Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor slpi
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Epithelial cells (ECs) transport class-switched immunoglobulin G (IgG) and IgA antibodies across mucous membranes. Whether ECs initiate class switching remains unknown. Here we
found that ECs lining tonsillar crypts formed pockets populated by B cells expressing activation-induced cytidine deaminase (AID), an enzyme associated with ongoing class switching. ECs
released B cell–activating AID-inducing factors after sensing microbial products through Toll-like receptors. The resulting class switching was amplified by thymic stromal lymphopoietin, an
epithelial interleukin 7–like cytokine that enhanced the B cell 'licensing' function of dendritic cells, and was restrained by secretory leukocyte protease inhibitor, an epithelial
homeostatic protein that inhibited AID induction in B cells. Thus, ECs may function as mucosal 'guardians' orchestrating frontline IgG and IgA class switching through a Toll-like
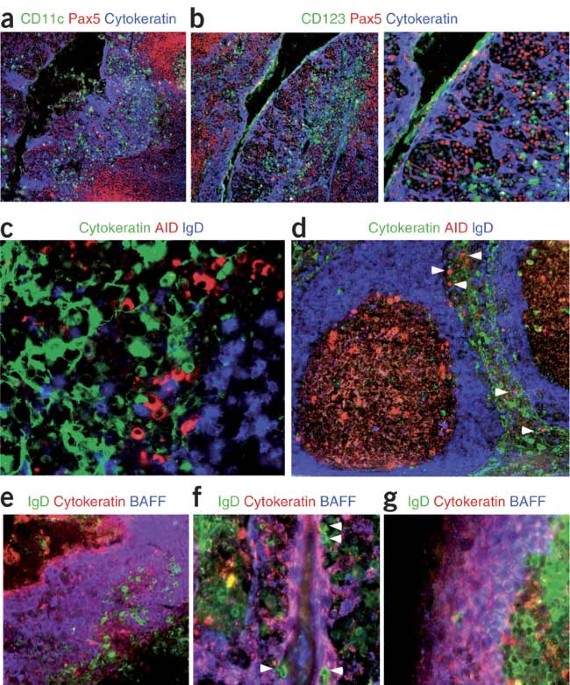
receptor–inducible signaling program regulated by secretory leukocyte protease inhibitor. NOTE: In the version of this article initially published online, the middle label above Figure 6c is
incorrect. The correct label should be ‘BAFF’. The error has been corrected for all versions of the article. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only
$17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS AN INTEGRIN ΑEΒ7-DEPENDENT
MECHANISM OF IGA TRANSCYTOSIS REQUIRES DIRECT PLASMA CELL CONTACT WITH INTESTINAL EPITHELIUM Article Open access 20 August 2021 EPITHELIAL IFNΓ SIGNALLING AND COMPARTMENTALIZED ANTIGEN
PRESENTATION ORCHESTRATE GUT IMMUNITY Article 22 November 2023 IL-22 INITIATES AN IL-18-DEPENDENT EPITHELIAL RESPONSE CIRCUIT TO ENFORCE INTESTINAL HOST DEFENCE Article Open access 15
February 2022 CHANGE HISTORY * _ 08 FEBRUARY 2007 In the version of this article initially published online, the middle label above Figure 6c is incorrect. The correct label should be
‘BAFF’. The error has been corrected for all versions of the article. _ REFERENCES * Nagler-Anderson, C. Man the barrier! Strategic defences in the intestinal mucosa. _Nat. Rev. Immunol._ 1,
59–67 (2001). Article CAS Google Scholar * Neutra, M.R., Mantis, N.J. & Kraehenbuhl, J.P. Collaboration of epithelial cells with organized mucosal lymphoid tissues. _Nat. Immunol._
2, 1004–1009 (2001). Article CAS Google Scholar * Hornef, M.W., Wick, M.J., Rhen, M. & Normark, S. Bacterial strategies for overcoming host innate and adaptive immune responses. _Nat.
Immunol._ 3, 1033–1040 (2002). Article CAS Google Scholar * Kiyono, H. & Fukuyama, S. NALT- versus Peyer's-patch-mediated mucosal immunity. _Nat. Rev. Immunol._ 4, 699–710
(2004). Article CAS Google Scholar * Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. _Annu. Rev. Immunol._ 21, 335–376 (2003). Article CAS Google Scholar * Abreu, M.T.,
Fukata, M. & Arditi, M. TLR signaling in the gut in health and disease. _J. Immunol._ 174, 4453–4460 (2005). Article CAS Google Scholar * Rakoff-Nahoum, S., Paglino, J.,
Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. _Cell_ 118, 229–241 (2004). Article
CAS Google Scholar * Ganz, T. & Lehrer, R.I. Antimicrobial peptides of vertebrates. _Curr. Opin. Immunol._ 10, 41–44 (1998). Article CAS Google Scholar * Hooper, L.V., Stappenbeck,
T.S., Hong, C.V. & Gordon, J.I. Angiogenins: a new class of microbicidal proteins involved in innate immunity. _Nat. Immunol._ 4, 269–273 (2003). Article CAS Google Scholar *
Hiemstra, P.S., Fernie-King, B.A., McMichael, J., Lachmann, P.J. & Sallenave, J.M. Antimicrobial peptides: mediators of innate immunity as templates for the development of novel
anti-infective and immune therapeutics. _Curr. Pharm. Des._ 10, 2891–2905 (2004). Article CAS Google Scholar * Yang, D. et al. Beta-defensins: linking innate and adaptive immunity through
dendritic and T cell CCR6. _Science_ 286, 525–528 (1999). Article CAS Google Scholar * Biragyn, A. et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2.
_Science_ 298, 1025–1029 (2002). Article CAS Google Scholar * Kelsall, B.L. & Rescigno, M. Mucosal dendritic cells in immunity and inflammation. _Nat. Immunol._ 5, 1091–1095 (2004).
Article CAS Google Scholar * Rescigno, M. et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. _Nat. Immunol._ 2, 361–367
(2001). Article CAS Google Scholar * Rimoldi, M. et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. _Nat. Immunol._ 6,
507–514 (2005). Article CAS Google Scholar * Fagarasan, S. & Honjo, T. Intestinal IgA synthesis: regulation of front-line body defences. _Nat. Rev. Immunol._ 3, 63–72 (2003). Article
CAS Google Scholar * Soumelis, V. et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. _Nat. Immunol._ 3, 673–680 (2002). Article CAS
Google Scholar * Watanabe, N. et al. Human thymic stromal lymphopoietin promotes dendritic cell–mediated CD4+ T cell homeostatic expansion. _Nat. Immunol._ 5, 426–434 (2004). Article CAS
Google Scholar * Brandtzaeg, P. et al. The B-cell system of human mucosae and exocrine glands. _Immunol. Rev._ 171, 45–87 (1999). Article CAS Google Scholar * Brandtzaeg, P., Baekkevold,
E.S. & Morton, H.C. From B to A the mucosal way. _Nat. Immunol._ 2, 1093–1094 (2001). Article CAS Google Scholar * Stavnezer, J. Antibody class switching. _Adv. Immunol._ 61, 79–146
(1996). Article CAS Google Scholar * Honjo, T., Kinoshita, K. & Muramatsu, M. Molecular mechanism of class switch recombination: linkage with somatic hypermutation. _Annu. Rev.
Immunol._ 20, 165–196 (2002). Article CAS Google Scholar * Calame, K.L. Plasma cells: finding new light at the end of B cell development. _Nat. Immunol._ 2, 1103–1108 (2001). Article CAS
Google Scholar * Kunkel, E.J. et al. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. _J. Clin. Invest._ 111, 1001–1010 (2003).
Article CAS Google Scholar * Wilson, E. & Butcher, E.C. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the
neonate. _J. Exp. Med._ 200, 805–809 (2004). Article CAS Google Scholar * Suzuki, K., Meek, B., Doi, Y., Honjo, T. & Fagarasan, S. Two distinctive pathways for recruitment of naive
and primed IgM+ B cells to the gut lamina propria. _Proc. Natl. Acad. Sci. USA_ 102, 2482–2486 (2005). Article CAS Google Scholar * Fagarasan, S., Kinoshita, K., Muramatsu, M., Ikuta, K.
& Honjo, T. _In situ_ class switching and differentiation to IgA-producing cells in the gut lamina propria. _Nature_ 413, 639–643 (2001). Article CAS Google Scholar * Macpherson, A.J.
et al. IgA production without μ or δ chain expression in developing B cells. _Nat. Immunol._ 2, 625–631 (2001). Article CAS Google Scholar * Litinskiy, M.B. et al. DCs induce
CD40-independent immunoglobulin class switching through BLyS and APRIL. _Nat. Immunol._ 3, 822–829 (2002). Article CAS Google Scholar * Macpherson, A.J. & Lamarre, A. BLySsful
interactions between DCs and B cells. _Nat. Immunol._ 3, 798–800 (2002). Article CAS Google Scholar * Macpherson, A.J. & Uhr, T. Induction of protective IgA by intestinal dendritic
cells carrying commensal bacteria. _Science_ 303, 1662–1665 (2004). Article CAS Google Scholar * Fagarasan, S. & Honjo, T. T-Independent immune response: new aspects of B cell
biology. _Science_ 290, 89–92 (2000). Article CAS Google Scholar * Woof, J.M. & Mestecky, J. Mucosal immunoglobulins. _Immunol. Rev._ 206, 64–82 (2005). Article CAS Google Scholar
* Brandtzaeg, P. & Prydz, H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. _Nature_ 311, 71–73 (1984). Article
CAS Google Scholar * Mostov, K.E. & Simister, N.E. Transcytosis. _Cell_ 43, 389–390 (1985). Article CAS Google Scholar * Mostov, K.E. & Deitcher, D.L. Polymeric immunoglobulin
receptor expressed in MDCK cells transcytoses IgA. _Cell_ 46, 613–621 (1986). Article CAS Google Scholar * Johansen, F.E. et al. Absence of epithelial immunoglobulin A transport, with
increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. _J. Exp. Med._ 190, 915–922 (1999). Article CAS Google Scholar * Spiekermann, G.M. et
al. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. _J. Exp. Med._ 196, 303–310 (2002). Article CAS
Google Scholar * Yoshida, M. et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. _Immunity_ 20,
769–783 (2004). Article CAS Google Scholar * Kinoshita, K., Harigai, M., Fagarasan, S., Muramatsu, M. & Honjo, T. A hallmark of active class switch recombination: transcripts directed
by I promoters on looped-out circular DNAs. _Proc. Natl. Acad. Sci. USA_ 98, 12620–12623 (2001). Article CAS Google Scholar * Graeme-Cook, F., Bhan, A.K. & Harris, N.L.
Immunohistochemical characterization of intraepithelial and subepithelial mononuclear cells of the upper airways. _Am. J. Pathol._ 143, 1416–1422 (1993). CAS PubMed PubMed Central Google
Scholar * Banchereau, J. & Steinman, R. Dendritic cells and the control of immunity. _Nature_ 392, 245–252 (1998). Article CAS Google Scholar * Liu, Y.J. IPC: professional type 1
interferon-producing cells and plasmacytoid dendritic cell precursors. _Annu. Rev. Immunol._ 23, 275–306 (2005). Article CAS Google Scholar * Cerutti, A., Qiao, X. & He, B.
Plasmacytoid dendritic cells and the regulation of immunoglobulin heavy chain class switching. _Immunol. Cell Biol._ 83, 554–562 (2005). Article CAS Google Scholar * Ziegler, S.F. &
Liu, Y.J. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. _Nat. Immunol._ 7, 709–714 (2006). Article CAS Google Scholar * Jin, F.Y., Nathan, C.,
Radzioch, D. & Ding, A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. _Cell_ 88, 417–426 (1997). Article CAS
Google Scholar * Zhu, J. et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. _Cell_ 111, 867–878 (2002). Article CAS Google
Scholar * Taggart, C.C. et al. Secretory leucoprotease inhibitor binds to NF-κB binding sites in monocytes and inhibits p65 binding. _J. Exp. Med._ 202, 1659–1668 (2005). Article CAS
Google Scholar * Petersen, S. et al. AID is required to initiate Nbs1/γ-H2AX focus formation and mutations at sites of class switching. _Nature_ 414, 660–665 (2001). Article CAS Google
Scholar * He, B., Qiao, X. & Cerutti, A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with
IL-10. _J. Immunol._ 173, 4479–4491 (2004). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank C. Nathan (Weill Medical College of Cornell University) and M.
Rescigno (European Institute of Oncology) for reagents and discussions. Supported by the National Institutes of Health (AI057653 to A.C.; and T32 AI07621, supporting W.X.). AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Pathology and Laboratory Medicine, Weill Medical College of Cornell University, New York, 10021, New York, USA Weifeng Xu, Bing He, April
Chiu, Amy Chadburn, Malwina Buldys, Daniel M Knowles, Paul A Santini & Andrea Cerutti * Department of Immunology and Microbiology, Weill Medical College of Cornell University, New York,
10021, New York, USA Meimei Shan & Aihao Ding * Graduate Program of Immunology and Microbial Pathogenesis, Weill Medical College of Cornell University, New York, 10021, New York, USA
Aihao Ding & Andrea Cerutti Authors * Weifeng Xu View author publications You can also search for this author inPubMed Google Scholar * Bing He View author publications You can also
search for this author inPubMed Google Scholar * April Chiu View author publications You can also search for this author inPubMed Google Scholar * Amy Chadburn View author publications You
can also search for this author inPubMed Google Scholar * Meimei Shan View author publications You can also search for this author inPubMed Google Scholar * Malwina Buldys View author
publications You can also search for this author inPubMed Google Scholar * Aihao Ding View author publications You can also search for this author inPubMed Google Scholar * Daniel M Knowles
View author publications You can also search for this author inPubMed Google Scholar * Paul A Santini View author publications You can also search for this author inPubMed Google Scholar *
Andrea Cerutti View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS W.X. designed and did research and analyzed data; B.H., M.S. and M.D. did
research; A. Chiu provided tissue samples and discussed data; A. Chadburn and D.M.K. provided tissue samples; A.D. provided reagents, discussed data and edited the paper; P.A.S. analyzed and
discussed data; and A. Cerutti designed research, analyzed data and wrote the paper. CORRESPONDING AUTHOR Correspondence to Andrea Cerutti. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIG. 1 Primary oral ECs lack contaminating DCs and express BAFF. (PDF 185 kb) SUPPLEMENTARY FIG. 2
Intestinal and epidermal ECs express BAFF and TLR3. (PDF 195 kb) SUPPLEMENTARY FIG. 3 Intestinal and epidermal ECs express TLR9. (PDF 153 kb) SUPPLEMENTARY FIG. 4 B cells up-regulate TLR3
expression upon exposure to viral RNA and BAFF. (PDF 194 kb) SUPPLEMENTARY FIG. 5 ECs stimulate IgD+ B cells to undergo IgG and IgA CSR. (PDF 120 kb) SUPPLEMENTARY FIG. 6 ECs stimulate IgD+
B cells to produce broadly reactive IgG and IgM antibodies. (PDF 135 kb) SUPPLEMENTARY FIG. 7 Phenotype of myeloid DCs. (PDF 126 kb) SUPPLEMENTARY FIG. 8 Tonsillar ECs and intraepithelial B
cells contain SLPI. (PDF 136 kb) SUPPLEMENTARY FIG. 9 SLPI inhibits IgG and IgA production in IgD+ B cells exposed to CD40L, IL-4 and IL-10. (PDF 65 kb) SUPPLEMENTARY FIG. 10 ECs induce
frontline IgG and IgA class switching through a TLR-inducible SLPI-regulated epithelial pathway. (PDF 108 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Xu, W., He, B., Chiu, A. _et al._ Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. _Nat Immunol_ 8, 294–303
(2007). https://doi.org/10.1038/ni1434 Download citation * Received: 12 September 2006 * Accepted: 21 December 2006 * Published: 28 January 2007 * Issue Date: March 2007 * DOI:
https://doi.org/10.1038/ni1434 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative