Distinct profiles of anxiety and dysphoria during spontaneous withdrawal from acute morphine exposure
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The negative motivational aspects of withdrawal include symptoms of both anxiety and depression, and emerge after termination of chronic drug use as well as after acute drug
exposure. States of acute withdrawal are an inherent part of intermittent drug use in humans, but the contribution of acute withdrawal to the development of addiction has received limited
systematic investigation, because of a lack of preclinical models for withdrawal states that emerge spontaneously after acute drug exposure. Here, we have characterized a spontaneous
increase in the magnitude of the acoustic startle reflex (ie, spontaneous withdrawal-potentiated startle) that emerges after acute morphine administration in rats, and compared the time
course of startle potentiation and place conditioning. We find that startle potentiation seems to be related to a decrease in opiate receptor occupancy and reflects an anxiety-like state
with a pharmacological profile similar to other signs of opiate withdrawal. Spontaneous startle potentiation emerges before the rewarding effects of morphine have subsided, even though
naloxone administration after a single morphine exposure causes both startle potentiation and conditioned place aversion (CPA). These results show that negative emotional signs of withdrawal
develop after just one exposure to morphine, and are likely a recurrent aspect of intermittent drug use that may contribute to the earliest adaptations underlying the development of
addiction. Furthermore, the dissociation between spontaneous startle potentiation and CPA suggests anxiogenic and dysphoric manifestations of opiate withdrawal may be mediated by distinct
neural mechanisms that are progressively engaged as withdrawal unfolds. SIMILAR CONTENT BEING VIEWED BY OTHERS AN ENDOGENOUS OPIOID CIRCUIT DETERMINES STATE-DEPENDENT REWARD CONSUMPTION
Article 13 October 2021 OXYTOCINERGIC INPUT FROM THE PARAVENTRICULAR NUCLEUS TO THE NUCLEUS ACCUMBENS CORE MODULATES METHAMPHETAMINE-CONDITIONED PLACE PREFERENCE Article Open access 23 May
2025 MESOLIMBIC DOPAMINE RELEASE PRECEDES ACTIVELY SOUGHT AVERSIVE STIMULI IN MICE Article Open access 27 April 2023 INTRODUCTION The negative motivational aspects of withdrawal from chronic
drug exposure contribute to the maintenance of established drug addiction (Koob and Le Moal, 2008), but negative emotional states also emerge after acute drug exposure (Breiter et al, 1997;
Kirby and Stitzer, 1993; Van Dyke and Byck, 1982). These episodes of acute withdrawal are a recurrent and integral component of human drug use (Baker et al, 2004), emerging after occasional
drug use or when ongoing drug intake is interrupted by sleep or periods when drug supply is limited (Dole et al, 1966; Haertzen and Hooks, 1969). Alleviation of acute withdrawal may
motivate further drug use, and changes in neural activity during acute withdrawal could contribute to drug-induced alterations in physiology and brain function (Houshyar et al, 2003;
Houshyar et al, 2004). Withdrawal from acute opiate exposure can be precipitated by opiate receptor antagonists (for review, see Harris and Gewirtz, 2005), but in the context of human opiate
abuse, withdrawal emerges spontaneously in the absence of an antagonist. Despite this fact, surprisingly few preclinical models have been developed to study the spontaneous emergence of
withdrawal after acute opiate exposure. This study describes distinct profiles of anxiety and dysphoria in rats that emerge spontaneously after acute exposure to morphine. Withdrawal from
chronic drug use produces symptoms of both anxiety and depression, including restlessness, irritability, dysphoria, and anhedonia (American Psychiatric Association, 2000; Haertzen and Hooks,
1969). The acoustic startle reflex is a validated measure of anxiety in both animals and humans (Lang et al, 2000), and is elevated in rodents during spontaneous withdrawal from acute
morphine exposure (ie, withdrawal-potentiated startle; Harris and Gewirtz, 2004a). Other spontaneous signs of acute morphine withdrawal in rodents, including conditioned place aversion (CPA)
(Bechara et al, 1995) and increased thresholds for intracranial self-stimulation (ICSS) (Liu and Schulteis, 2004), may reflect states of dysphoria or anhedonia associated with depression
(Barr et al, 2002; Carlezon and Chartoff, 2007; Land et al, 2008b). Given the growing number of experimental dissociations between anxiety- and depression-like behavior in rodents (Bosch et
al, 2008; Land et al, 2008a; Nestler and Carlezon, 2006; Sahuque et al, 2006; Wallace et al, 2009), as well as distinctions between clinical disorders of anxiety and depression (American
Psychiatric Association, 2000; Goldberg, 2008; Kessler et al, 2008; Krueger, 1999), it is important to distinguish between these specific negative affective components of opiate withdrawal,
as they may not necessarily coincide with one another. To address these issues, we have further characterized spontaneous withdrawal-potentiated startle and compared its time course with
that of spontaneous CPA. We show that spontaneous withdrawal-potentiated startle seems to be related to a decrease in opiate receptor occupancy and has an anxiety-like pharmacological
profile that resembles other measures of opiate withdrawal. However, startle potentiation emerges while rats still exhibit conditioned place preference (CPP), showing an increase in
anxiety-like behavior before the rewarding effects of morphine have subsided. In contrast, withdrawal-potentiated startle and CPA develop concurrently when withdrawal is precipitated by
naloxone (an opiate receptor antagonist). These results indicate that anxiogenic and dysphoric manifestations of acute morphine withdrawal reflect changes in distinct neural systems. These
negative emotional states accompany the earliest stages of drug exposure, are likely a recurrent feature of intermittent drug use in humans, and thus may contribute significantly to the
development of addiction. MATERIALS AND METHODS SUBJECTS Male Sprague–Dawley rats (Harlan) were housed in groups of 4–5 in metal cages with a 12 h light–dark cycle (light on 0800–2000 hours)
and free access to food and water except during testing. Rats were allowed to acclimate to housing conditions for 2 weeks after arrival, were gently handled for two consecutive days before
any testing, and weighed 250–350 _g_ at the beginning of each experiment. All procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and
were approved by the University of Minnesota Institutional Animal Care and Use Committee. DRUGS Morphine sulfate was provided by the National Institute on Drug Abuse (Rockville, MD).
Naloxone, chlordiazepoxide, and R,S-propranolol hydrochlorides were obtained from Sigma (St. Louis, MO). LY235959 was obtained from Tocris (Ellisville, MO). All drugs were dissolved in 0.9%
saline (except propranolol, which was dissolved in water) and injected (i.p.) in a volume of 1 ml/kg body weight. Over the course of these studies, we shifted to s.c. administration of
morphine and naloxone to be consistent with the majority of other work in this field (Houshyar et al, 2003; Houshyar et al, 2004; Schulteis et al, 1994). We directly compared i.p. and s.c.
morphine injections in several experiments and found no significant differences between routes of administration (data not shown), so results from both routes of administration have been
pooled. All drug doses are expressed as the weight of the salt. ACOUSTIC STARTLE Acoustic startle was tested in four identical plastic cages (17 × 8.5 × 11 cm) resting on compression springs
and located within individual ventilated sound-attenuating chambers. Cage movement resulted in displacement of a piezoelectronic accelerometer (Model ACH-01, Measurement Specialties, Valley
Forge, PA) attached to each cage. Voltage output from the accelerometer was filtered and amplified by a custom-built signal processor, digitized on a scale of arbitrary units ranging from
0–1000 (National Instruments SCB100 and PCI-6071E boards), and recorded using Matlab (The MathWorks, Natick, MA). Startle amplitude was defined as the peak accelerometer voltage during the
first 200 ms after onset of the startle stimulus. High-frequency speakers (Radio Shack Supertweeters, range=5–40 kHz) located 10 cm beside each cage delivered the startle stimuli, which were
50-ms bursts of filtered white noise (low pass: 22 kHz, rise-decay <5 ms) at intensities of 95 or 105 dB. Ventilating fans elevated background noise to ∼60 dB. Acoustic startle was
tested on each of 2 days before drug exposure. For each test session, rats were placed in the startle chambers for a 5-min acclimation period, and then presented with 40 startle stimuli (20
each at 95 or 105 dB in semi-random order) with a 30-s inter-stimulus interval. Average startle amplitudes from the second day were used to match animals into experimental groups with
similar overall mean startle amplitudes. Each day of drug testing began with a baseline startle session before any drug injections. Several experiments involved startle testing over multiple
days using Latin Square or crossover designs; details are provided in figure legends. All drug injections were given in the colony, and rats remained in their home cage between drug
injections and startle tests. PLACE CONDITIONING Our place conditioning apparatus and procedure were developed according to published recommendations (Bardo and Bevins, 2000; Carlezon, 2003;
Cunningham et al, 2006). The apparatus consists of a rectangular plastic cage (40 × 20 × 20 cm) divided into two sides by a central partition. Each side has a distinct floor texture and
wall color: metal bars paired with white walls, and wire mesh paired with black striped walls. Each rat's position within the apparatus was monitored by an overhead video camera
connected to a computer running AnyMaze software (Stoelting, Wood Dale, IL). Rats were transported to the place conditioning room and allowed to acclimate for at least 10 min before every
experimental session. Each experiment began with a 10-min baseline session in which rats were free to move between both sides of the apparatus. The rats used in these studies spent an
average of 320 s (53.4%) on the bar side during the baseline session; two rats with >75% baseline preference for one side were excluded from further study. The side of the apparatus
paired with drug treatment was counterbalanced within each experiment, yielding an unbiased procedure in which rats spend ∼50% of the baseline session on the side to be paired with drug.
Daily conditioning sessions began 24 h after the baseline session. Details concerning the number, duration, and order of conditioning sessions are provided in figure legends. Twenty-four
hours after the last conditioning session, a 10-min test session was conducted in which rats were free to move between both sides of the apparatus. We chose to express place conditioning
results in terms of percent time spent on the drug-paired side, rather than using a difference score measured in seconds, because percentage measures are relatively independent of the length
of the testing session and thus facilitate comparisons across studies. DATA ANALYSIS Startle data were collapsed across both intensities (95/105 dB) before statistical analysis (Harris and
Gewirtz, 2004a), as the magnitude of withdrawal-potentiated startle was not affected by stimulus intensity (data not shown). In each experiment, we first conducted one-way analysis of
variance (ANOVA) to verify similar baseline startle amplitude between experimental groups; there were no differences in baseline startle between groups in any experiment (data not shown).
Changes in startle after morphine administration were calculated as percent change from baseline on the same day (Walker and Davis, 2002b). For experiments that utilized a crossover design,
baseline startle amplitude was similar on both days of testing, so an average baseline value was used to calculate percent change on each individual day. An area under the curve measure for
total withdrawal severity was calculated for each individual subject by adding together percent change in startle across all time points tested; mean and standard error were then calculated
for all subjects in each group. All data were analyzed using factorial ANOVA, with repeated measures on within-subject factors. For main effects or interactions involving repeated measures,
the Huynh–Feldt correction was applied to control for potential violations of the sphericity assumption. Significant interactions were followed with tests for simple effects (Keppel and
Wickens, 2004). When appropriate, significant main effects were followed with polynomial trend analysis. All statistical analysis was conducted using SPSS (version 13.0) with a Type I error
rate of _α_=0.05 (two-tailed). Group sizes for each experiment are indicated in figure legends. RESULTS STARTLE TIME COURSE DURING SPONTANEOUS AND NALOXONE-PRECIPITATED WITHDRAWAL Startle
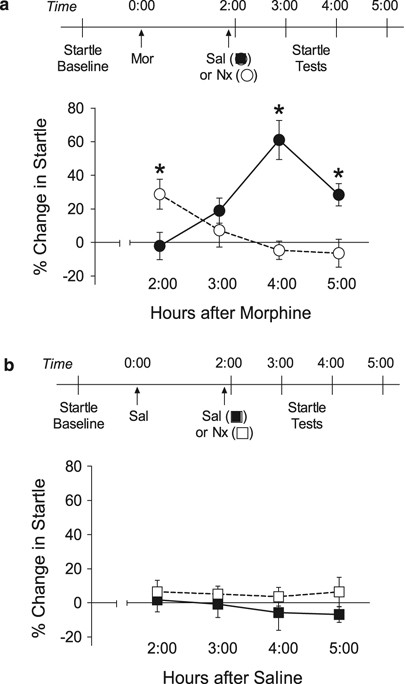
was tested 2–5 h after acute administration of morphine (10 mg/kg), with some rats receiving naloxone (2.5 mg/kg) just before the 2:00 startle test (Figure 1a). Startle was significantly
potentiated 4–5 h after injection of morphine alone, consistent with our earlier report that also showed startle returns to baseline 6 h after this dose of morphine (Harris and Gewirtz,
2004a). Naloxone caused an immediate but transient potentiation of startle at 2:00, with no change from baseline 3–5 h after morphine injection (Naloxone × time interaction: F3,78=12.19,
_p_<0.001). This dose of naloxone was selected based on our earlier work (Harris et al, 2004b) in an effort to completely displace morphine from the opiate receptor. There were no
significant changes in startle after injection of saline or naloxone alone (Figure 1b) (Naloxone × time interaction, F3,66 <1). It is noteworthy that the peak magnitude of spontaneous
withdrawal (4:00: 61.0±11.6%) was significantly larger than the peak magnitude of naloxone-precipitated withdrawal (2:00: 29.8±9.0%) (_t_28=2.23, _p_=0.034). This difference was more
pronounced when comparing total withdrawal severity, measured as area under the curve across all time points tested (spontaneous: 102.2±19.5%; precipitated: 24.9±21.0%) (_t_28=2.67,
_p_=0.013]. The difference in total withdrawal severity was partly driven by the absence of spontaneous startle potentiation at later time points after naloxone administration (Figure 1b).
MORPHINE RE-EXPOSURE DELAYS STARTLE POTENTIATION If startle potentiation represents a withdrawal effect, it should be blocked by re-exposure to morphine (Figure 2a). Indeed, the startle
potentiation normally observed 4 h after an initial morphine injection was prevented by a second injection of morphine 3 h after initial injection (Figure 2b) (initial injection × second
injection interaction: F1,32=11.07, _p_=0.002). We conducted a second startle test in the same animals 7 h after the initial injection (Figure 2c). Both groups that received a second
injection of morphine (ie, 4 h earlier) showed significant startle potentiation at this time (main effect of second injection: F1,32=76.11, _p_<0.001; interaction: F1,32 <1). Thus,
morphine re-exposure does not prevent startle potentiation, but delays its onset until opiate receptor occupancy eventually decreases. PHARMACOLOGICAL PROFILE OF SPONTANEOUS
WITHDRAWAL-POTENTIATED STARTLE We next examined whether anxiolytic drugs attenuate spontaneous withdrawal-potentiated startle. Both chlordiazepoxide (a benzodiazepine) and propranolol (a
β-adrenergic receptor antagonist) prevent other forms of startle potentiation (Walker and Davis, 2002a) at the same doses used here (10 mg/kg), and also decrease anxiety-like behavior in a
number of other behavioral paradigms (Cole and Koob, 1988; Harris and Aston-Jones, 1993a, 1993b; Knoll et al, 2007; Rodriguez-Romaguera et al, 2009). Administration of chlordiazepoxide
prevented spontaneous withdrawal-potentiated startle (Figure 3a) (morphine × chlordiazepoxide interaction: F1,30=5.49, _p_=0.026), as did administration of propranolol (Figure 3b) (morphine
× propranolol interaction: F1,20=5.91, _p_=0.025). Neither anxiolytic drug affected startle amplitude in the absence of morphine. NMDA receptor antagonists also prevent signs of opiate
withdrawal in rodents (Harris et al, 2008; Kawasaki et al, 2005; Rasmussen, 1995). We examined the effects of LY235959, a competitive NMDA receptor antagonist shown to attenuate precipitated
morphine withdrawal (Jones et al, 2002), using doses (1–3 mg/kg) that prevent tolerance to morphine analgesia (Bilsky et al, 1996) and sensitization to morphine-induced locomotion (Mendez
and Trujillo, 2008). LY235959 produced a dose-dependent attenuation of startle potentiation (Figure 4) (morphine × LY235959 interaction: F2,33=14.27, _p_<0.001). There was a significant
linear effect of LY235959 dose after morphine injection (_p_=0.012), but no effect after saline injection (_p_=0.56). STARTLE POTENTIATION AFTER THE FIRST MORPHINE EXPOSURE As the preceding
experiments utilized Latin Square and crossover designs, sometimes involving multiple exposures to morphine, we sought to clarify whether startle was potentiated after the very first
exposure to morphine. We pooled control data from the preceding experiments in which startle was tested 4 h after an animal's first exposure to morphine (_n_=33) or saline (_n_=31), and
found a highly reliable potentiation of startle after morphine injection (51.2±6.6%) that was significantly greater than the change in startle after saline injection (5.3±4.6%; _t_62=5.62,
_p_<0.001). PLACE CONDITIONING TIME COURSE AFTER ACUTE MORPHINE EXPOSURE A delayed CPA has been reported after acute exposure to morphine (Bechara et al, 1995). We next determined the
time course of place conditioning after injection of 10-mg/kg morphine (Figure 5). Each group spent ∼50% time on the drug side during the baseline session, confirming the unbiased nature of
our place conditioning procedure. ANOVA indicated a significant session × time interaction (F6,87=4.89, _p_<0.001). As expected, CPP was observed immediately after morphine injection
(0:00) (_t_11=2.85, _p_=0.016), and was maintained at 2:00 (_t_11=4.29, _p_=0.001) and 4:00 (_t_10=3.70, _p_=0.004). The 4:00 time point is when we observe peak startle potentiation after
this same dose of morphine (cf. Figure 1), indicating that rats are still experiencing a state of reward when anxiety-like behavior emerges. There was no effect of place conditioning at
6:00, and a non-significant tendency for CPA at 8:00 (_t_22=1.46, _p_=0.16). A trend toward CPP was also observed at 10:00 (_t_11=2.18, _p_=0.052). There was a significant fit to
fourth-order polynomial trend across time (_p_=0.003), suggesting the emergence of aversion after the initial preference. PLACE CONDITIONING AND STARTLE DURING NALOXONE-PRECIPITATED
WITHDRAWAL Because spontaneous withdrawal-potentiated startle and CPA emerged at different times after acute morphine injection, we asked whether these two behavioral effects could be
dissociated under other conditions. As 2.5-mg/kg naloxone produces startle potentiation when administered 2 h after 10-mg/kg morphine (cf. Figure 1), we examined whether naloxone causes CPA
under these same conditions (Figure 6a). ANOVA indicated a significant session × group interaction (F2,28=10.04, _p_=0.001). Exposure to morphine alone caused CPP (_t_8=2.49, _p_=0.038),
whereas naloxone had no effect in the absence of morphine (_t_7 <1). However, naloxone administration 2 h after morphine caused CPA (_t_13=3.90, _p_=0.002). Earlier studies have shown
that naloxone still causes CPA when administered 24 h after a single morphine injection (Araki et al, 2004; Parker and Joshi, 1998), and we also replicated this effect (Figure 6b). At the 24
h time point, ANOVA indicated a trend towards a session × group interaction (F2,44=2.15, _p_=0.13). Planned comparisons showed that administration of morphine followed by naloxone caused
CPA (_t_14=2.47, _p_=0.027), whereas there was no effect of either morphine alone (_t_15 <1) or naloxone alone (_t_15 <1). The effect of naloxone on acoustic startle has not been
examined 24 h after a single exposure to morphine. We found that naloxone still produced startle potentiation 24 h after a single morphine injection (Figure 7) (_t_10=2.57, _p_=0.028),
similar to its effect 2 h after acute morphine injection. Startle potentiation and CPA thus develop concurrently when naloxone is administered either 2 or 24 h after a single morphine
injection. DISCUSSION Our results show that anxiety-like behavior (ie, startle potentiation) emerges spontaneously after a single exposure to morphine, seems to be related to a decrease in
opiate receptor occupancy, and shares a pharmacological profile with other forms of opiate withdrawal. Startle potentiation develops before the rewarding effects of morphine have subsided,
clearly dissociating increased anxiety-like behavior from decreased reward system activity. This study represents the first direct demonstration that anxiogenic and dysphoric manifestations
of opiate withdrawal may be mediated by distinct neural mechanisms, which are progressively engaged during withdrawal after acute exposure to morphine. THE NATURE OF SPONTANEOUS
WITHDRAWAL-POTENTIATED STARTLE Spontaneous withdrawal-potentiated startle emerged and peaked 4 h after injection of 10-mg/kg morphine, consistent with our earlier report (Harris and Gewirtz,
2004a). This corresponds to a time at which morphine levels in blood and brain have declined substantially (Barjavel et al, 1995; Hipps et al, 1976), and the direct behavioral and
neurochemical effects of morphine have already peaked and are returning to baseline (Babbini and Davis, 1972; Barjavel et al, 1995; Di Chiara and Imperato, 1988; Hipps et al, 1976). This
suggests startle elevation emerges as morphine metabolism leads to falling drug levels and reduced opiate receptor occupancy. Startle was potentiated by naloxone administration 2 h after
morphine, whereas morphine re-exposure delayed the onset of startle potentiation, suggesting a link between startle potentiation and decreased opiate receptor occupancy. Spontaneous
withdrawal-potentiated startle was also blocked by chlordiazepoxide and propranolol, two anxiolytic drugs shown earlier to attenuate increases in startle amplitude caused by conditioned fear
cues and exposure to bright light (de Jongh et al, 2002; Risbrough et al, 2003; Walker and Davis, 2002a). Chlordiazepoxide prevents other forms of anxiety-like behavior in rodents (eg,
Knoll et al, 2007), whereas propranolol has been shown to reduce affective signs of opiate withdrawal (Harris and Aston-Jones, 1993a, 1993b). NMDA receptor antagonists also alleviate signs
of opiate withdrawal (Harris et al, 2008; Kawasaki et al, 2005; Rasmussen, 1995). LY235959, a competitive NMDA receptor antagonist that reduces precipitated morphine withdrawal (Jones et al,
2002), produced a dose-dependent attenuation of startle potentiation. These results clearly indicate that startle elevation shares a pharmacological profile with other measures of opiate
withdrawal. RELATIONSHIP BETWEEN STARTLE POTENTIATION AND PLACE CONDITIONING A delayed CPA has been observed after administration of morphine (Bechara et al, 1995), as well as other opiates
(Pain et al, 2008) and other addictive drugs (Ettenberg and Bernardi, 2007; Morse et al, 2000; Pliakas et al, 2001). We assessed the time course of place conditioning after administration of
10-mg/kg morphine, and found that CPP persisted up to 4 h after morphine injection (see also White et al, 2005). This time course closely parallels the elevation of extracellular dopamine
levels in the nucleus accumbens (Di Chiara and Imperato, 1988), consistent with the role of nucleus accumbens dopamine in generating morphine CPP (Fenu et al, 2006). A tendency for CPA
emerged 8 h after morphine injection. Other studies have reported robust CPA 11–16 h after acute exposure to 20-mg/kg morphine (Bechara et al, 1995; Vargas-Perez et al, 2007), which is
likely related to the differences in morphine dose and the number and timing of conditioning sessions. There was a significant fourth-order polynomial trend across time, suggesting the acute
rewarding effects of morphine were followed by delayed aversive effects. We speculate that the tendency toward CPP at 10 h could reflect alleviation of an aversive withdrawal state. We were
surprised to find CPP 4 h after 10-mg/kg morphine, the same time point at which we observed peak startle potentiation. To determine whether a similar dissociation was observed under other
conditions, we examined the effect of naloxone on acoustic startle and place conditioning at different times after a single morphine injection. We found that injection of 2.5-mg/kg naloxone
generated CPA (as well as startle potentiation) 2 h after morphine injection. These results are consistent with human studies showing that naloxone can precipitate withdrawal symptoms as
soon as 45 min after acute morphine administration (Heishman et al, 1989). Several signs of withdrawal (Eisenberg, 1982; Gellert and Sparber, 1977), including CPA (Araki et al, 2004; Parker
and Joshi, 1998), are still observed when naloxone is administered 24–48 h after a single exposure to morphine. We observed both startle potentiation and CPA when naloxone was administered
24 h after one morphine injection, showing an additional similarity between startle potentiation and other measures of withdrawal. A dissociation between the emergence of startle
potentiation and CPA was only observed when withdrawal was allowed to unfold spontaneously. These results provide an important example in which antagonist-precipitated withdrawal does not
precisely recapitulate the conditions of spontaneous withdrawal. Precipitated withdrawal is a useful experimental tool for controlling the timing of withdrawal and studying states of
dependence. However, in the context of human opiate abuse, withdrawal develops spontaneously in the absence of an opiate receptor antagonist. Our findings highlight the importance of further
developing models of spontaneous opiate withdrawal in rodents, to examine potential similarities and distinctions between spontaneous and precipitated withdrawal states. In future studies,
it will be important to examine the opiate receptor subtypes mediating different behavioral changes during spontaneous and precipitated withdrawal from acute morphine exposure. Specific
antagonists of the mu-opioid receptor (MOR) can precipitate signs of withdrawal after chronic morphine exposure (Le Guen et al, 2003; Maldonado et al, 1992), suggesting that loss of MOR
occupancy may cause spontaneous withdrawal. However, morphine also has a lower affinity for the kappa-opioid receptor (KOR) (Goldstein and Naidu, 1989), and some effects of morphine can be
mediated by KOR activation (Nobre et al, 2000; Sante et al, 2000; Yamada et al, 2006). As KOR agonists produce signs of anxiety, dysphoria, and anhedonia in humans and rodents (Land et al,
2008b; Motta et al, 1995; Nestler and Carlezon, 2006; Pfeiffer et al, 1986; Sante et al, 2000; Shippenberg et al, 2007), signaling cascades triggered by KOR activation could also contribute
to spontaneous withdrawal. On the other hand, KOR antagonists can in some cases exacerbate the severity of opiate withdrawal (Spanagel et al, 1994). The potential contributions of MOR and
KOR could be addressed in future studies using techniques that directly measure receptor occupancy, such as autoradiography, to examine changes in the occupancy of MOR and KOR across
multiple brain structures in the hours after acute morphine administration. POTENTIAL NEURAL SUBSTRATES FOR ANXIETY AND DYSPHORIA During spontaneous withdrawal from acute morphine exposure,
startle potentiation emerges before the rewarding effects of morphine have subsided, and thus before the onset of CPA. Our data suggest that startle potentiation may be caused by decreased
opiate receptor occupancy. In contrast, CPA may instead represent an opponent process to the acute rewarding effects of morphine (Vargas-Perez et al, 2007). As such, CPA may reflect a
decrease below baseline activity in the mesolimbic dopamine system. This type of change has been shown during withdrawal from chronic opiate exposure (Diana et al, 1995; Rossetti et al,
1992; Spanagel et al, 1994), as well as acute amphetamine exposure (Barr et al, 2002), although we are not aware of similar studies performed after acute morphine administration. Adaptations
within the reward system are thought to play a role in depression (Nestler and Carlezon, 2006), and depression-like changes during opiate withdrawal may be manifested as CPA (Vargas-Perez
et al, 2007) and elevated ICSS thresholds (Liu and Schulteis, 2004). In contrast, startle potentiation emerges while the reward system is still active, but its activity has decreased from
peak levels (Di Chiara and Imperato, 1988). This suggests the anxiety-like manifestations of opiate withdrawal may be closely tied to a relative decrease in hedonic state (ie, a negative
slope), rather than an absolute decrease below baseline. Portions of the extended amygdala, including the bed nucleus of the stria terminalis and central nucleus of the amygdala, play a
general role in states of withdrawal (Koob and Le Moal, 2008) and anxiety (Walker et al, 2003), and are specifically involved in antagonist-precipitated withdrawal from acute morphine
administration (Cabral et al, 2009; Criner et al, 2007; Harris et al, 2006). This involvement could result from a direct and local effect of morphine, or may be secondary to morphine-induced
elevations in extracellular dopamine (Carboni et al, 2000). The specific role of these circuits in different emotional manifestations of spontaneous withdrawal will be an important topic
for future research. This framework may help explain why baseline startle amplitude is not elevated during precipitated withdrawal from chronic opiate administration (Fendt and Mucha, 2001;
Kalinichev and Holtzman, 2003; Mansbach et al, 1992). These conditions produce dramatic decreases in mesolimbic dopamine system activity (Diana et al, 1995; Pothos et al, 1991; Rossetti et
al, 1992) and brain reward function (Schulteis et al, 1994). The resulting depression-like state could obscure or overwhelm the expression of anxiogenic manifestations of withdrawal. Indeed,
human patients diagnosed with depressive illness do not exhibit increases in startle amplitude under conditions that normally produce startle potentiation in control subjects (Dichter and
Tomarken, 2008; Forbes et al, 2005; Lang and McTeague, 2009). IMPLICATIONS FOR ADDICTION Our results clearly indicate that withdrawal is a complex and multifaceted construct. We have
described distinct time courses for two specific emotional manifestations of acute withdrawal (ie, startle potentiation and CPA). The spontaneous evolution of other signs of acute withdrawal
may parallel one of the time courses we have described, or may follow other unique time courses. For example, spontaneous increases in ICSS threshold have been reported 24 h after acute
morphine exposure (Liu and Schulteis, 2004). We also note that spontaneous hyperalgesia has been observed after a single exposure to heroin (Laulin et al, 1998) or morphine (Sweitzer et al,
2004). The time course of hyperalgesia is complex, as it emerges and dissipates in the hours after opiate administration, then reappears 24 h later and lasts several days (Laulin et al,
1998). Thus, the various emotional and physical manifestations of spontaneous withdrawal likely result from a cascade of numerous neurobiological events, which develop and evolve as a
function of time. It is important to note that anxiety-like signs of spontaneous withdrawal may represent one of the earliest manifestations of the withdrawal syndrome, developing before CPA
and changes in ICSS threshold. As spontaneous startle potentiation was delayed by re-exposure to morphine, relief or prevention of anxiety may be particularly important for motivating
continued drug use. The relief of anxiety states may provide primary negative reinforcement for ongoing drug use, perhaps by maintaining dopaminergic tone within the extended amygdala, as D1
receptor antagonism in the amygdala can enhance cocaine intake, even while dopamine levels in NAc remain elevated (Hurd et al, 1997). In addition, anxiety-like states may motivate drug use
because they predict the subsequent emergence of depression-like states, thus serving as secondary negative reinforcers. Finally, anxiety-like states generated by stressful experience could
also contribute to stress-induced relapse, particularly given the common neural circuitry involved in stress-induced reinstatement (Shaham et al, 2003) and potentiated acoustic startle
(Walker et al, 2003). Events that occur during spontaneous withdrawal may also contribute to some of the unique effects of intermittent opiate exposure. For example, intermittent injections
of morphine produce physiological changes similar to those caused by chronic stress (Houshyar et al, 2003; Houshyar et al, 2004), changes not observed when morphine is administered
continuously. The acute withdrawal state that follows each intermittent morphine injection may contribute to this stress-like profile. Indeed, changes in brain activity during spontaneous
withdrawal could contribute to any difference between the consequences of continuous and intermittent opiate exposure. As human drug abuse is routinely interrupted by drug-free periods
(Baker et al, 2004; Dole et al, 1966), it will be important to examine whether events that occur during spontaneous withdrawal contribute to adaptations in brain function during intermittent
drug exposure. These results add to a growing number of dissociations between anxiety- and depression-like behavior under a variety of experimental conditions (Bosch et al, 2008; Land et
al, 2008a; Nestler and Carlezon, 2006; Sahuque et al, 2006; Wallace et al, 2009), and raise important considerations for future research. First, signs of withdrawal develop spontaneously
after just one exposure to morphine, and are likely expressed after each intermittent exposure to an opiate. This means withdrawal is not unique to the termination of chronic drug use, but
is an intrinsic component of drug taking that may play an important but often neglected role in the development of addiction. Second, anxiety-like manifestations of withdrawal emerge while
the animal is still experiencing a state of reward. As dysphoria and other depression-like manifestations of withdrawal likely reflect decreases below baseline in reward system activity,
symptoms of anxiety and depression may develop at different times as withdrawal unfolds, which could have important treatment implications. Therapeutic interventions that ameliorate symptoms
of anxiety and depression, such as KOR antagonists (Knoll et al, 2007; Land et al, 2008a; Land et al, 2008b; Nestler and Carlezon, 2006), may prove particularly effective. A clearer
understanding of the neurobiological underpinnings of opiate withdrawal could potentially advance our understanding of mood and anxiety disorders, in addition to improving treatment of
addiction itself. REFERENCES * American Psychiatric Association (2000). _Diagnostic and statistical manual of mental disorders : DSM-IV-TR_, 4th edn. American Psychiatric Association:
Washington, DC, xxxvii, 943 p.pp. * Araki H, Kawakami KY, Jin C, Suemaru K, Kitamura Y, Nagata M _et al_ (2004). Nicotine attenuates place aversion induced by naloxone in single-dose,
morphine-treated rats. _Psychopharmacology (Berl)_ 171: 398–404. Article CAS Google Scholar * Babbini M, Davis WM (1972). Time-dose relationships for locomotor activity effects of
morphine after acute or repeated treatment. _Br J Pharmacol_ 46: 213–224. Article CAS PubMed PubMed Central Google Scholar * Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC
(2004). Addiction motivation reformulated: an affective processing model of negative reinforcement. _Psychol Rev_ 111: 33–51. Article PubMed Google Scholar * Bardo MT, Bevins RA (2000).
Conditioned place preference: what does it add to our preclinical understanding of drug reward? _Psychopharmacology (Berl)_ 153: 31–43. Article CAS Google Scholar * Barjavel MJ,
Scherrmann JM, Bhargava HN (1995). Relationship between morphine analgesia and cortical extracellular fluid levels of morphine and its metabolites in the rat: a microdialysis study. _Br J
Pharmacol_ 116: 3205–3210. Article CAS PubMed PubMed Central Google Scholar * Barr AM, Markou A, Phillips AG (2002). A ‘crash’ course on psychostimulant withdrawal as a model of
depression. _Trends Pharmacol Sci_ 23: 475–482. Article CAS PubMed Google Scholar * Bechara A, Nader K, van der Kooy D (1995). Neurobiology of withdrawal motivation: evidence for two
separate aversive effects produced in morphine-naive _vs_ morphine-dependent rats by both naloxone and spontaneous withdrawal. _Behav Neurosci_ 109: 91–105. Article CAS PubMed Google
Scholar * Bilsky EJ, Inturrisi CE, Sadee W, Hruby VJ, Porreca F (1996). Competitive and non-competitive NMDA antagonists block the development of antinociceptive tolerance to morphine, but
not to selective mu or delta opioid agonists in mice. _Pain_ 68: 229–237. Article CAS PubMed Google Scholar * Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ (2008). The CRF system
mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. _Neuropsychopharmacology_ 34: 1406–1415. Article PubMed CAS Google Scholar
* Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD _et al_ (1997). Acute effects of cocaine on human brain activity and emotion. _Neuron_ 19: 591–611. Article CAS
PubMed Google Scholar * Cabral A, Ruggiero RN, Nobre MJ, Brandao ML, Castilho VM (2009). GABA and opioid mechanisms of the central amygdala underlie the withdrawal-potentiated startle from
acute morphine. _Prog Neuropsychopharmacol Biol Psychiatry_ 33: 334–344. Article CAS PubMed Google Scholar * Carboni E, Silvagni A, Rolando MT, Di Chiara G (2000). Stimulation of _in
vivo_ dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. _J Neurosci_ 20: RC102. Article CAS PubMed PubMed Central Google Scholar * Carlezon Jr WA
(2003). Place conditioning to study drug reward and aversion. _Methods Mol Med_ 84: 243–249. CAS PubMed Google Scholar * Carlezon Jr WA, Chartoff EH (2007). Intracranial self-stimulation
(ICSS) in rodents to study the neurobiology of motivation. _Nat Protoc_ 2: 2987–2995. Article CAS PubMed Google Scholar * Cole BJ, Koob GF (1988). Propranolol antagonizes the enhanced
conditioned fear produced by corticotropin releasing factor. _J Pharmacol Exp Ther_ 247: 902–910. CAS PubMed Google Scholar * Criner SH, Liu J, Schulteis G (2007). Rapid neuroadaptation
in the nucleus accumbens and bed nucleus of the stria terminalis mediates suppression of operant responding during withdrawal from acute opioid dependence. _Neuroscience_ 144: 1436–1446.
Article CAS PubMed Google Scholar * Cunningham CL, Gremel CM, Groblewski PA (2006). Drug-induced conditioned place preference and aversion in mice. _Nat Protoc_ 1: 1662–1670. Article
CAS PubMed Google Scholar * de Jongh R, Groenink L, van Der Gugten J, Olivier B (2002). The light-enhanced startle paradigm as a putative animal model for anxiety: effects of
chlordiazepoxide, flesinoxan and fluvoxamine. _Psychopharmacology (Berl)_ 159: 176–180. Article CAS Google Scholar * Di Chiara G, Imperato A (1988). Drugs abused by humans preferentially
increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. _Proc Natl Acad Sci USA_ 85: 5274–5278. Article CAS PubMed PubMed Central Google Scholar *
Diana M, Pistis M, Muntoni A, Gessa G (1995). Profound decrease of mesolimbic dopaminergic neuronal activity in morphine withdrawn rats. _J Pharmacol Exp Ther_ 272: 781–785. CAS PubMed
Google Scholar * Dichter GS, Tomarken AJ (2008). The chronometry of affective startle modulation in unipolar depression. _J Abnorm Psychol_ 117: 1–15. Article PubMed PubMed Central
Google Scholar * Dole VP, Nyswander ME, Kreek MJ (1966). Narcotic blockade. _Arch Intern Med_ 118: 304–309. Article CAS PubMed Google Scholar * Eisenberg RM (1982). Further studies on
the acute dependence produced by morphine in opiate naive rats. _Life Sci_ 31: 1531–1540. Article CAS PubMed Google Scholar * Ettenberg A, Bernardi RE (2007). Effects of buspirone on the
immediate positive and delayed negative properties of intravenous cocaine as measured in the conditioned place preference test. _Pharmacol Biochem Behav_ 87: 171–178. Article CAS PubMed
PubMed Central Google Scholar * Fendt M, Mucha RF (2001). Anxiogenic-like effects of opiate withdrawal seen in the fear-potentiated startle test, an interdisciplinary probe for
drug-related motivational states. _Psychopharmacology (Berl)_ 155: 242–250. Article CAS Google Scholar * Fenu S, Spina L, Rivas E, Longoni R, Di Chiara G (2006). Morphine-conditioned
single-trial place preference: role of nucleus accumbens shell dopamine receptors in acquisition, but not expression. _Psychopharmacology (Berl)_ 187: 143–153. Article CAS Google Scholar
* Forbes EE, Miller A, Cohn JF, Fox NA, Kovacs M (2005). Affect-modulated startle in adults with childhood-onset depression: relations to bipolar course and number of lifetime depressive
episodes. _Psychiatry Res_ 134: 11–25. Article PubMed Google Scholar * Gellert VF, Sparber SB (1977). A comparison of the effects of naloxone upon body weight loss and suppression of
fixed-ratio operant behavior in morphine-dependent rats. _J Pharmacol Exp Ther_ 201: 44–54. CAS PubMed Google Scholar * Goldberg D (2008). Towards DSM-V: the relationship between
generalized anxiety disorder and major depressive episode. _Psychol Med_ 38: 1671–1675. Article CAS PubMed Google Scholar * Goldstein A, Naidu A (1989). Multiple opioid receptors: ligand
selectivity profiles and binding site signatures. _Mol Pharmacol_ 36: 265–272. CAS PubMed Google Scholar * Haertzen CA, Hooks Jr NT (1969). Changes in personality and subjective
experience associated with the chronic administration and withdrawal of opiates. _J Nerv Ment Dis_ 148: 606–614. Article CAS PubMed Google Scholar * Harris AC, Atkinson DM, Aase DM,
Gewirtz JC (2006). Double dissociation in the neural substrates of acute opiate dependence as measured by withdrawal-potentiated startle. _Neuroscience_ 139: 1201–1210. Article CAS PubMed
Google Scholar * Harris AC, Gewirtz JC (2004a). Elevated startle during withdrawal from acute morphine: a model of opiate withdrawal and anxiety. _Psychopharmacology (Berl)_ 171: 140–147.
Article CAS Google Scholar * Harris AC, Gewirtz JC (2005). Acute opioid dependence: characterizing the early adaptations underlying drug withdrawal. _Psychopharmacology (Berl)_ 178:
353–366. Article CAS Google Scholar * Harris AC, Hanes SL, Gewirtz JC (2004b). Potentiated startle and hyperalgesia during withdrawal from acute morphine: effects of multiple opiate
exposures. _Psychopharmacology (Berl)_ 176: 266–273. Article CAS Google Scholar * Harris AC, Rothwell PE, Gewirtz JC (2008). Effects of the NMDA receptor antagonist memantine on the
expression and development of acute opiate dependence as assessed by withdrawal-potentiated startle and hyperalgesia. _Psychopharmacology (Berl)_ 196: 649–660. Article CAS Google Scholar
* Harris GC, Aston-Jones G (1993a). Beta-adrenergic antagonists attenuate somatic and aversive signs of opiate withdrawal. _Neuropsychopharmacology_ 9: 303–311. Article CAS PubMed Google
Scholar * Harris GC, Aston-Jones G (1993b). Beta-adrenergic antagonists attenuate withdrawal anxiety in cocaine- and morphine-dependent rats. _Psychopharmacology (Berl)_ 113: 131–136.
Article CAS Google Scholar * Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA (1989). Acute opioid physical dependence in humans: effect of varying the morphine-naloxone interval. I. _J
Pharmacol Exp Ther_ 250: 485–491. CAS PubMed Google Scholar * Hipps PP, Eveland MR, Meyer ER, Sherman WR, Cicero TJ (1976). Mass fragmentography of morphine: relationship between brain
levels and analgesic activity. _J Pharmacol Exp Ther_ 196: 642–648. CAS PubMed Google Scholar * Houshyar H, Gomez F, Manalo S, Bhargava A, Dallman MF (2003). Intermittent morphine
administration induces dependence and is a chronic stressor in rats. _Neuropsychopharmacology_ 28: 1960–1972. Article CAS PubMed Google Scholar * Houshyar H, Manalo S, Dallman MF (2004).
Time-dependent alterations in mRNA expression of brain neuropeptides regulating energy balance and hypothalamo–pituitary–adrenal activity after withdrawal from intermittent morphine
treatment. _J Neurosci_ 24: 9414–9424. Article CAS PubMed PubMed Central Google Scholar * Hurd YL, McGregor A, Ponten M (1997). _In vivo_ amygdala dopamine levels modulate cocaine
self-administration behaviour in the rat: D1 dopamine receptor involvement. _Eur J Neurosci_ 9: 2541–2548. Article CAS PubMed Google Scholar * Jones KL, Zhu H, Jenab S, Du T, Inturrisi
CE, Barr GA (2002). Attenuation of acute morphine withdrawal in the neonatal rat by the competitive NMDA receptor antagonist LY235959. _Neuropsychopharmacology_ 26: 301–310. Article CAS
PubMed Google Scholar * Kalinichev M, Holtzman SG (2003). Changes in urination/defecation, auditory startle response, and startle-induced ultrasonic vocalizations in rats undergoing
morphine withdrawal: similarities and differences between acute and chronic dependence. _J Pharmacol Exp Ther_ 304: 603–609. Article CAS PubMed Google Scholar * Kawasaki Y, Jin C,
Suemaru K, Kawasaki H, Shibata K, Choshi T _et al_ (2005). Effect of glutamate receptor antagonists on place aversion induced by naloxone in single-dose morphine-treated rats. _Br J
Pharmacol_ 145: 751–757. Article CAS PubMed PubMed Central Google Scholar * Keppel G, Wickens TD (2004). _Design and Analysis : A Researcher's Handbook_. Pearson Prentice Hall:
Upper Saddle River, NJ. xii, 611 p.pp. Google Scholar * Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA (2008). Co-morbid major depression and generalized anxiety disorders
in the National Comorbidity Survey follow-up. _Psychol Med_ 38: 365–374. CAS PubMed Google Scholar * Kirby KC, Stitzer ML (1993). Opioid physical dependence development in humans: effect
of time between agonist pretreatments. _Psychopharmacology (Berl)_ 112: 511–517. Article CAS Google Scholar * Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon Jr WA (2007).
Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. _J Pharmacol Exp Ther_ 323: 838–845. Article CAS PubMed Google Scholar *
Koob GF, Le Moal M (2008). Addiction and the brain antireward system. _Annu Rev Psychol_ 59: 29–53. Article PubMed Google Scholar * Krueger RF (1999). The structure of common mental
disorders. _Arch Gen Psychiatry_ 56: 921–926. Article CAS PubMed Google Scholar * Land BB, Bruchas MR, Lemos JC, Chavkin C (2008a). Distinct roles for CRF receptor subtypes 1 and 2 in
producing dynorphin/kappa opioid receptor-dependent anxiety and conditioned place aversion. _Abstr Soc Neurosci_ 34: 187.1. Google Scholar * Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ,
Chavkin C (2008b). The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. _J Neurosci_ 28: 407–414. Article CAS PubMed PubMed Central Google
Scholar * Lang PJ, Davis M, Ohman A (2000). Fear and anxiety: animal models and human cognitive psychophysiology. _J Affect Disord_ 61: 137–159. Article CAS PubMed Google Scholar * Lang
PJ, McTeague LM (2009). The anxiety disorder spectrum: fear imagery, physiological reactivity, and differential diagnosis. _Anxiety Stress Coping_ 22: 5–25. Article PubMed PubMed Central
Google Scholar * Laulin JP, Larcher A, Celerier E, Le Moal M, Simonnet G (1998). Long-lasting increased pain sensitivity in rat following exposure to heroin for the first time. _Eur J
Neurosci_ 10: 782–785. Article CAS PubMed Google Scholar * Le Guen S, Gestreau C, Besson JM (2003). Morphine withdrawal precipitated by specific mu, delta or kappa opioid receptor
antagonists: a c-Fos protein study in the rat central nervous system. _Eur J Neurosci_ 17: 2425–2437. Article PubMed Google Scholar * Liu J, Schulteis G (2004). Brain reward deficits
accompany naloxone-precipitated withdrawal from acute opioid dependence. _Pharmacol Biochem Behav_ 79: 101–108. Article CAS PubMed Google Scholar * Maldonado R, Negus S, Koob GF (1992).
Precipitation of morphine withdrawal syndrome in rats by administration of mu-, delta- and kappa-selective opioid antagonists. _Neuropharmacology_ 31: 1231–1241. Article CAS PubMed Google
Scholar * Mansbach RS, Gold LH, Harris LS (1992). The acoustic startle response as a measure of behavioral dependence in rats. _Psychopharmacology (Berl)_ 108: 40–46. Article CAS Google
Scholar * Mendez IA, Trujillo KA (2008). NMDA receptor antagonists inhibit opiate antinociceptive tolerance and locomotor sensitization in rats. _Psychopharmacology (Berl)_ 196: 497–509.
Article CAS Google Scholar * Morse AC, Schulteis G, Holloway FA, Koob GF (2000). Conditioned place aversion to the ‘hangover’ phase of acute ethanol administration in the rat. _Alcohol_
22: 19–24. Article CAS PubMed Google Scholar * Motta V, Penha K, Brandao ML (1995). Effects of microinjections of mu and kappa receptor agonists into the dorsal periaqueductal gray of
rats submitted to the plus maze test. _Psychopharmacology (Berl)_ 120: 470–474. Article CAS Google Scholar * Nestler EJ, Carlezon Jr WA (2006). The mesolimbic dopamine reward circuit in
depression. _Biol Psychiatry_ 59: 1151–1159. Article CAS PubMed Google Scholar * Nobre MJ, Ribeiro dos Santos N, Aguiar MS, Brandao ML (2000). Blockade of mu- and activation of
kappa-opioid receptors in the dorsal periaqueductal gray matter produce defensive behavior in rats tested in the elevated plus-maze. _Eur J Pharmacol_ 404: 145–151. Article CAS PubMed
Google Scholar * Pain L, Oberling P, Mainsongeon M, Moulinoux JP, Simonnet G (2008). Delayed aversive effects of high-dose fentanyl. Prevention by a polyamine-deficient diet. _Behav Brain
Res_ 190: 119–123. Article CAS PubMed Google Scholar * Parker LA, Joshi A (1998). Naloxone-precipitated morphine withdrawal induced place aversions: effect of naloxone at 24 h
postmorphine. _Pharmacol Biochem Behav_ 61: 331–333. Article CAS PubMed Google Scholar * Pfeiffer A, Brantl V, Herz A, Emrich HM (1986). Psychotomimesis mediated by kappa opiate
receptors. _Science_ 233: 774–776. Article CAS PubMed Google Scholar * Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon Jr WA (2001). Altered responsiveness to cocaine
and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. _J Neurosci_ 21: 7397–7403. Article CAS
PubMed PubMed Central Google Scholar * Pothos E, Rada P, Mark GP, Hoebel BG (1991). Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine,
naloxone-precipitated withdrawal and clonidine treatment. _Brain Res_ 566: 348–350. Article CAS PubMed Google Scholar * Rasmussen K (1995). The role of the locus coeruleus and
N-methyl-D-aspartic acid (NMDA) and AMPA receptors in opiate withdrawal. _Neuropsychopharmacology_ 13: 295–300. Article CAS PubMed Google Scholar * Risbrough VB, Brodkin JD, Geyer MA
(2003). GABA-A and 5-HT1A receptor agonists block expression of fear-potentiated startle in mice. _Neuropsychopharmacology_ 28: 654–663. Article CAS PubMed Google Scholar *
Rodriguez-Romaguera J, Sotres-Bayon F, Mueller D, Quirk GJ (2009). Systemic propranolol acts centrally to reduce conditioned fear in rats without impairing extinction. _Biol Psychiatry_ 65:
887–892. Article CAS PubMed PubMed Central Google Scholar * Rossetti ZL, Hmaidan Y, Gessa GL (1992). Marked inhibition of mesolimbic dopamine release: a common feature of ethanol,
morphine, cocaine and amphetamine abstinence in rats. _Eur J Pharmacol_ 221: 227–234. Article CAS PubMed Google Scholar * Sahuque LL, Kullberg EF, McGeehan AJ, Kinder JR, Hicks MP,
Blanton MG _et al_ (2006). Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes.
_Psychopharmacology (Berl)_ 186: 122–132. Article CAS Google Scholar * Sante AB, Nobre MJ, Brandao ML (2000). Place aversion induced by blockade of mu or activation of kappa opioid
receptors in the dorsal periaqueductal gray matter. _Behav Pharmacol_ 11: 583–589. Article CAS PubMed Google Scholar * Schulteis G, Markou A, Gold LH, Stinus L, Koob GF (1994). Relative
sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. _J Pharmacol Exp Ther_ 271: 1391–1398. CAS PubMed Google Scholar * Shaham Y,
Shalev U, Lu L, De Wit H, Stewart J (2003). The reinstatement model of drug relapse: history, methodology and major findings. _Psychopharmacology (Berl)_ 168: 3–20. Article CAS Google
Scholar * Shippenberg TS, Zapata A, Chefer VI (2007). Dynorphin and the pathophysiology of drug addiction. _Pharmacol Ther_ 116: 306–321. Article CAS PubMed PubMed Central Google
Scholar * Spanagel R, Almeida OF, Bartl C, Shippenberg TS (1994). Endogenous kappa-opioid systems in opiate withdrawal: role in aversion and accompanying changes in mesolimbic dopamine
release. _Psychopharmacology (Berl)_ 115: 121–127. Article CAS Google Scholar * Sweitzer SM, Allen CP, Zissen MH, Kendig JJ (2004). Mechanical allodynia and thermal hyperalgesia upon
acute opioid withdrawal in the neonatal rat. _Pain_ 110: 269–280. Article CAS PubMed Google Scholar * Van Dyke C, Byck R (1982). Cocaine. _Sci Am_ 246: 128–141. Article CAS PubMed
Google Scholar * Vargas-Perez H, Ting AKRA, Heinmiller A, Sturgess JE, van der Kooy D (2007). A test of the opponent-process theory of motivation using lesions that selectively block
morphine reward. _Eur J Neurosci_ 25: 3713–3718. Article PubMed Google Scholar * Walker DL, Davis M (2002a). Light-enhanced startle: further pharmacological and behavioral
characterization. _Psychopharmacology (Berl)_ 159: 304–310. Article CAS Google Scholar * Walker DL, Davis M (2002b). Quantifying fear potentiated startle using absolute _vs_ proportional
increase scoring methods: implications for the neurocircuitry of fear and anxiety. _Psychopharmacology (Berl)_ 164: 318–328. Article CAS Google Scholar * Walker DL, Toufexis DJ, Davis M
(2003). Role of the bed nucleus of the stria terminalis _vs_ the amygdala in fear, stress, and anxiety. _Eur J Pharmacol_ 463: 199–216. Article CAS PubMed Google Scholar * Wallace DL,
Han MH, Graham DL, Green TA, Vialou V, Iniguez SD _et al_ (2009). CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. _Nat Neurosci_ 12:
200–209. Article CAS PubMed PubMed Central Google Scholar * White DA, Hwang ML, Holtzman SG (2005). Naltrexone-induced conditioned place aversion following a single dose of morphine in
the rat. _Pharmacol Biochem Behav_ 81: 451–458. Article CAS PubMed Google Scholar * Yamada H, Shimoyama N, Sora I, Uhl GR, Fukuda Y, Moriya H _et al_ (2006). Morphine can produce
analgesia via spinal kappa opioid receptors in the absence of mu opioid receptors. _Brain Res_ 1083: 61–69. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We
thank Bonnie LaCroix and Malaak Moussa for expert technical assistance, and Dr Andrew Harris and members of the Gewirtz and Thomas labs for helpful comments. This work was supported by
funding from the University of Minnesota Graduate School (to PER) and Grants from NIDA (DA007234 and DA023750 to PER, DA019666 to MJT, and DA018784 to JCG), the Whitehall Foundation (to
MJT), and NARSAD (to JCG). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Graduate Program in Neuroscience and Departments of Neuroscience and Psychology, University of Minnesota,
Minneapolis, MN, USA Patrick E Rothwell, Mark J Thomas & Jonathan C Gewirtz Authors * Patrick E Rothwell View author publications You can also search for this author inPubMed Google
Scholar * Mark J Thomas View author publications You can also search for this author inPubMed Google Scholar * Jonathan C Gewirtz View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Jonathan C Gewirtz. ADDITIONAL INFORMATION DISCLOSURE/CONFLICT OF INTEREST The authors declare that, except for income
received from their primary employers, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional
service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Rothwell, P., Thomas, M. & Gewirtz, J. Distinct Profiles of Anxiety and Dysphoria during Spontaneous Withdrawal from Acute Morphine Exposure.
_Neuropsychopharmacol_ 34, 2285–2295 (2009). https://doi.org/10.1038/npp.2009.56 Download citation * Received: 12 March 2009 * Revised: 25 April 2009 * Accepted: 26 April 2009 * Published:
03 June 2009 * Issue Date: September 2009 * DOI: https://doi.org/10.1038/npp.2009.56 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * morphine
* withdrawal * anxiety * dysphoria * acoustic startle * place conditioning