Enhanced insulin sensitivity, energy expenditure and thermogenesis in adipose-specific Pten suppression in mice
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Pten is an important phosphatase, suppressing the phosphatidylinositol-3 kinase/Akt pathway. Here, we generated adipose-specific Pten-deficient (AdipoPten-KO) mice, using newly generated
Acdc promoter–driven Cre transgenic mice. AdipoPten-KO mice showed lower body and adipose tissue weights despite hyperphagia and enhanced insulin sensitivity with induced phosphorylation of
Akt in adipose tissue. AdipoPten-KO mice also showed marked hyperthermia and increased energy expenditure with induced mitochondriagenesis in adipose tissue, associated with marked reduction
of p53, inactivation of Rb, phosphorylation of cyclic AMP response element binding protein (CREB) and increased expression of Ppargc1a, the gene that encodes peroxisome proliferative
activated receptor gamma coactivator 1 alpha. Physiologically, adipose Pten mRNA decreased with exposure to cold and increased with obesity, which were linked to the mRNA alterations of
mitochondriagenesis. Our results suggest that altered expression of adipose Pten could regulate insulin sensitivity and energy expenditure. Suppression of adipose Pten may become a
beneficial strategy to treat type 2 diabetes and obesity.
We thank Y. Matsuzawa and K. Sugihara for their encouragement, and K. Nishida, K. Oiki and S. Mizuno for technical assistance. This work was supported in part by grants from the Suzuken
Memorial Foundation, The Nakajima Foundation, Kanae Foundation for Life and Socio-Medical Science, The Tokyo Biochemical Research Foundation, Takeda Medical Research Foundation, Uehara
Memorial Foundation, Takeda Science Foundation, Novartis Foundation (Japan) for the Promotion of Science, The Cell Science Research Foundation, The Mochida Memorial Foundation for Medical
and Pharmaceutical Research, a Grant-in-Aid from the Japan Medical Association, The Naito Foundation, a grant from the Japan Heart Foundation Research, Kato Memorial Bioscience Foundation,
Japan Research Foundation for Clinical Pharmacology, a grant from the Ministry of Health, Labor and Welfare, Japan, and Grants-in-Aid from COE Research and Scientific Research on Priority
Areas from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Department of Social and Environmental Medicine, Graduate School of Frontier Bioscience, Osaka University, 2-2 Yamadaoka, Suita, 565-0871, Osaka
Department of Medicine and Pathophysiology, Graduate School of Frontier Bioscience, Osaka University, 2-2 Yamadaoka, Suita, 565-0871, Osaka
Morihiro Matsuda, Masanori Iwaki, Toshiyuki Takagi & Iichiro Shimomura
Division of Food Science and Biotechnology, Laboratory of Nutrition Chemistry, Graduate School of Agriculture, Kyoto University, Kitashirakawa, Sakyo-ku, 606-8502, Kyoto
Department of Dermatology, Osaka University, 2-2 Yamadaoka, Suita, 565-0871, Osaka
Department of Biochemistry, Akita University, 1-1 Hondou, Akita, 010-8543, Akita, Japan
Advanced Medical Discovery Institute, University of Toronto, Toronto, M5G 2Cl, Ontario, Canada
Department of Pathology, Graduate School of Medicine, Osaka University, 2-2 Yamadaoka, Suita, 565-0871, Osaka
Center for Advanced Science and Innovation, Osaka University, 2-2 Yamadaoka, Suita, 565-0871, Osaka
Department of Internal Medicine and Molecular Science, Osaka University, 2-2 Yamadaoka, Suita, 565-0871, Osaka
PREST, Japan Science and Technology Agency, 4-1-8 Honcho, Kawaguchi, 332-0012, Saitama, Japan
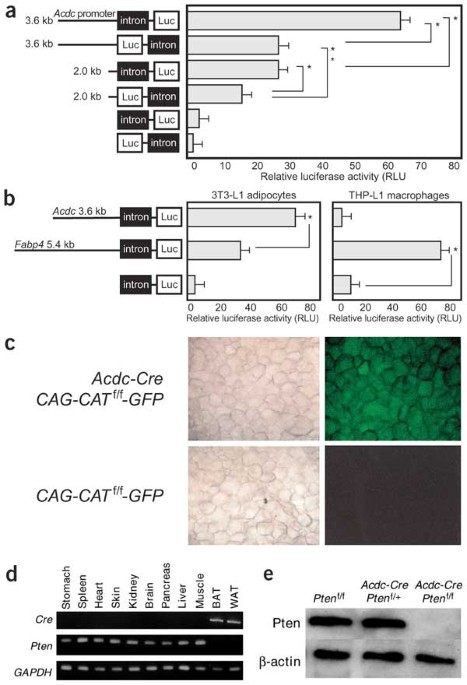
Expression analyses of Cre in Adiponectin promoter-driven Cre transgenic mice. (PDF 34 kb)
Anyone you share the following link with will be able to read this content: