubMed PubMed Central Google Scholar * Zhang, L. et al.
Hippocampal synaptic metaplasticity requires inhibitory autophosphorylation of Ca2+/calmodulin-dependent kinase II. _J. Neurosci._ 25, 7697–7707 (2005). THIS PAPER PROVIDED A KEY
DEMONSTRATION THAT PHOSPHORYLATION OF THE THR305/THR306 SITE ON ΑCAMKII MEDIATES THE METAPLASTIC INHIBITION OF LTP BY SYNAPTIC ACTIVITY. Article CAS PubMed PubMed Central Google Scholar
* Cooke, S. F. et al. Autophosphorylation of αCaMKII is not a general requirement for NMDA receptor-dependent LTP in the adult mouse. _J. Physiol._ 574, 805–818 (2006). Article CAS
PubMed PubMed Central Google Scholar * Cohen, A. S., Coussens, C. M., Raymond, C. R. & Abraham, W. C. Long-lasting increase in cellular excitability associated with the priming of LTP
induction in rat hippocampus. _J. Neurophysiol._ 82, 3139–3148 (1999). Article CAS PubMed Google Scholar * Ireland, D. R. & Abraham, W. C. Group I mGluRs increase excitability of
hippocampal CA1 pyramidal neurons by a PLC-independent mechanism. _J. Neurophysiol._ 88, 107–116 (2002). Article CAS PubMed Google Scholar * Ireland, D. R., Guevremont, D., Williams, J.
M. & Abraham, W. C. Metabotropic glutamate receptor-mediated depression of the slow afterhyperpolarization is gated by tyrosine phosphatases in hippocampal CA1 pyramidal neurons. _J.
Neurophysiol._ 92, 2811–2819 (2004). Article CAS PubMed Google Scholar * Oh, M. C., Derkach, V. A., Guire, E. S. & Soderling, T. R. Extrasynaptic membrane trafficking regulated by
GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. _J. Biol. Chem._ 281, 752–758 (2006). THIS STUDY PROVIDED AN IMPORTANT HIPPOCAMPAL DEMONSTRATION THAT
TRAFFICKING OF AMPARS TO THE EXTRASYNAPTIC MEMBRANE CAN PRIME SYNAPSES FOR ENHANCED LTP IN RESPONSE TO A SUBSEQUENT HFS. Article CAS PubMed Google Scholar * Gao, C., Sun, X. & Wolf,
M. E. Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. _J. Neurochem._ 98,
1664–1677 (2006). Article CAS PubMed Google Scholar * Grooms, S. Y. et al. Activity bidirectionally regulates AMPA receptor mRNA abundance in dendrites of hippocampal neurons. _J.
Neurosci._ 26, 8339–8351 (2006). Article CAS PubMed PubMed Central Google Scholar * O'Connor, J. J., Rowan, M. J. & Anwyl, R. Long-lasting enhancement of NMDA receptor-mediated
synaptic transmission by metabotropic glutamate receptor activation. _Nature_ 367, 557–559 (1994). Article CAS PubMed Google Scholar * MacDonald, J. F., Jackson, M. F. & Beazely, M.
A. G protein-coupled receptors control NMDARs and metaplasticity in the hippocampus. _Biochim. Biophys. Acta_ 1768, 941–951 (2007). Article CAS PubMed Google Scholar * Christie, B. R.,
Stellwagen, D. & Abraham, W. C. Reduction of the threshold for long-term potentiation by prior theta-frequency synaptic activity. _Hippocampus_ 5, 52–59 (1995). Article CAS PubMed
Google Scholar * Cohen, A. S., Raymond, C. R. & Abraham, W. C. Priming of long-term potentiation induced by activation of metabotropic glutamate receptors coupled to phospholipase C.
_Hippocampus_ 8, 160–170 (1998). Article CAS PubMed Google Scholar * Blank, T., Nijholt, I., Eckart, K. & Spiess, J. Priming of long-term potentiation in mouse hippocampus by
corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. _J. Neurosci._ 22, 3788–3794 (2002). Article CAS PubMed PubMed Central Google Scholar *
Cohen, A. S. & Abraham, W. C. Facilitation of long-term potentiation by prior activation of metabotropic glutamate receptors. _J. Neurophysiol._ 76, 953–962 (1996). Article CAS PubMed
Google Scholar * Matsuda, Y., Marzo, A. & Otani, S. The presence of background dopamine signal converts long-term synaptic depression to potentiation in rat prefrontal cortex. _J.
Neurosci._ 26, 4803–4810 (2006). Article CAS PubMed PubMed Central Google Scholar * Sun, X., Zhao, Y. & Wolf, M. E. Dopamine receptor stimulation modulates AMPA receptor synaptic
insertion in prefrontal cortex neurons. _J. Neurosci._ 25, 7342–7351 (2005). Article CAS PubMed PubMed Central Google Scholar * Bortolotto, Z. A., Bashir, Z. I., Davies, C. H. &
Collingridge, G. L. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. _Nature_ 368, 740–743 (1994). THIS PAPER PROVIDED THE
FIRST DEMONSTRATION THAT ACTIVATION OF MGLURS CAN METAPLASTICALLY PROMOTE SUBSEQUENT LTP INDUCTION, IN THIS CASE BY CHANGING THE STATE OF THE SYNAPSES SO AS TO RENDER FURTHER ACTIVATION OF
MGLURS UNNECESSARY FOR LTP. Article CAS PubMed Google Scholar * Bortolotto, Z. A. et al. Studies on the role of metabotropic glutamate receptors in long-term potentiation: some
methodological considerations. _J. Neurosci. Methods_ 59, 19–24 (1995). Article CAS PubMed Google Scholar * Bortolotto, Z. A. et al. The regulation of hippocampal LTP by the molecular
switch, a form of metaplasticity, requires mGlu5 receptors. _Neuropharmacology_ 49, 13–25 (2005). Article CAS PubMed Google Scholar * Bortolotto, Z. A. & Collingridge, G. L.
Involvement of calcium/calmodulin-dependent protein kinases in the setting of a molecular switch involved in hippocampal LTP. _Neuropharmacology_ 37, 535–544 (1998). Article CAS PubMed
Google Scholar * Bortolotto, Z. A. & Collingridge, G. L. A role for protein kinase C in a form of metaplasticity that regulates the induction of long-term potentiation at CA1 synapses
of the adult rat hippocampus. _Eur. J. Neurosci._ 12, 4055–4062 (2000). Article CAS PubMed Google Scholar * Raymond, C. R., Thompson, V. L., Tate, W. P. & Abraham, W. C. Metabotropic
glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation. _J. Neurosci._ 20, 969–976 (2000). Article CAS PubMed PubMed Central Google Scholar *
Maggio, N. & Segal, M. Unique regulation of long term potentiation in the rat ventral hippocampus. _Hippocampus_ 17, 10–25 (2007). Article CAS PubMed Google Scholar * Mellentin, C.,
Jahnsen, H. & Abraham, W. C. Priming of long-term potentiation mediated by ryanodine receptor activation in rat hippocampal slices. _Neuropharmacology_ 52, 118–125 (2007). Article CAS
PubMed Google Scholar * Klann, E. & Dever, T. E. Biochemical mechanisms for translational regulation in synaptic plasticity. _Nature Rev. Neurosci._ 5, 931–942 (2004). Article CAS
Google Scholar * Tsokas, P., Ma, T., Iyengar, R., Landau, E. M. & Blitzer, R. D. Mitogen-activated protein kinase upregulates the dendritic translation machinery in long-term
potentiation by controlling the mammalian target of rapamycin pathway. _J. Neurosci._ 27, 5885–5894 (2007). Article CAS PubMed PubMed Central Google Scholar * Huang, F., Chotiner, J. K.
& Steward, O. The mRNA for elongation factor 1α is localized in dendrites and translated in response to treatments that induce long-term depression. _J. Neurosci._ 25, 7199–7209 (2005).
Article CAS PubMed PubMed Central Google Scholar * Ouyang, Y., Rosenstein, A., Kreiman, G., Schuman, E. M. & Kennedy, M. B. Tetanic stimulation leads to increased accumulation of
Ca2+/calmodulin-dependent protein kinase II via dendritic protein synthesis in hippocampal neurons. _J. Neurosci._ 19, 7823–7833 (1999). Article CAS PubMed PubMed Central Google Scholar
* Rush, A. M., Wu, J. Q., Rowan, M. J. & Anwyl, R. Group I metabotropic glutamate receptor (mGluR)-dependent long-term depression mediated via p38 mitogen-activated protein kinase is
inhibited by previous high-frequency stimulation and activation of mGluRs and protein kinase C in the rat dentate gyrus _in vitro_. _J. Neurosci._ 22, 6121–6128 (2002). Article CAS PubMed
PubMed Central Google Scholar * Bienenstock, E. L., Cooper, L. N. & Munro, P. W. Theory for the development of neuron selectivity: orientation specificity and binocular interaction
in visual cortex. _J. Neurosci._ 2, 32–48 (1982). THIS SEMINAL PAPER DESCRIBED A MODEL DESIGNED TO ACCOUNT FOR THE VARIOUS FEATURES OF EXPERIENCE-DEPENDENT PLASTICITY IN THE VISUAL CORTEX.
NOW KNOWN AS THE BCM MODEL, THE WORK INSPIRED NUMEROUS EXPERIMENTAL TESTS OF ITS ESSENTIAL PRINCIPLES. Article CAS PubMed PubMed Central Google Scholar * Holland, L. L. & Wagner, J.
J. Primed facilitation of homosynaptic long-term depression and depotentiation in rat hippocampus. _J. Neurosci._ 18, 887–894 (1998). Article CAS PubMed PubMed Central Google Scholar *
Wang, H. & Wagner, J. J. Priming-induced shift in synaptic plasticity in the rat hippocampus. _J. Neurophysiol._ 82, 2024–2028 (1999). Article CAS PubMed Google Scholar *
Roth-Alpermann, C., Morris, R. G. M., Korte, M. & Bonhoeffer, T. Homeostatic shutdown of long-term potentiation in the adult hippocampus. _Proc. Natl Acad. Sci. USA_ 103, 11039–11044
(2006). Article CAS PubMed PubMed Central Google Scholar * Abraham, W. C., Mason-Parker, S. E., Bear, M. F., Webb, S. & Tate, W. P. Heterosynaptic metaplasticity in the hippocampus
_in vivo_: a BCM-like modifiable threshold for LTP. _Proc. Natl Acad. Sci. USA_ 98, 10924–10929 (2001). Article CAS PubMed PubMed Central Google Scholar * Frey, U. & Morris, R. G.
M. Synaptic tagging and long-term potentiation. _Nature_ 385, 533–536 (1997). THIS PAPER DESCRIBED THE CONCEPT OF SYNAPTIC TAGGING FOR THE FIRST TIME. EXPERIMENTAL EVIDENCE WAS PRESENTED FOR
AN INTERACTION BETWEEN A STRONGLY ACTIVATED INPUT PATHWAY AND A WEAKLY ACTIVATED PATHWAY. THIS INTERACTION PROMOTES PROTEIN-SYNTHESIS-DEPENDENT LTP IN THE WEAK PATHWAY. Article CAS PubMed
Google Scholar * Frey, U. & Morris, R. G. M. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. _Trends Neurosci._ 21, 181–188 (1998). Article
CAS PubMed Google Scholar * Frey, S., Bergado-Rosado, J., Seidenbecher, T., Pape, H. C. & Frey, J. U. Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by
stimulation of the basolateral amygdala: heterosynaptic induction mechanisms of late-LTP. _J. Neurosci._ 21, 3697–3703 (2001). Article CAS PubMed PubMed Central Google Scholar *
Sajikumar, S. & Frey, J. U. Resetting of 'synaptic tags' is time- and activity-dependent in rat hippocampal Ca1 _in vitro_. _Neuroscience_ 129, 503–507 (2004). Article CAS
PubMed Google Scholar * Young, J. Z. & Nguyen, P. V. Homosynaptic and heterosynaptic inhibition of synaptic tagging and capture of long-term potentiation by previous synaptic activity.
_J. Neurosci._ 25, 7221–7231 (2005). Article CAS PubMed PubMed Central Google Scholar * Young, J. Z., Isiegas, C., Abel, T. & Nguyen, P. V. Metaplasticity of the late-phase of
long-term potentiation: a critical role for protein kinase A in synaptic tagging. _Eur. J. Neurosci._ 23, 1784–1794 (2006). Article PubMed PubMed Central Google Scholar * Sajikumar, S.,
Navakkode, S. & Frey, J. U. Identification of compartment- and process-specific molecules required for “synaptic tagging” during long-term potentiation and long-term depression in
hippocampal CA1. _J. Neurosci._ 27, 5068–5080 (2007). Article CAS PubMed PubMed Central Google Scholar * Sajikumar, S., Navakkode, S., Sacktor, T. C. & Frey, J. U. Synaptic tagging
and cross-tagging: the role of protein kinase Mζ in maintaining long-term potentiation but not long-term depression. _J. Neurosci._ 25, 5750–5756 (2005). Article CAS PubMed PubMed Central
Google Scholar * Kauderer, B. S. & Kandel, E. R. Capture of a protein synthesis-dependent component of long-term depression. _Proc. Natl Acad. Sci. USA_ 97, 13342–13347 (2000).
Article CAS PubMed PubMed Central Google Scholar * Sajikumar, S. & Frey, J. U. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. _Neurobiol. Learn.
Mem._ 82, 12–25 (2004). Article CAS PubMed Google Scholar * Govindarajan, A., Kelleher, R. J. & Tonegawa, S. A clustered plasticity model of long-term memory engrams. _Nature Rev.
Neurosci._ 7, 575–583 (2006). Article CAS Google Scholar * Sajikumar, S., Navakkode, S., Korz, V. & Frey, J. U. Cognitive and emotional information processing: protein synthesis and
gene expression. _J. Physiol._ 584, 389–400 (2007). Article CAS PubMed PubMed Central Google Scholar * Barco, A., Alarcon, J. M. & Kandel, E. R. Expression of constitutively active
CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. _Cell_ 108, 689–703 (2002). Article CAS PubMed Google Scholar * Woo, N. H. & Nguyen,
P. V. Protein synthesis is required for synaptic immunity to depotentiation. _J. Neurosci._ 23, 1125–1132 (2003). Article CAS PubMed PubMed Central Google Scholar * Akirav, I. &
Richter-Levin, G. Priming stimulation in the basolateral amygdala modulates synaptic plasticity in the rat dentate gyrus. _Neurosci. Lett._ 270, 83–86 (1999). Article CAS PubMed Google
Scholar * Nakao, K., Matsuyama, K., Matsuki, N. & Ikegaya, Y. Amygdala stimulation modulates hippocampal synaptic plasticity. _Proc. Natl Acad. Sci. USA_ 101, 14270–14275 (2004).
Article CAS PubMed PubMed Central Google Scholar * Frey, S., Bergado, J. A. & Frey, J. U. Modulation of late phases of long-term potentiation in rat dentate gyrus by stimulation of
the medial septum. _Neuroscience_ 118, 1055–1062 (2003). Article CAS PubMed Google Scholar * Bergado, J. A., Frey, S., Lopez, J., Almaguer-Melian, W. & Frey, J. U. Cholinergic
afferents to the locus coeruleus and noradrenergic afferents to the medial septum mediate LTP-reinforcement in the dentate gyrus by stimulation of the amygdala. _Neurobiol. Learn. Mem._ 88,
331–341 (2007). Article CAS PubMed Google Scholar * Zhang, W. & Linden, D. J. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. _Nature Rev.
Neurosci._ 4, 885–900 (2003). Article CAS Google Scholar * Magee, J. C. & Johnston, D. Plasticity of dendritic function. _Curr. Opin. Neurobiol._ 15, 334–342 (2005). Article CAS
PubMed Google Scholar * Marder, E. & Goaillard, J.-M. Variability, compensation and homeostasis in neuron and network function. _Nature Rev. Neurosci._ 7, 563–574 (2006). Article CAS
Google Scholar * Kim, S. J. & Linden, D. J. Ubiquitous plasticity and memory storage. _Neuron_ 56, 582–592 (2007). Article CAS PubMed Google Scholar * Sah, P. & Bekkers, J. M.
Apical dendritic location of slow afterhyperpolarization current in hippocampal pyramidal neurons: implications for the integration of long-term potentiation. _J. Neurosci._ 16, 4537–4542
(1996). Article CAS PubMed PubMed Central Google Scholar * Blitzer, R. D., Wong, T., Nouranifar, R., Iyengar, R. & Landau, E. M. Postsynaptic cAMP pathway gates early LTP in
hippocampal CA1 region. _Neuron_ 15, 1403–1414 (1995). Article CAS PubMed Google Scholar * Le Ray, D., De Sevilla, D. F., Porto, A. B., Fuenzalida, M. & Buno, W. Heterosynaptic
metaplastic regulation of synaptic efficacy in CA1 pyramidal neurons of rat hippocampus. _Hippocampus_ 14, 1011–1025 (2004). Article PubMed Google Scholar * Kim, J., Jung, S.-C., Clemens,
A. M., Petralia, R. S. & Hoffman, D. A. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. _Neuron_
54, 933–947 (2007). Article CAS PubMed PubMed Central Google Scholar * Wang, Z. R., Xu, N. L., Wu, C. P., Duan, S. M. & Poo, M. M. Bidirectional changes in spatial dendritic
integration accompanying long-term synaptic modifications. _Neuron_ 37, 463–472 (2003). Article CAS PubMed Google Scholar * Mittmann, W. & Hausser, M. Linking synaptic plasticity and
spike output at excitatory and inhibitory synapses onto cerebellar purkinje cells. _J. Neurosci._ 27, 5559–5570 (2007). Article CAS PubMed PubMed Central Google Scholar * Freund, T. F.
& Buzsaki, G. Interneurons of the hippocampus. _Hippocampus_ 6, 347–470 (1996). Article CAS PubMed Google Scholar * Kullmann, D. M. & Lamsa, K. P. Long-term synaptic plasticity
in hippocampal interneurons. _Nature Rev. Neurosci._ 8, 687–699 (2007). Article CAS Google Scholar * Pitler, T. A. & Alger, B. E. Postsynaptic spike firing reduces synaptic GABAA
responses in hippocampal pyramidal cells. _J. Neurosci._ 12, 4122–4132 (1992). Article CAS PubMed PubMed Central Google Scholar * Wigstrom, H. & Gusstafsson, B. Facilitated
induction of long-lasting potentiation during blockade of inhibition. _Nature_ 301, 603–604 (1983). Article CAS PubMed Google Scholar * Kerr, D. S. & Abraham, W. C. Cooperative
interactions among afferents govern the induction of homosynaptic long-term depression in the hippocampus. _Proc. Natl Acad. Sci. USA_ 92, 11637–11641 (1995). Article CAS PubMed PubMed
Central Google Scholar * Liu, Y. B., Disterhoft, J. F. & Slater, N. T. Activation of metabotropic glutamate receptors induces long-term depression of GABAergic inhibition in
hippocampus. _J. Neurophysiol._ 69, 1000–1004 (1993). Article CAS PubMed Google Scholar * Marsicano, G. et al. The endogenous cannabinoid system controls extinction of aversive memories.
_Nature_ 418, 530–534 (2002). Article CAS PubMed Google Scholar * Alger, B. E. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. _Prog.
Neurobiol._ 68, 247–286 (2002). Article CAS PubMed Google Scholar * Wilson, R. I. & Nicoll, R. A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses.
_Nature_ 410, 588–592 (2001). Article CAS PubMed Google Scholar * Carlson, G., Wang, Y. & Alger, B. E. Endocannabinoids facilitate the induction of LTP in the hippocampus. _Nature
Neurosci._ 5, 723–724 (2002). Article CAS PubMed Google Scholar * Azad, S. C. et al. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. _J.
Neurosci._ 24, 9953–9961 (2004). Article CAS PubMed PubMed Central Google Scholar * Chevaleyre, V. & Castillo, P. E. Endocannabinoid-mediated metaplasticity in the hippocampus.
_Neuron_ 43, 871–881 (2004). THIS PAPER CHARACTERIZED A NOVEL MECHANISM OF METAPLASTICITY, NAMELY THE ENDOCANNABINOID-MEDIATED LONG-TERM DEPRESSION OF GABA RELEASE. Article CAS PubMed
Google Scholar * Chevaleyre, V., Heifets, B. D., Kaeser, P. S., Sudhof, T. C. & Castillo, P. E. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1α.
_Neuron_ 54, 801–812 (2007). Article CAS PubMed PubMed Central Google Scholar * Harvey, C. D. & Svoboda, K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites.
_Nature_ 450, 1195–1200 (2007). Article CAS PubMed PubMed Central Google Scholar * Van Praag, H., Christie, B. R., Sejnowski, T. J. & Gage, F. H. Running enhances neurogenesis,
learning, and long-term potentiation in mice. _Proc. Natl Acad. Sci. USA_ 96, 13427–13431 (1999). Article CAS PubMed PubMed Central Google Scholar * Duffy, S. N., Craddock, K. J., Abel,
T. & Nguyen, P. V. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. _Learn. Mem._ 8, 26–34 (2001). Article CAS PubMed
PubMed Central Google Scholar * Foster, T. C., Fugger, H. N. & Cunningham, S. G. Receptor blockade reveals a correspondence between hippocampal-dependent behavior and
experience-dependent synaptic enhancement. _Brain Res._ 871, 39–43 (2000). Article CAS PubMed Google Scholar * Kim, J. J., Foy, M. R. & Thompson, R. F. Behavioral stress modifies
hippocampal plasticity through _N_-methyl-D-aspartate receptor activation. _Proc. Natl Acad. Sci. USA_ 93, 4750–4753 (1996). Article CAS PubMed PubMed Central Google Scholar * Garcia,
R., Musleh, W., Tocco, G., Thompson, R. F. & Baudry, M. Time-dependent blockade of STP and LTP in hippocampal slices following acute stress in mice. _Neurosci. Lett._ 233, 41–44 (1997).
Article CAS PubMed Google Scholar * Maggio, N. & Segal, M. Striking variations in corticosteroid modulation of long-term potentiation along the septotemporal axis of the hippocampus.
_J. Neurosci._ 27, 5757–5765 (2007). Article CAS PubMed PubMed Central Google Scholar * Kim, J. J. & Yoon, K. S. Stress: metaplastic effects in the hippocampus. _Trends Neurosci._
21, 505–509 (1998). Article CAS PubMed Google Scholar * Diamond, D. M., Park, C. R. & Woodson, J. C. Stress generates emotional memories and retrograde amnesia by inducing an
endogenous form of hippocampal LTP. _Hippocampus_ 14, 281–291 (2004). Article PubMed Google Scholar * Abraham, W. C. Stress-related phenomena. _Hippocampus_ 14, 675–676 (2004). Article
PubMed Google Scholar * Shors, T. J. & Thompson, R. F. Acute stress impairs (or induces) synaptic long-term potentiation (LTP) but does not affect paired-pulse facilitation in the
stratum-radiatum of rat hippocampus. _Synapse_ 11, 262–265 (1992). Article CAS PubMed Google Scholar * Sacchetti, B. et al. Long-lasting hippocampal potentiation and contextual memory
consolidation. _Eur. J. Neurosci._ 13, 2291–2298 (2001). Article CAS PubMed Google Scholar * Sacchetti, B., Scelfo, B., Tempia, F. & Strata, P. Long-term synaptic changes induced in
the cerebellar cortex by fear conditioning. _Neuron_ 42, 973–982 (2004). Article CAS PubMed Google Scholar * Sacchetti, B. et al. Time-dependent inhibition of hippocampal LTP _in vitro_
following contextual fear conditioning in the rat. _Eur. J. Neurosci._ 15, 143–150 (2002). Article PubMed Google Scholar * Kirkwood, A., Rioult, M. G. & Bear, M. F.
Experience-dependent modification of synaptic plasticity in visual cortex. _Nature_ 381, 526–528 (1996). Article CAS PubMed Google Scholar * Philpot, B. D., Espinosa, J. S. & Bear,
M. F. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. _J. Neurosci._ 23, 5583–5588 (2003). Article CAS PubMed PubMed Central Google Scholar *
Quinlan, E. M., Olstein, D. H. & Bear, M. F. Bidirectional, experience-dependent regulation of _N_-methyl-_D_-aspartate receptor subunit composition in the rat visual cortex during
postnatal development. _Proc. Natl Acad. Sci. USA_ 96, 12876–12880 (1999). Article CAS PubMed PubMed Central Google Scholar * Quinlan, E. M., Philpot, B. D., Huganir, R. L. & Bear,
M. F. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex _in vivo_. _Nature Neurosci._ 2, 352–357 (1999). Article CAS PubMed Google Scholar * Bellone, C.
& Nicoll, R. A. Rapid bidirectional switching of synaptic NMDA receptors. _Neuron_ 55, 779–785 (2007). Article CAS PubMed Google Scholar * Erreger, K., Dravid, S. M., Banke, T. G.,
Wyllie, D. J. A. & Traynelis, S. E. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. _J. Physiol._ 563, 345–358 (2005).
Article CAS PubMed PubMed Central Google Scholar * Philpot, B. D., Cho, K. K. A. & Bear, M. F. Obligatory role of NR2A for metaplasticity in visual cortex. _Neuron_ 53, 495–502
(2007). THIS WORK PROVIDED AN ELEGANT DEMONSTRATION THAT THE SUBUNIT COMPOSITION OF NMDARS IS CRITICAL FOR EXPERIENCE-DEPENDENT ALTERATIONS IN PLASTICITY THRESHOLDS IN THE VISUAL CORTEX.
Article CAS PubMed PubMed Central Google Scholar * Disterhoft, J. F., Golden, D. T., Read, H. R., Coulter, D. A. & Alkon, D. L. AHP reduction in rabbit hippocampal neurons during
conditioning correlate with acquisition of the learned response. _Brain Res._ 462, 118–125 (1988). Article CAS PubMed Google Scholar * Alkon, D. L. Voltage-dependent calcium and
potassium ion conductances: a contingency mechanism for an associative learning model. _Science_ 205, 810–816 (1979). Article CAS PubMed Google Scholar * Kandel, E. R. & Schwartz, J.
H. Molecular biology of learning: modulation of transmitter release. _Science_ 218, 433–443 (1982). Article CAS PubMed Google Scholar * Moyer, J. R. Jr, Thompson, L. T. &
Disterhoft, J. F. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. _J. Neurosci._ 16, 5536–5546 (1996). Article CAS PubMed Google
Scholar * Oh, M. M., Kuo, A. G., Wu, W. W., Sametsky, E. A. & Disterhoft, J. F. Watermaze learning enhances excitability of CA1 pyramidal neurons. _J. Neurophysiol._ 90, 2171–2179
(2003). Article PubMed Google Scholar * Saar, D., Grossman, Y. & Barkai, E. Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased
learning capability during operant conditioning. _Eur. J. Neurosci._ 10, 1518–1523 (1998). Article CAS PubMed Google Scholar * Cohen-Matsliah, S. I., Brosh, I., Rosenblum, K. &
Barkai, E. A novel role for extracellular signal-regulated kinase in maintaining long-term memory-relevant excitability changes. _J. Neurosci._ 27, 12584–12589 (2007). Article CAS PubMed
PubMed Central Google Scholar * Brosh, I., Rosenblum, K. & Barkai, E. Learning-induced modulation of SK channels-mediated effect on synaptic transmission. _Eur. J. Neurosci._ 26,
3253–3260 (2007). Article PubMed Google Scholar * Lebel, D., Grossman, Y. & Barkai, E. Olfactory learning modifies predisposition for long-term potentiation and long-term depression
induction in the rat piriform (olfactory) cortex. _Cereb. Cortex_ 11, 485–489 (2001). Article CAS PubMed Google Scholar * Quinlan, E. M., Lebel, D., Brosh, I. & Barkai, E. A
molecular mechanism for stabilization of learning-induced synaptic modifications. _Neuron_ 41, 185–192 (2004). Article CAS PubMed Google Scholar * Disterhoft, J. F. & Oh, M. M.
Learning, aging and intrinsic neuronal plasticity. _Trends Neurosci._ 29, 587–599 (2006). Article CAS PubMed Google Scholar * Zelcer, I. et al. A cellular correlate of learning-induced
metaplasticity in the hippocampus. _Cereb. Cortex_ 16, 460–468 (2006). THIS PAPER PROVIDED CLEAR EVIDENCE FOR A TYPE OF LEARNING-INDUCED METAPLASTICITY THAT PROMOTES SUBSEQUENT LEARNING OF
OTHER BEHAVIOURS, MEDIATED BY REGULATION OF THE SAHP. Article PubMed Google Scholar * Levenson, J. M. et al. Regulation of histone acetylation during memory formation in the hippocampus.
_J. Biol. Chem._ 279, 40545–40559 (2004). Article CAS PubMed Google Scholar * Levenson, J. M. et al. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the
hippocampus. _J. Biol. Chem._ 281, 15763–15773 (2006). Article CAS PubMed Google Scholar * Miller, C. A., Campbell, S. L. & Sweatt, J. D. DNA methylation and histone acetylation
work in concert to regulate memory formation and synaptic plasticity. _Neurobiol. Learn. Mem._ 17 Sep 2007 [epub ahead of print]. * Clem, R. L., Celikel, T. & Barth, A. L. Ongoing _in
vivo_ experience triggers synaptic metaplasticity in the neocortex. _Science_ 319, 101–104 (2008). Article CAS PubMed Google Scholar * Oddo, S. et al. Triple-transgenic model of
Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. _Neuron_ 39, 409–421 (2003). THIS PAPER PROVIDED AN IMPORTANT DEMONSTRATION THAT SENSORY
EXPERIENCE CAN CAUSE NMDAR-DEPENDENT LTP. THE LTP SATURATES OWING TO AN NMDAR-DEPENDENT INHIBITION OF ADDITIONAL MGLUR-MEDIATED POTENTIATION. Article CAS PubMed Google Scholar * Albensi,
B. C. & Janigro, D. Traumatic brain injury and its effects on synaptic plasticity. _Brain Inj._ 17, 653–663 (2003). Article PubMed Google Scholar * Schwarzbach, E., Bonislawski, D.
P., Xiong, G. & Cohen, A. S. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. _Hippocampus_ 16, 541–550 (2006). Article CAS PubMed
PubMed Central Google Scholar * Wang, S., Kee, N., Preston, E. & Wojtowicz, J. M. Electrophysiological correlates of neural plasticity compensating for ischemia-induced damage in the
hippocampus. _Exp. Brain Res._ 165, 250–260 (2005). Article PubMed Google Scholar * Goussakov, I. V., Fink, K., Elger, C. E. & Beck, H. Metaplasticity of mossy fiber synaptic
transmission involves altered release probability. _J. Neurosci._ 20, 3434–3441 (2000). Article CAS PubMed PubMed Central Google Scholar * Kleschevnikov, A. M. et al. Hippocampal
long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. _J. Neurosci._ 24, 8153–8160 (2004). Article CAS PubMed PubMed Central
Google Scholar * Huber, K. M., Gallagher, S. M., Warren, S. T. & Bear, M. F. Altered synaptic plasticity in a mouse model of fragile X mental retardation. _Proc. Natl Acad. Sci. USA_
99, 7746–7750 (2002). Article CAS PubMed PubMed Central Google Scholar * Picconi, B. et al. Pathological synaptic plasticity in the striatum: implications for Parkinson's disease.
_Neurotoxicology_ 26, 779–783 (2005). Article CAS PubMed Google Scholar * Kung, V. W. S., Hassam, R., Morton, A. J. & Jones, S. Dopamine-dependent long term potentiation in the
dorsal striatum is reduced in the R6/2 mouse model of Huntington's disease. _Neuroscience_ 146, 1571–1580 (2007). Article CAS PubMed Google Scholar * Turrigiano, G. G. & Nelson,
S. B. Homeostatic plasticity in the developing nervous system. _Nature Rev. Neurosci._ 5, 97–107 (2004). Article CAS Google Scholar * Thiagarajan, T. C., Lindskog, M. & Tsien, R. W.
Adaptation to synaptic inactivity in hippocampal neurons. _Neuron_ 47, 725–737 (2005). Article CAS PubMed Google Scholar * Slutsky, I., Sadeghpour, S., Li, B. & Liu, G. S.
Enhancement of synaptic plasticity through chronically reduced Ca2+ flux during uncorrelated activity. _Neuron_ 44, 835–849 (2004). Article CAS PubMed Google Scholar * Eid, T., Kovacs,
I., Spencer, D. D. & de Lanerolle, N. C. Novel expression of AMPA-receptor subunit GluR1 on mossy cells and CA3 pyramidal neurons in the human epileptogenic hippocampus. _Eur. J.
Neurosci._ 15, 517–527 (2002). Article PubMed Google Scholar * He, H. Y., Hodos, W. & Quinlan, E. M. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual
cortex. _J. Neurosci._ 26, 2951–2955 (2006). Article CAS PubMed PubMed Central Google Scholar * Hofer, S. B., Mrsic-Flogel, T. D., Bonhoeffer, T. & Hubener, M. Prior experience
enhances plasticity in adult visual cortex. _Nature Neurosci._ 9, 127–132 (2006). THIS IMPORTANT PAPER DEMONSTRATED THAT THE ADULT VISUAL CORTEX IS CAPABLE OF SUBSTANTIAL
EXPERIENCE-DEPENDENT PLASTICITY IF IT HAS PREVIOUSLY BEEN PRIMED BY BRIEF EXPOSURE TO ALTERED VISUAL EXPERIENCE. Article CAS PubMed Google Scholar * He, H.-Y., Ray, B., Dennis, K. &
Quinlan, E. M. Experience-dependent recovery of vision following chronic deprivation amblyopia. _Nature Neurosci._ 10, 1134–1136 (2007). THIS PAPER PROVIDED A CLINICALLY IMPORTANT
DEMONSTRATION THAT AMBLYOPIA THAT HAS BEEN INDUCED BY MD CAN BE REVERSED IF THE CORTEX IS FIRST PRIMED BY PUTTING ANIMALS IN A DARK ENVIRONMENT JUST PRIOR TO THE REVERSAL PROCEDURES. Article
CAS PubMed Google Scholar * Sale, A. et al. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. _Nature Neurosci._ 10,
679–681 (2007). Article CAS PubMed Google Scholar * Ullal, G. R., Ninchoji, T. & Uemura, K. Low frequency stimulation induces an increase in after-discharge thresholds in hippocampal
and amygdaloid kindling. _Epilepsy Res._ 3, 236–247 (1989). Article Google Scholar * Hesp, B. R., Clarkson, A. N., Sawant, P. M. & Kerr, D. S. Domoic acid preconditioning and seizure
induction in young and aged rats. _Epilepsy Res._ 76, 103–112 (2007). Article CAS PubMed Google Scholar * Gidday, J. M. Cerebral preconditioning and ischaemic tolerance. _Nature Rev.
Neurosci._ 7, 437–448 (2006). Article CAS Google Scholar * Schaller, B. & Graf, R. Cerebral ischemic preconditioning. _J. Neurol._ 249, 1503–1511 (2002). Article CAS PubMed Google
Scholar * Dirnagl, U., Simon, R. P. & Hallenbeck, J. M. Ischemic tolerance and endogenous neuroprotection. _Trends Neurosci._ 26, 248–254 (2003). Article CAS PubMed Google Scholar *
Thiagarajan, T. C., Lindskog, M., Malgaroli, A. & Tsien, R. W. LTP and adaptation to inactivity: overlapping mechanisms and implications for metaplasticity. _Neuropharmacology_ 52,
156–175 (2007). Article CAS PubMed Google Scholar * Fusi, S., Drew, P. J. & Abbott, L. F. Cascade models of synaptically stored memories. _Neuron_ 45, 599–611 (2005). THIS PAPER
DETAILED AN INNOVATIVE NETWORK MODEL THAT INCORPORATES MULTIPLE METAPLASTIC STATES INTO THE FUNCTIONALITY OF THE SYNAPSES SO AS TO PROLONG THE DURATION OF MEMORIES STORED IN THE NETWORK.
Article CAS PubMed Google Scholar * Fusi, S. & Abbott, L. F. Limits on the memory storage capacity of bounded synapses. _Nature Neurosci._ 10, 485–493 (2007). Article CAS PubMed
Google Scholar * Montgomery, J. M. & Madison, D. V. Discrete synaptic states define a major mechanism of synapse plasticity. _Trends Neurosci._ 27, 744–750 (2004). Article CAS PubMed
Google Scholar * Ward, B. et al. State-dependent mechanisms of LTP expression revealed by optical quantal analysis. _Neuron_ 52, 649–661 (2006). Article CAS PubMed Google Scholar *
Yang, X. D. & Faber, D. S. Initial synaptic efficacy influences induction and expression of long-term changes in transmission. _Proc. Natl Acad. Sci. USA_ 88, 4299–4303 (1991). Article
CAS PubMed PubMed Central Google Scholar * Barrionuevo, G., Schottler, F. & Lynch, G. The effects of repetitive low-frequency stimulation on control and 'potentiated'
synaptic responses in the hippocampus. _Life Sci._ 27, 2385–2391 (1980). Article CAS PubMed Google Scholar * Lee, H. K., Barbarosie, M., Kameyama, K., Bear, M. F. & Huganir, R. L.
Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. _Nature_ 405, 955–959 (2000). Article CAS PubMed Google Scholar * Benuskova, L. &
Abraham, W. STDP rule endowed with the BCM sliding threshold accounts for hippocampal heterosynaptic plasticity. _J. Comp. Neurosci._ 22, 129–133 (2007). Article Google Scholar *
Benuskova, L., Diamond, M. E. & Ebner, F. F. Dynamic synaptic modification threshold: computational model of experience-dependent plasticity in adult rat barrel cortex. _Proc. Natl Acad.
Sci. USA_ 91, 4791–4795 (1994). Article CAS PubMed PubMed Central Google Scholar * Izhikevich, E. M. & Desai, N. S. Relating STDP to BCM. _Neural Comput._ 15, 1511–1523 (2003).
Article PubMed Google Scholar * Bear, M. F., Cooper, L. N. & Ebner, F. F. A physiological basis for a theory of synapse modification. _Science_ 237, 42–48 (1987). Article CAS PubMed
Google Scholar * Shouval, H. Z., Bear, M. F. & Cooper, L. N. A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. _Proc. Natl Acad. Sci. USA_ 99, 10831–10836
(2002). Article CAS PubMed PubMed Central Google Scholar * Shouval, H. Z., Castellani, G. C., Blais, B. S., Yeung, L. C. & Cooper, L. N. Converging evidence for a simplified
biophysical model of synaptic plasticity. _Biol. Cybern._ 87, 383–391 (2002). Article PubMed Google Scholar * Yeung, L. C., Shouval, H. Z., Blais, B. S. & Cooper, L. N. Synaptic
homeostasis and input selectivity follow from a calcium-dependent plasticity model. _Proc. Natl Acad. Sci. USA_ 101, 14943–14948 (2004). Article CAS PubMed PubMed Central Google Scholar
Download references ACKNOWLEDGEMENTS Preparation of this Review was assisted by a James Cook Fellowship from the Royal Society of New Zealand. Metaplasticity research in the author's
laboratory has been supported by grants from the Health Research Council of New Zealand, the New Zealand Marsden Fund and the University of Otago Research Committee. I thank M. Bear for many
years of discussion and collaboration on metaplasticity topics. I thank D. Ireland, J. Wagner and E. Quinlan for comments on an earlier version of this manuscript. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Department of Psychology and the Brain Health and Repair Research Centre, University of Otago, BOX 56, Dunedin, 9054, New Zealand Wickliffe C. Abraham Authors *
Wickliffe C. Abraham View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Wickliffe C. Abraham. RELATED LINKS RELATED
LINKS DATABASES OMIM Alzheimer's disease Down syndrome Fragile X-linked mental retardation syndrome Huntington's disease Parkinson's disease FURTHER INFORMATION Wickliffe
Abraham's homepage GLOSSARY * Long-term potentiation (LTP). A long-lasting and activity-dependent increase in synaptic efficacy. Canonically it requires activation of the NMDAR subtype
of glutamate receptors; however, different forms of LTP caused by the activation of other receptor subtypes also occur. * Long-term depression (LTD). The converse of LTP: in LTD there is a
long-lasting and activity-dependent decrease in synaptic efficacy. * Excitotoxicity Cellular toxicity involving the activation of glutamate receptors in the CNS. Excessive activation of
these receptors by high concentrations of glutamate or by neurotoxins leads to cell death. * Tetanus A bout of HFS used to elicit activity-dependent synaptic plasticity. The frequency and
duration of the stimulation varies across protocols. * Uncaging The release of a molecule from a photolabile binding partner known as a cage. Cages typically inhibit the biological activity
of the bound ('caged') molecule. A brief flash of light of the appropriate wavelength can photochemically disrupt the structure of the binding partner and render the now uncaged
molecule biologically active. * Calcium/calmodulin-dependent protein kinase II (CaMKII). A multi-functional serine/threonine kinase that is activated by a Ca2+/calmodulin complex. Once
activated, CaMKII can autophosphorylate, leading to autonomous (Ca2+-independent) activity and calmodulin trapping. The α isoform is a major component of the postsynaptic density and a key
component of the LTP induction process. * Slow afterhyperpolarization (slow AHP). A type of membrane hyperpolarization that can last for seconds. It is mediated by the opening of
Ca2+-dependent K+ channels and is generated in response to the firing of one or more postsynaptic Na+ or Ca2+ action potentials. * G-protein-coupled receptors (GPCRs). A large family of
transmembrane receptors that couple extracellular signalling molecules to an intracellular signalling cascade which they trigger by activating a G protein. * Depotentiation A reversal of LTP
that brings synaptic efficacy to a baseline level. There is growing evidence that this process involves mechanisms that are different to those that mediate LTD. * Antidromic stimulation The
activation of neuronal cell bodies and dendrites by back-propagating action potentials triggered by electrical stimulation of the cells' axons. * Plasticity-related proteins (PRPs).
Proteins that are synthesized in response to synaptic activation or postsynaptic activity and that are necessary for establishing the persistent forms of LTP and LTD. * Back-propagating
action potentials Action potentials that are initiated at the soma or the axon hillock and that propagate back into the dendrites, where they shape the integration of synaptic activity and
influence the induction of synaptic plasticity. * Active zone A portion of the presynaptic membrane that faces the postsynaptic density across the synaptic cleft. It is the site of synaptic
vesicle clustering and docking and resultant neurotransmitter release. * Critical period A finite but modifiable developmental time window during which experience provides information that
is essential for normal development and permanently alters brain structure and performance. * Eye-blink conditioning A classical conditioning paradigm that is commonly used for the study of
learning. In it, an eye-blink, or the retraction of the nictitating membrane over the eye, is reflexively conditioned by pairing a conditioned neutral stimulus such as a tone with an
aversive stimulus such as an air-puff to the eye. After sufficient pairings the conditioned stimulus can elicit the eye-blink response by itself. * Memory consolidation A
protein-synthesis-dependent process of memory stabilization occuring over hours in animals and for up to years in humans that renders the memory resistant to change. * Amblyopia Poor vision,
usually occurring in one eye, that is associated with a prolonged period of indistinct visual stimulation or visual system dysfunction during development. * Ischaemic preconditioning (IPC).
A phenomenon observed both clinically and experimentally whereby a mild ischaemic event 'primes' a tissue by activating endogenous cellular protective mechanisms that amelioriate
the neurotoxic outcome of a later, more severe ischaemic event. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Abraham, W. Metaplasticity: tuning
synapses and networks for plasticity. _Nat Rev Neurosci_ 9, 387 (2008). https://doi.org/10.1038/nrn2356 Download citation * Issue Date: May 2008 * DOI: https://doi.org/10.1038/nrn2356 SHARE
THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative