Metallodendrimers in three oxidation states with electronically interacting metals and stabilization of size-selected gold nanoparticles
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Metallodendrimers containing redox-robust centres may have applications in molecular redox recognition, sensing, biosensing and catalysis. So far, however, no metallodendrimer is
known in several oxidation states. Here we report metallodendrimers with two electronically communicating iron centres that are stable and isolated in both the FeII and FeIII oxidation
states, and in addition as class-II mixed-valent FeIIFeIII complexes. These dendrons are branched to arene-centred dendrimer cores either by Sonogashira coupling or ‘click’ reactions. The
latter reaction involves the introduction of intradendritic 1,2,3-triazolyl ligands, which allows investigation of the selective role of these ligands in intradendritic AuIII coordination
and Au0 nanoparticle stabilization. As a result, and using the various metallodendrimers with different oxidation states, small Au0 nanoparticles are intradendritically stabilized by the
triazole ligands, whereas with the related non-‘click’ dendrimers large Au0 nanoparticles are formed outside the dendrimers and stabilized by a group of dendrimers. You have full access to
this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS UNDERSTANDING LIGAND-PROTECTED NOBLE METAL NANOCLUSTERS AT WORK Article 20 February 2023 ULTRA-STABLE
AND HIGHLY REACTIVE COLLOIDAL GOLD NANOPARTICLE CATALYSTS PROTECTED USING MULTI-DENTATE METAL OXIDE NANOCLUSTERS Article Open access 06 February 2024 EFFICIENT SYNTHESIS OF BENZOACRIDINES
AND INDENOQUINOLINES CATALYZED BY ACIDIC MAGNETIC DENDRIMER Article Open access 16 April 2024 INTRODUCTION To date, dendrimers and metal nanoparticles (NPs) have been largely involved in
materials science including nanomedicine, photonics and supramolecular chemistry1,2,3,4,5, and in particular metallodendrimers and dendrimer-containing NPs2,3,4,6,7,8,9,10,11 have been
efficiently designed for catalysis2,3,4, photophysics7,8,9,10,11 and sensing9,10,11,12,13. Redox-stable metallodendrimers are a specific class of materials allowing redox anion recognition,
sensing2,3,4,5,6,7,8,9,10,11,12,13,14,15,16 and biosensing14,15 that have been used essentially with ferrocene derivatives12,13,14,15. Although biferrocene- and silicon-bridged
biferrocene-terminated dendrimers are known5,14,15,16, no dendrimers have yet been isolated in several oxidation states owing to instability of the odd-electron species. The flexibility of
oxidation-state variation with the maintenance of stability for a large collection of metal centres located at the metallodendrimer periphery is highly desirable, however, because it would
allow the design of nanodevices towards applications in molecular nanoelectronics including nanobatteries17 and polyelectrolytes18. Here we present the synthesis and redox, electronic and
NP-stabilization properties of new metallodendrimers in three robust oxidation states including mixed-valent metallodendrimers. The metal centres located at the dendritic periphery are
organized in dendrons in such a way that the two metal centres of the dendrons are electronically communicating. Redox reactions of transition-metal complexes19,20,21,22 are essential to
organize molecular electronic communication in nanomaterials23. The redox-rich iron centres in the chosen system are Cp*FeII(dppe)-alkynyl units (Cp*=η5-C5Me5,
dppe=1,2-bis(diphenylphosphino)ethane)24 that are isolobal to ferrocene and linked in meta position of an aryl bridge. In 1,3,5-trimetallaalkynylbenzene derivatives24,25,26,27, and in such
Cp*FeII(dppe) derivatives in particular25,26, it has been shown by density functional theory calculation and electrochemistry that, unlike in the 1,3,5-triferrocenylalkynylbenzene
derivatives28, the three metals are electronically communicating. We synthesize the dendrons with only two iron-ethynyl groups in 1,3 position, leaving the third ethynyl position free for
binding to the dendritic core either by Sonogashira coupling29,30 or copper-catalysed azide-alkyne cycloaddition (CuAAC, ‘click’) reactions31,32,33 with appropriately functionalized
dendritic core termini. In this way, metallodendrimers in the three oxidation states FeIIFeII, FeIIFeIII and FeIIIFeIII are synthetically accessible with and without 1,2,3-triazolyl ligands
on the branches, and the latter parameter strongly influences their ability to stabilize and isolate gold NPs (AuNPs) on HAuCl4 reduction. As a result, we demonstrate how the presence and
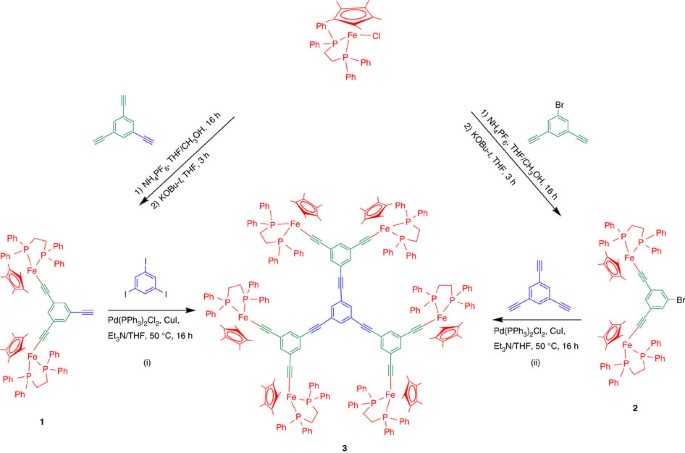
number of these intradendritic ligands is crucial to form well-defined, very small AuNPs. RESULTS DENDRON AND DENDRIMER SYNTHESIS Both new bimetallic dendrons 1 and 2 were prepared from the
precursor complex [FeCp*(dppe)Cl] according to Fig. 1. Treatment of one equiv. of 1,3,5-triethynylbenzene with two equiv. of [FeCp*(dppe)Cl] and NH4PF6 in a methanol/tetrahydrofuran (THF)
mixture (1:1), followed by deprotonation with KOBu_t_ produced 1-ethynyl-3,5-bis(iron)alkynylbenzene, 1, with 60% yield, and 1-bromo-3,5-bis(iron)alkynylbenzene, 2, was obtained upon
reaction of [FeCp*(dppe)Cl] with 1-bromo-3,5-bis(ethynyl)benzene in 70% yield (Fig. 1). Both complexes 1 and 2 were isolated as orange microcrystals following standard column chromatography.
The characterizations of complexes 1 and 2 are gathered, respectively, in the Supplementary Figs 1–6 and 7–12. The hexanuclear compound 3 was obtained as deep orange microcrystals using two
methods shown in Fig. 1: (i) Sonogashira reaction between dendron 1 and 1,3,5-triiodobenzene catalysed by [Pd(PPh3)2Cl2] and CuI (Fig. 1) giving 3 with 50% yield and (ii) reaction of
dendron 2 with 1,3,5-triethynylbenzene under the same conditions (55% yield). The 31P NMR spectrum (C6D6) of 3 exhibits a broad signal (99.39 δp.p.m.) due to the steric hindrance in the
hexanuclear complex revealing slow rotation on the NMR timescale. The characterizations of the complex 3 are gathered in Supplementary Figs 13–18. The dodecairon complex 4 was synthesized
from the reaction between dendron 1 and hexakis(4-iodophenyl)-benzene catalysed by [Pd(PPh3)2Cl2] and CuI (Fig. 2a). Its infrared spectra show the disappearance of the absorption band around
2,275 cm−1 corresponding to the free alkynyl absorption (_ν_C≡C). The characterizations of the complex 4 are gathered in Supplementary Figs 19–24. From the known dendritic cores
1,3,5{C[(CH2)3SiMe2CH2I]3}3-C6H3, G0-CH2I, containing 9 terminal iodomethylsilyl branches (Fig. 2b), and the 27 iodomethylsilyl-terminated analogue G1-CH2I (Fig. 3), azido- (Figs. 4 and 5)
and _p_.iodophenyl-terminated (Figs 2 and 3) dendrimers were synthesized with 9 and 27 branches by I/N3 substitution or Williamson reactions with _p_.iodophenol, respectively. These
dendritic precursors were then functionalized with dendron 1 according to Sonogashira (Figs 2 and 3) and ‘click’ reactions (Figs 4 and 5), respectively. The characterizations of complexes
G0-PHI and 5A are gathered in Supplementary Figs 25–27 and 28–33, respectively. Those of complexes G1-PHI and 6A are gathered in Supplementary Figs 34–36 and 37–41, respectively, and those
of complexes 5B and 6B are gathered in Supplementary Figs 42–47 and 48–53, respectively. Diffusion-ordered spectroscopy experiments were carried out for the dendrimers 3, 4, 5A, 5B, 6A and
6B. The main goal was to determine the diffusion coefficient D leading to the hydrodynamic radii of the dendrimer. The spectra also reflected the purity of the products (the measurements
were reproducible). The dendrimer size was found to regularly increase with the increase of the number of Fe(II) units at the dendrimer periphery. Also, the values obtained from dynamic
light scattering (DLS) were much larger than the sizes obtained from diffusion-ordered spectroscopy and simulation owing to intermolecular associations34. The data are gathered in Table 1.
The cyclic voltammograms of all the metallodendrons and metallodendrimers (Supplementary Fig. 54) were recorded. They showed two reversible waves around −0.5 and −0.6 V, respectively, with a
wave separation (Δ_E_p) of 0.11–0.13 V for the metallodendrons (Table 2) with a slight solvent effect and only around 0.070–0.080 V for the metallodendrimers. For the later, the cyclic
voltammogram waves are broad with significant adsorption owing to their large size, which prevents clear wave separation and accuracy in the determination of the potentials, although the
comproportionation constants (Table 2) are sufficient for further syntheses of the mixed-valent complexes (_vide infra_). SYNTHESIS OF THE OXIDIZED METALLODENDRIMERS Mixed-valent complexes
that are indicators of electronic communication between neighbouring redox sites35 are useful tools and models of electronic communication in nanomaterials36. The neutral FeII
metallodendrimers 3, 4, 5B and 6B have been oxidized in dichloromethane (DCM) to the deep-blue fully oxidized (FeIIIFeIII) metallodendrimers 3(PF6)6, 4(PF6)12, 5B(PF6)18, 6B(PF6)54 using one
equiv. ferricinium hexafluorophosphate (FcH+ PF6−) per Fe centre. Oxidation to the green mixed-valent metallodendrimers 3(PF6)3, 4(PF6)6, 5B(PF6)9 and 6B(PF6)27 was conducted using only one
equiv. FcH+PF6− per metallodendron. Alternatively, these mixed-valent metallodendrimers were synthesized on mixing equimolecular amounts of the FeII metallodendrimers and the fully oxidized
FeIII metallodendrimers (Figs 6 and 7). Ferrocene that was formed in these reactions was removed on washing with pentane, and the oxidized metallodendrimers were characterized by Fourier
transform infrared (Supplementary Figs 55–70 and Supplementary Table 1), Mössbauer spectroscopies (Supplementary Fig. 71 and Supplementary Table 2), near-infrared (Supplementary Fig. 72 and
Supplementary Table 3), ultraviolet–visible (Supplementary Table 4) and elemental analysis. The infrared spectra of all the multinuclear iron(II) and iron(III) complexes display a single C≡C
stretching band around 2,040 and 2,000 cm−1, respectively (Supplementary Table 1). In contrast, the infrared spectra of all the mixed-valent complexes show two distinct C≡C stretching bands
at these wavenumbers, providing evidence for localized oxidation states at 1013 s−1. The Mössbauer spectra of all the mixed-valent complexes recorded at 78 K show two quadrupole doublets of
approximately equal intensities corresponding to the FeII and FeIII states (Supplementary Table 2), indicating localized mixed valency at 10−7 s−1. The intervalence charge transfer (IVCT)
band (Supplementary Table 3) of the four mixed-valent complexes was found in the near-infrared region in CD3COCD3. The full width at half height of the band is very close to the theoretical
value derived from the near-infrared band position _λ_max according to Hush’s relationship for symmetrical mixed-valent complexes. The ratios (Δ_ν_1/2)obs/(Δ_ν_1/2)calc were less than 1.15,
falling in the 1.1–1.4 range of class-II mixed-valent complexes36,37,38. The UV-vis spectra of all the multinuclear complexes (Supplementary Table 4) display intense absorption bands in the
range 250–380 nm (LC π–π* transition) and around 400 nm (metal to ligand charge transfer (MLCT) transition) responsible for the orange colour of the neutral compounds and also present in the
deep colour of the mixed-valent/fully oxidized products, whereas the oxidized complexes present two transitions above 550–580 and 645–680 nm explaining their green/blue colour38. The
mixed-valent properties of all the mixed-valent dendrimers are further discussed in the Supplementary Notes 1–5. STABILIZATION OF AUNPS The availability of metallodendrimers in three
oxidation states with 54 Cp*Fe(dppe)alkynyl termini with interiors containing or not 1,2,3-triazole ligands offered the opportunity to examine the influence of the intradendritic presence of
these triazole ligands on the stabilization of AuNPs39,40,41,42. Indeed, it was possible to start either from FeIIFeII metallodendrimers to reduce AuIII into AuNPs (equation (1)) or from
FeIIIFeIII metallodendrimers and AuIII and to reduce the mixture using excess NaBH4 to FeIIFeII and AuNPs (equation (2)). These experiments were conducted with the dendrimer 6A and 6B and
with dendron 1 for comparison, and the results are gathered in Table 3 and Supplementary Figs 73–82. The AuNPs formed from 6A gave a much more intense plasmon absorption in the
ultraviolet–visible spectrum than 6B around 530 nm indicating much larger AuNPs (Fig. 8). Accordingly, the smallest AuNPs (cores: 1.4 and 1.9 nm from equation (1) resp. equation (2)) were
obtained with the ‘click’ metallodendrimer 6B. The Sonogashira metallodendrimer 6A gave larger AuNPs (cores: 3.4 and 3.7 nm from equation (1) resp. equation (2)), and the metallodendron 1
gave large AuNPs with equation (1) (20 nm core), but small AuNPs (2 nm core) according to equation (2) (Fig. 9). DISCUSSION In this work, the syntheses and characterizations of
metallodendrimers in three oxidation states have been achieved for the first time, allowing redox interplay exemplified by the formation of AuNPs of selective size depending on the presence
and number of intradendritic 1,2,3-triazole ligands. The metallodendrimers have been designed using interacting metal centres in new bimetallic dendrons. It is the electronic communication
between these two iron centres within each bimetallic dendron that provides the occurrence of another stable oxidation state that is class-II mixed-valent in the Robin–Day classification as
shown by the splitting of the C≡C stretching band in infrared and presence of a near-infrared intervalent band. The choice of the method of dendron connection to dendrimer cores, Sonogashira
versus ‘click’ reaction, further results in the presence or absence of ‘clicked’ 1,2,3-triazolyl intradendritic ligands. This dendrimer engineering providing electronic flexibility is
unique in that the stability and isolation of different dendrimer oxidation states allow examining the influence of the presence and number of these triazole ligands on the intradendritic
coordination of AuIII moieties. As a consequence, it could be shown here that the intradendritic AuNP stabilization specifically induces the formation of small AuNPs. The results are well
taken into account by coordination of Au3+ by the triazole ring in both equations (1) and (2) experiments followed by reduction to small monodisperse AuNPs (1.4±0.3 nm) having a core size
slightly higher than 27 Au atoms inside a dendrimer, owing to some leaching of Au atoms among dendrimers (the diameter of a 27-atom AuNP is 1 nm). The triazole loosely interacts with the
AuNP surface, which contributes to its intradendritic stabilization of these small AuNPs. On the contrary, the larger AuNPs stabilized by the Sonagashira dendrimer 6B gather a number of
atoms around 1,400, which involves considerable distribution around dendrimer peripheries and final stabilization of each AuNP by several dendrimers, because AuIII reduction essentially
proceeds outside dendrimers, and there are no heteroatom-containing ligand retaining AuNPs inside the dendrimers (Fig. 10). The small dendrons allow the formation of a very large AuNP core
surrounded by a large number of dendrons because small dendrons are much less efficient than large dendrimers for NP stabilization. Remarkably, the AuNP core size of the
AuIII-‘click’-dendrimer complexes does not vary considerably with the mode of formation for the dendrimers 6A and 6B whatever the synthetic route (equation (1) versus equation (2)). Indeed,
the AuNP core size approximately corresponds to the number of Au atoms generated from the triazolyl-coordinated AuIII precursors in a single dendrimer. It does for dendron 1, however,
because AuNP stabilization by borohydride then predominates in equation (2) in the absence of strong dendritic stabilization. On the other hand, the related non-‘click’ (Sonogashira)
dendrimers generate much larger AuNPs formed outside the metallodendrimers, but also stabilized by a group of metallodendrimers around the AuNP (Supplementary Notes 6 and 7). Such a
nanomaterial engineering strategy should be most useful for the design of sophisticated nanosensors, nanocatalysts, nanobatteries and nanopolyelectrolytes in the near future6. METHODS
SYNTHESIS AND CHARACTERIZATIONS OF DENDRON 1 1,3,5-Triethynylbenzene (150 mg, 1 mmol) in 10 ml distilled THF was added to a solution of [FeCp*(dppe)Cl] (1.56 g, 2.5 mmol) and NH4PF6 (326 mg,
2 mmol) in 12 ml CH3OH. The suspension was stirred at room temperature (r.t.) for 16 h. The solvent was evaporated _in vacuo_. The residual solid was dissolved in 5 ml distilled DCM, 100 ml
distilled pentane was added and the precipitation was washed with pentane for another two times. The residual solid was dissolved in 10 ml distilled THF, and then potassium _tert_-butoxide
(224 mg, 2 mmol) was added in a single portion. The mixture was stirred 3 h under r.t., the solvent was removed _in vacuo_ and the residue was extracted with distilled diethylether to form
the crude compound that was further purified by column chromatography (pentane/diethylether=5/1, _R_f=0.2) providing dendron 1 as an orange powder (795.6 mg, yield=60%). Matrix-assisted
laser desorption/ionization time of flight (MALDI-TOF): calc. _m_/_z_ for M+ (C84H82Fe2P4) 1,327.1, found 1,326.4. Anal. calcd. for C84H82Fe2P4: C, 76.02; H, 6.23. Found: C, 76.32; H, 6.38.
For dendron 2, a similar procedure was used. See Supplementary Methods for remaining syntheses; full characterization of all other complexes are not described herein. SYNTHESIS OF DENDRIMER
6A USING A SONOGASHIRA REACTION A mixture of dendron 1 (121 mg, 1.5 equiv. for each branch), G1-PhI (25 mg, 1 equiv. for each branch) and catalytic amount of Pd(PPh3)2Cl2 (8.5 mg, 0.2 equiv.
for each branch) and CuI (4.6 mg, 0.1 equiv. for each branch) were dissolved in the mixed solvent of distilled diisopropylamine (DIPA)/THF 10 ml. The solution was stirred overnight at 50 °C
under N2. The solvent was evaporated _in vacuo_. The residual solid was extracted with PhCH3 30 ml, the solution was dried with anhydrous Na2SO4, the solvent was removed in vacuo and the
dark red residue was washed three times with distilled diethyl ether 50 ml to remove the excess free ligand. Then the residual solid was dissolved in 1 ml of dry THF, 100 ml of dry CH3CN was
added to the mixture and compound 6A precipitated as a deep orange solid (66 mg, yield=70%). Anal. calcd. for C2718H2838Fe54P108Si36O36: C, 75.13; H, 6.58. Found: C, 75.44; H, 6.47.
SYNTHESIS OF DENDRIMER 6B BY ‘CLICK’ REACTION To a mixture of G1-N3 (20 mg, 3.6 × 10−3 mmol, 1 equiv.) and dendron 1 (193 mg, 0.146 mmol, 1.5 equiv. for each branch) in THF 20 ml, 10 ml of
distilled H2O was added dropwise, and a solution of CuSO4·5 H2O (36.5 mg, 0.146 mmol, 1.5 equiv. for each branch) in 1 ml of H2O was slowly added to the mixture at 0 °C, which provoked a
colour change from orange to dark brown. The solution of sodium ascorbate (57.8 mg, 0.292 mmol, 3 equiv. for each branch) was dropwise added to this mixture at 0 °C, which resulted in a
colour change from dark brown to orange. Stirring was continued at 50 °C under N2 for another 16 h, the solvent was evaporated _in vacuo_ until only the aqueous phase was left, 50 ml of
toluene, 50 ml of H2O and 3 ml of ammonium hydroxide was added to the solution and the mixture was stirred at r.t. for 30 min for extraction of the Cu catalyst. After separation, the organic
phase was dried with anhydrous Na2SO4, and the solvent was removed _in vacuo_, which gave a deep orange solid that was washed three times with 300 ml diethyl ether to remove the excess free
ligands. This procedure yielded the ‘click’ dendrimer as an orange solid (89.2 mg, 60%). Anal. calcd. for C2556H2757Fe54P108N81O9Si36: C, 72.87; H, 6.60; N, 2.69. Found: C, 72.69; H, 6.40;
N, 2.45. The synthesis and characterizations of 5B were conducted under similar conditions. OXIDATION OF THE COMPLEX 6B TO 6B(PF6)27 The dendrimer 6B (82 mg, 2 × 10−3 mmol, 1 equiv.) was
dissolved in 5 ml distilled DCM, and [FeCp2] [PF6] (17.7 mg, 0.0535, mmol, 27 equiv.) was added in a single portion to this solution at r.t., which provoked an immediate colour change from
orange to dark green, the solution was stirred for 4 h at r.t. under N2, the solvent was removed in vacuo until 0.5 ml was left, then 50 ml of distilled pentane was added to this dark green
solution and the mixed-valent dendrimer was precipitated. After filtration, the residual solid was washed twice more with 100 ml pentane, and the residue was dried under vacuum to give the
mixed-valent dendrimer as a dark solid (80.9 mg, 90% yield. Anal. calcd. for C2556H2757Fe54P108N81O9Si36(PF6)27: C, 66.68; H, 6.04; N, 2.46. Found: C, 66.77; H, 6.20; N, 2.45. The oxidation
of 3, 4, 5B and 6B by [FeCp2] [PF6] to the mixed-valent or fully oxidized dendrimers were conducted identically. SYNTHESIS OF AUNPS STABILIZED BY DENDRIMER 6B27+ The neutral dendrimer 6B (10
mg, 2.419 × 10−4 mmol, 1 equiv. per branch) was dissolved in 2 ml DCM, and the solution of HAuCl4 (0.86 mg, 2.175 × 10−3 mmol, 1/3 equiv.) in 2 ml of acetone was slowly added to the mixture
at r.t., which provoked a colour change from orange to deep green. Stirring was continued for another 30 min. Infrared (KBr): 2,036 cm−1 (_ν_C≡C-Fe(II)), 2,009 cm−1 (_ν_C≡C-Fe(III)).
Ultraviolet–visible: _λ_max 1=410 nm (νΠ-Π*), _λ_max 2/_λ_max 3=580/665 nm (νLMCT). Transmission electron microscopy: 1.4±0.3 nm. The synthesis and characterizations of AuNPs stabilized by
ionic mixed-valent dendron 1+ or dendrimer 6A27+ were analogous. SYNTHESIS OF AUNPS STABILIZED BY NEUTRAL DENDRIMER 6B The neutral dendrimer 6B (5 mg, 1.15 × 10−4 mmol, 1 equiv.) was
dissolved in 2 ml of DCM, and [FeCp2] [PF6] (2.1 mg, 6.22 × 10−3 mmol, 54 equiv) was added under stirring, which resulted in an instantaneous colour change from orange to deep blue, and the
mixture was stirred at r.t. during 4 h. Then, the solvent was removed _in vacuo_ until 0.5 ml was left, 50 ml of distilled pentane was added to the mixture and the fully oxidized complex
precipitated as a dark product. After filtration, this solid was dried under vacuum, dissolved in 2 ml of distilled DCM and a solution of HAuCl4 (1 mg, 2.54 × 10−3 mmol) in 2 ml methanol was
added to the mixture under stirring, forming a blue solution. Then, a solution of NaBH4 (2.35 mg, 6.2 × 10−2 mmol, three equiv.) in 1 ml methanol was added dropwise under stirring, leading
to a colour change from dark blue to deep violet, which indicated the formation of the AuNPs. The mixture was stirred at r.t. for another 10 min. Infrared (KBr): 2,045 cm−1 (_ν_C≡C-Fe(II)).
Ultraviolet–visible: _λ_max 1=410 nm (_ν_Π-Π* ), _λ_max 2=545 nm (plasmon band). Transmission electron microscopy: 1.9±0.3 nm. The synthesis and characterizations of AuNPs stabilized by the
neutral dendron 1 or the dendrimer 6A were conducted analogously. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Wang, Y. _et al._ Metallodendrimers in three oxidation states with
electronically interacting metals and stabilization of size-selected gold nanoparticles. _Nat. Commun._ 5:3489 doi: 10.1038/ncomms4489 (2014). REFERENCES * Newkome, G. R. & Shreiner, C.
Dendrimers derived from 1→3 branching motifs. _Chem. Rev._ 110, 6338–6442 (2010). Article CAS Google Scholar * Myers, V. S. et al. Dendrimer-encapsulated nanoparticles: new synthetic and
characterization methods and catalytic applications. _Chem. Sci._ 2, 1632–1646 (2011). Article CAS Google Scholar * Scott, R. J. W., Wilson, O. M. & Crooks, R. M. Synthesis,
characterization and application of dendrimer-encapsulated nanoparticles. _J. Phys. Chem. B_ 109, 692–704 (2005). Article CAS Google Scholar * Astruc, D., Boisselier, E. & Ornelas, C.
Dendrimers designed for functions: from physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine.
_Chem. Rev._ 110, 1857–1959 (2010). Article CAS Google Scholar * Casado, C. M., Alonso, B., Losada, J. & Garcia-Armada, M. P. in_Designing Dendrimers_ (eds Campagna S., Ceroni P.,
Puntoriero F.) 219–262 (Wiley, 2012). * Ochi, Y. et al. Controlled storage of ferrocene derivatives as redox-active molecules in dendrimers. _J. Am. Chem. Soc._ 132, 5061–5069 (2010).
Article CAS Google Scholar * Andronov, A. & Fréchet, J. M. J. Light-harvesting dendrimers. _Chem. Commun._ 1701–1710 (2000). * Balzani, V., Bergamini, G. & Ceroni, P. Chapter
3–Photochemistry and photophysics of metal complexes with dendritic ligands. _Adv. Inorg. Chem._ 63, 105–135 (2011). Article CAS Google Scholar * Tomalia, D. A., Uppuluri, S., Swanson, D.
R. & Li, J. Dendrimers as reactive modules for the synthesis of new structure-controlled, higher-complexity megamers. _Pure Appl. Chem._ 72, 2343 (2000). Article CAS Google Scholar *
Abruna, H. D. Redox and photoactive dendrimers in solution and on surfaces. _Anal. Chem._ 76, 310–319 (2004). Google Scholar * Wang, S.-H., Shreiner, C. D., Moorefield, C. N. &
Newkome, G. R. Recent progress and applications for metallodendrimers. _New J. Chem._ 31, 1192–1217 (2007). Article Google Scholar * Astruc, D. Electron-transfer processes in dendrimers
and their implication in biology, catalysis, sensing and nanotechnology. _Nat. Chem._ 4, 255–267 (2012). Article CAS Google Scholar * Beer, P. D. & Gale, P. A. Anion recognition and
sensing: the state of the art and future perspectives. _Angew. Chem. Int. Ed._ 40, 486–516 (2001). Article CAS Google Scholar * Casado, C. M. et al. Redox-active ferrocenyl dendrimers and
polymers in solution and immobilised on electrode surfaces. _Coord. Chem. Rev._ 185–186, 53–80 (1999). Article Google Scholar * Armada, M. P. G. et al. Electrocatalytical properties of
polymethylferrocenyl dendrimers and their applications in biosensing. _Bioelectrochemistry_ 69, 65–73 (2006). Article CAS Google Scholar * Djeda, R. et al. Click syntheses of
1,2,3-triazolylbiferrocenyl dendrimers and the selective roles of the inner and outer ferrocenyl groups in the redox recognition of ATP2− and Pd2+. _Angew. Chem. Int. Ed._ 49, 8152–8156
(2010). Article CAS Google Scholar * Arico, A. S., Bruce, P., Scrosati, B., Tarascon, J.-M. & Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage
devices. _Nat. Mater._ 4, 366–377 (2005). Article CAS ADS Google Scholar * Tatykhanova, G. et al. Metal complexes of amphoteric cryogels based on allylamine and methacrylic acid.
_Macromol. Symp._ 317–318, 18–27 (2012). Article Google Scholar * Flanagan, J. B., Margel, S. & Bard, A. Electron transfer to and from molecules containing multiple, noninteracting
redox centers. Electrochemical oxidation of poly(vinylferrocene). _J. Am. Chem. Soc._ 100, 4248–4253 (1978). Article CAS Google Scholar * Connelly, N. J. & Geiger, W. E. Chemical
redox agents for organometallic chemistry. _Chem. Rev._ 96, 877–910 (1996). Article CAS Google Scholar * Geiger, W. E. Organometallic electrochemistry: origins, development, and future.
_Organometallics_ 26, 5738–5765 (2007). Article CAS Google Scholar * Nguyen, P., Gomez-Elipe, P. & Manners, I. Organometallic polymers with transition metals in the main chain. _Chem.
Rev._ 99, 1515–1548 (1999). Article CAS Google Scholar * Eloi, J. C., Chabanne, L., Whittell, G. R. & Manners, I. Resistive switching in transition metal oxides. _Mater. Today_ 11,
28–36 (2008). Article CAS Google Scholar * Paul, F. & Lapinte, C. Organometallic molecular wires and other nanoscale-sized devices: an approach using the organoiron (dppe)Cp*Fe
building block. _Coord. Chem. Rev._ 178–180, 431–509 (1998). Article Google Scholar * Fink, H. et al. Ethynylferrocene compounds of 1,3,5-tribromobenzene. _Organometallics_ 16, 2646–2650
(1997). Article CAS Google Scholar * Weyland, T. et al. Bi- and trimetallic σ-acetylide complexes connected through a phenyl ring in the Fe(Cp*)(dppe) series. _Organometallics_ 16,
2024–2031 (1997). Article CAS Google Scholar * Halet, J.-F. & Lapinte, C. Charge delocalization versus localization in carbon-rich iron mixed-valence complexes: a subtle interplay
between the carbon spacer and the (dppe)Cp*Fe organometallic electrophore. _Coord. Chem. Rev._ 257, 1584–1613 (2013). Article CAS Google Scholar * Diallo, A. K., Absalon, C., Ruiz, J.
& Astruc, D. Ferrocenyl-terminated redox stars: synthesis and electrostatic effects in mixed-valence stabilization. _J. Am. Chem. Soc._ 133, 629–641 (2011). Article CAS Google Scholar
* Sonogashira, K., Tohda, Y. & Hagihara, N. A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines.
_Tetrahedron Lett._ 16, 4467–4470 (1975). Article Google Scholar * Chinchilla, R. & Najera, C. The Sonogashira reaction: a booming methodology in synthetic organic chemistry. _Chem.
Rev._ 107, 874–922 (2007). Article CAS Google Scholar * Meldal, M. & Tornøe, C. W. Cu-catalyzed azide−alkyne cycloaddition. _Chem. Rev._ 108, 2952–3015 (2008). Article CAS Google
Scholar * Hein, J. E. & Fokin, V. V. Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides. _Chem. Soc. Rev._ 39, 1302–1315 (2010).
Article CAS Google Scholar * Liang, L. & Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) ‘click’ reaction and its applications. AN overview. _Coord. Chem. Rev._
255, 2933–2945 (2011). Article CAS Google Scholar * Lee, H. & Ooya, T. Generation-dependent host–guest interactions: solution states of polyglycerol dendrimers of generations 3 and 4
modulate the localization of a guest molecule. _Chem. Eur. J._ 18, 10624–10629 (2012). Article CAS Google Scholar * Robin, M. B., Melvin, B. & Day, P. Mixed-valence chemistry—a survey
and classification. _Adv. Inorg. Chem. Radiochem._ 10, 247–403 (1967). Article CAS Google Scholar * Allen, G. C. & Hush, N. S. Intervalence-transfer absorption. I. Qualitative
evidence for intervalence-transfer absorption in inorganic systems in solution and in the solid state. _Prog. Inorg. Chem._ 8, 357–390 (1967). CAS Google Scholar * Richardson, D. E. &
Taube, H. Mixed-valence molecules: electronic delocalization and stabilization. _Coord. Chem. Rev._ 60, 107–129 (1984). Article CAS Google Scholar * Weyland, T., Costuas, K., Toupet, L.,
Halet, J.-F. & Lapinte, C. Organometallic mixed-valence systems. Two-center and three-center compounds with meta connections around a central phenylene ring. _Organometallics_ 19,
4228–4239 (2000). Article CAS Google Scholar * Chen, X. & Mao, S. S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. _Chem. Rev._ 107,
2891–2959 (2007). Article CAS Google Scholar * Xia, Y., Xiong, Y., Lim, B. & Skrabalak, S. E. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics?
_Angew. Chem. Int. Ed._ 48, 60–103 (2009). Article CAS Google Scholar * Saha, K., Agasti, S. S., Kim, C., Li, X. & Rotello, V. M. Gold nanoparticles in chemical and biological
sensing. _Chem. Rev._ 112, 2739–2779 (2012). Article CAS Google Scholar * Daniel, M.-C. & Astruc, D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related
properties, and applications toward biology, catalysis, and nanotechnology. _Chem. Rev._ 104, 293–346 (2004). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We gratefully
acknowledge the financial support by the China Scholarship Council (CSC) from China (PhD grant to Y.W.), the University of Bordeaux and the Centre National de la Recherche Scientifique
(CNRS). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * ISM, UMR CNRS No. 5255, Université de Bordeaux, Talence, 33405, France Yanlan Wang, Jaime Ruiz & Didier Astruc * LCC, CNRS, 205
Route de Narbonne, Toulouse, 31077, France Lionel Salmon Authors * Yanlan Wang View author publications You can also search for this author inPubMed Google Scholar * Lionel Salmon View
author publications You can also search for this author inPubMed Google Scholar * Jaime Ruiz View author publications You can also search for this author inPubMed Google Scholar * Didier
Astruc View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.W. and D.A. conceived and designed the experiments and wrote the paper. Y.W.
performed the experiments. Y.W. and J.R. conducted the electrochemical measurements. L.S. conducted Mössbauer and transmission electron microscopic experiments. CORRESPONDING AUTHOR
Correspondence to Didier Astruc. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION
Supplementary Figures 1-82, Supplementary Tables 1-4, Supplementary Notes 1-7 and Supplementary Methods (PDF 4941 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE
THIS ARTICLE Wang, Y., Salmon, L., Ruiz, J. _et al._ Metallodendrimers in three oxidation states with electronically interacting metals and stabilization of size-selected gold nanoparticles.
_Nat Commun_ 5, 3489 (2014). https://doi.org/10.1038/ncomms4489 Download citation * Received: 22 November 2013 * Accepted: 21 February 2014 * Published: 01 April 2014 * DOI:
https://doi.org/10.1038/ncomms4489 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative