Histology and postural change during the growth of the ceratopsian dinosaur psittacosaurus lujiatunensis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT A few dinosaurs are inferred to have undergone an ontogenetic shift from quadrupedal-to-bipedal posture, or vice versa, based on skeletal allometry. The basal ceratopsian
_Psittacosaurus lujiatunensis_ is considered to have been mainly bipedal as an adult. Here we infer a postural shift in this species based on a novel combination of limb measurements and
histological data. The forelimb is strongly negatively allometric relative to the hindlimb, and patterns of vascular canal orientation provide evidence that growth of the hindlimb was
particularly rapid during the middle part of ontogeny. Histology also makes it possible to determine the ontogenetic ages of individual specimens, showing that the forelimb-to-hindlimb ratio
changed rapidly during the first or second year of life and thereafter decreased gradually. Occurrence of an ontogenetic shift from quadrupedality to bipedality was evidently widespread in
dinosaurs, and may even represent the ancestral condition for the entire group. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS
3D HINDLIMB JOINT MOBILITY OF THE STEM-ARCHOSAUR _EUPARKERIA CAPENSIS_ WITH IMPLICATIONS FOR POSTURAL EVOLUTION WITHIN ARCHOSAURIA Article Open access 21 September 2020 FUNCTIONAL
REORGANISATION OF THE CRANIAL SKELETON DURING THE CYNODONT–MAMMALIAFORM TRANSITION Article Open access 12 April 2023 A MISSISSIPPIAN (EARLY CARBONIFEROUS) TETRAPOD SHOWING EARLY
DIVERSIFICATION OF THE HINDLIMBS Article Open access 14 April 2022 INTRODUCTION The first dinosaurs were bipeds, but quadrupedality evolved secondarily in at least four groups: giant
sauropodomorphs, thyreophorans, derived ornithopods and ceratopsians1. However, ontogenetic studies have shown that even some dinosaurs that remained bipedal as adults had quadrupedal
offspring2, while conversely some dinosaurs that were quadrupedal when mature had bipedal offspring3,4. The primary evidence for these postural shifts has come from allometric studies
showing shortening or lengthening of the forelimbs, relative to the hindlimbs, over the course of ontogeny. However, no study has simultaneously investigated both limb bone allometry and
limb bone histology in any dinosaur, even though histological evidence would make it possible to establish the approximate ontogenetic ages of the specimens included in the analysis and
therefore address the timing of postural changes. Furthermore, patterns of differential growth should be reflected in the microstructure of the forelimb and hindlimb bones. Histology has
considerable potential as a supplement to allometric studies of dinosaurs, and can potentially provide a continuous record of the growth of an individual bone up to the time of an animal’s
death. By contrast, measurements of the dimensions of a limb bone offer only a ‘snapshot’ of the size the bone had reached at that time. However, an obstacle limiting this application of
histological analysis is the difficulty of obtaining a sufficiently large sample of individuals at different ontogenetic stages whose limb bones can be sectioned. _Psittacosaurus_ is among
the most diverse and abundant dinosaurs, known from 10 or more species and >1,000 specimens from the upper Lower Cretaceous of China, Mongolia, Russia and Thailand5. It is a basal member
of Ceratopsia, a group that subsequently diversified in the northern continents during the Late Cretaceous to produce a species-rich assemblage of large, horned herbivores. _Psittacosaurus_
is widely interpreted as an obligate or at least habitual biped as an adult6,7,8,9,10,11,12, and lay phylogenetically near or even within the transition to the obligate quadrupedality that
was characteristic of ceratopsids and possibly some of their closest relatives12,13,14. For these reasons, _Psittacosaurus_ is of intense interest from the perspective of dinosaurian
postural evolution, and its abundance in the Lower Cretaceous of Asia makes it a natural subject for palaeobiological analyses in which sample size is a factor. In this paper, we analyse the
limb proportions and histology of _Psittacosaurus_ based on many hatchling to adult specimens, to determine whether a postural shift took place during the growth of this dinosaur and
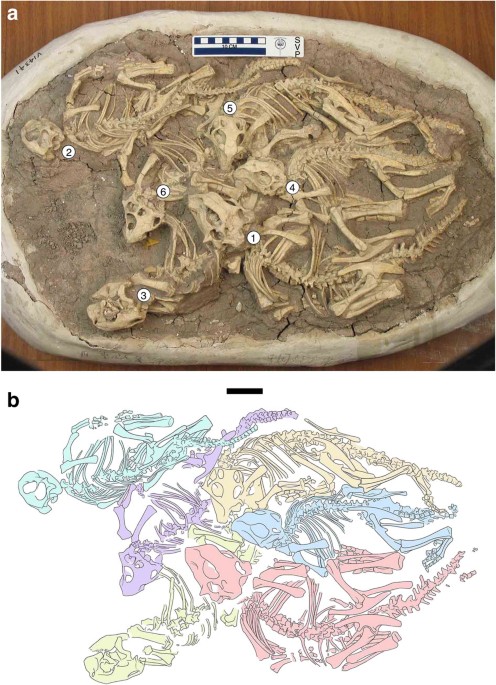
investigate the ontogenetic timing of any shift that can be inferred. All of the 16 individuals in our sample were collected from the Lujiatun Beds of the Lower Cretaceous Yixian Formation
exposed near Lujiatun Village, Beipiao City, Liaoning Province, China. The majority (10 skeletons) are juveniles that were preserved in clusters of individuals apparently representing the
same stage of growth. One of these clusters, accessioned as IVPP (Institute of Vertebrate Paleontology & Paleoanthropology, Beijing) V14341, has previously been interpreted15 as
resulting from burial in a volcanic debris flow that killed the animals instantly. Juvenile individuals were selected for analysis from IVPP V14341 and three similar clusters, but only some
of the specimens in each cluster were sufficiently well preserved for inclusion in the study. Almost all of the individuals included in this study, including the two from the cluster
described by Zhao _et al._15, are clearly referable to _P. lujiatunensis_ Zhou _et al._16 All of them have narrow prefrontals, an autapomorphy of _P. lujiatunensis_5,16. A shallow depression
on the jugal is also present in all the individuals in the sample. This feature is uniquely shared by _P. lujiatunensis_ and _Psittacosaurus major_, the only other _Psittacosaurus_ species
known from the Lujiatun Beds. However, _P. major_ has a characteristically narrow skull roof, a trait not evident in any of the specimens in this study. Other aspects of cranial morphology
observed in the specimens are consistent with their referral to _P. lujiatunensis_, based on published diagnoses5,16. The only possible exception is the largest individual in the sample
(IVPP V12617), which was originally described as an adult of _Hongshanosaurus houi_17. However, this specimen differs in important respects from the juvenile holotype of _H. houi_, and has
been more recently referred to _P. lujiatunensis_5,18. This conclusion is tentatively accepted in the present study. Allometric analysis of limb bone proportions within this large sample of
_P. lujiatunensis_ shows that the bones of the forelimb are negatively allometric relative to those of the hindlimb, and histological evidence reinforces this result by indicating
particularly rapid growth of the hindlimb during the middle part of ontogeny. _P. lujiatunensis_ appears to have undergone a shift from quadrupedality towards bipedality during ontogenetic
growth, a pattern that may have been widespread among dinosaurs. RESULTS DINOSAUR AGE PROFILES One specimen (IVPP V14342) in the data set was unavailable for histological sampling, but for
every other individual we took histological sections from the humerus, radius, ulna, femur, tibia and/or fibula. Across all individuals, a total of 41 bones were sectioned. Three of the
individuals were from a cluster (IVPP V16902) of very small juveniles, and sectioning their long bones revealed no lines of arrested growth (LAG). Accordingly, they appear to have been
hatchlings <1 year old at the time of death, and they probably represent the smallest _Psittacosaurus_ specimens ever reported (femur lengths 22 and 26 mm). The other individuals in the
sample were at least 1 year old. Of the six skeletons from the cluster IVPP V14341 (Fig. 1), five were found to be 2 years old whereas the sixth was found to be 3 years old (Supplementary
Fig. S1), indicating slight heterogeneity in the ages of the individuals in this apparent social group. Four of the individuals were from another cluster (ELDM, Erlianhaote Dinosaur Museum,
V1038, Supplementary Fig. S2), and were all found to be 2 years old. All individuals in the data set that were preserved in isolation (rather than as part of a cluster) and could be studied
histologically were at least 5 years old and ranged up to 10 years old (Supplementary Table S1), although the single isolated skeleton (IVPP V14342) that could not be studied histologically
was close in size to the 3 years old in cluster IVPP V14341. Conversely, all the specimens in clusters that were studied histologically were 3 years old or younger. LIMB ALLOMETRY Length
measurements from _P. lujiatunensis_ long bones demonstrate that the humerus, radius and ulna are negatively allometric relative to the femur, with allometric coefficients of 0.89, 0.72 and
0.78, respectively (Fig. 2, Table 1, Supplementary Table S2). The growth of the tibia and fibula relative to the femur is statistically distinguishable from isometry, but the coefficients
(0.97 and 0.94, respectively; Fig. 2, Table 1, Supplementary Table S2) indicate only minimal negative allometry. The tibia remained slightly longer than the femur (averaging 110% of femoral
length across the entire sample) throughout ontogeny. The forelimb became shorter relative to the hindlimb over the ontogeny of _P. lujiatunensis_ (Fig. 3), the allometric coefficient of
forelimb length relative to hindlimb length being 0.83 (Table 1, Supplementary Table S2). In the three hatchling (<1 year old) individuals the forelimb-to-hindlimb ratio (the ratio of the
combined length of the humerus and radius to that of the femur and tibia) averaged 0.84. The ratio averaged 0.70 in 1 year olds, but thereafter decreased gradually and steadily to an
average of 0.61 in 7 year olds. The single 10-year-old specimen had an anomalously large ratio of 0.66, suggesting unusual proportions. The anomaly might be interpreted to support the
original referral of this individual to _H. houi_17, but other aspects of this specimen’s morphology make a strong case for assigning it to _P. lujiatunensis_5,16. BONE HISTOLOGY The thin
sections of the 41 bones included in the histological analysis reveal many important details of vascular canal orientation and other aspects of microstructure. In all sections, the cortex is
composed of fibrolamellar bone. The degree of vascularization is high in early growth, but appears to decrease abruptly beginning at the age of 4 or 5. Similarly, only primary osteons are
present in younger individuals in the data set, but secondary osteons are visible in the long bones of all specimens that are at least 5 years old. Only the largest specimen shows evidence
of remodelling of the secondary osteons. Forelimb and hindlimb bones show different patterns of canal orientation during ontogeny (Fig. 4), although only limited information is available for
the radius and ulna. Throughout most of ontogeny a combination of reticular and longitudinal canals is present in the humerus, the proportion of reticular canals being higher during the
earlier half of growth and reaching a maximum in second-year bone (that is, bone deposited during the second year of life, between the first and second LAGs). In third-year bone both radial
and reticular canals, as well as longitudinal ones, are present (Fig. 5a). These patterns suggest that bone was deposited rapidly during early growth of the humerus, but that the rate of
deposition decreased in later ontogeny. For the radius and ulna, good histological evidence is available only for the first 3 years of growth, but bone formed during this period also shows a
relatively high proportion of reticular canals in these elements. In the radius, third-year bone also contains radial canals, as in the humerus (Fig. 5b). The evidence based on canal
orientation from the entire forelimb suggests rapid deposition of bone, consistent with a high level of vascularization during early growth. Patterns of canal orientation in the hindlimb,
however, are strikingly different. No hindlimb section shows radial canals at any stage of growth (Figs 5 and 6), and the proportion of reticular as opposed to longitudinal canals is highest
in bone formed during middle (approximately from ages three to six) rather than early ontogeny (Fig. 5c). The middle period of growth was therefore the time when bone was being deposited
most rapidly in the hindlimb. DISCUSSION The observed changes in vascular canal orientation during the growth of particular long bones suggest shifts in the rate of bone deposition that may
correlate with postural changes. This line of evidence indicates that deposition of bone was most rapid during early ontogeny in the forelimb elements. In the humerus, at least, growth
appears to have slowed following deposition of the third LAG. In the hindlimb bones, by contrast, growth appears to have been most rapid during the fourth through sixth years of life.
Unfortunately, histology does not provide a basis for directly comparing rates of bone deposition between the forelimb and the hindlimb. However, the fact that the forelimb appears to grow
most rapidly during early ontogeny, whereas the hindlimb grows most rapidly during middle ontogeny, suggests a postural shift from quadrupedality to bipedality during the growth of _P.
lujiatunensis_. In early ontogeny, the forelimb may have been growing rapidly, to maintain its proportional length and role in locomotion, whereas in middle ontogeny the simultaneous slowing
of forelimb growth and acceleration of hindlimb growth may have caused the hindlimb to increase in relative length and assume primary responsibility for locomotion. However, the nature of
the linkage between vascular canal orientation and the rate of length increase of a given bone requires further investigation, and the hypothesis that a postural shift took place in the
growth of _P. lujiatunensis_ requires further testing on the basis of limb bone measurements (see below). Histological patterns observed in this study (Fig. 4) differ in some respects from
those previously reported in a growth series of _Psittacosaurus mongoliensis_ from the Lower Cretaceous of Mongolia19. The largest hindlimb bones (femur and tibia) in this growth series,
representing individuals up to nine years old, show deposition of radially vascularized fibrolamellar bone along part of the mid-shaft circumference beginning at age 7. Other parts of the
mid-shaft circumference have only reticular or even longitudinal vascularization. This indicates that local apposition rate differed greatly along the circumference of the bone, presumably
reflecting osseous drift19. One possible explanation for the occurrence of radial vascularization is a postural shift ‘from bipedality to quadrupedality’19, but this is unlikely given the
strong evidence that adult individuals of _Psittacosaurus_ were mainly bipedal (see below). The fitted growth curve for _P. mongoliensis_19 indicates that at age 9 the largest sampled
individuals had reached perhaps 80% of final body mass, a conclusion consist with the lack of an external fundamental system in any of the sampled bones. The largest histologically sampled
individuals of _P. mongoliensis_ thus appear to have been well short of final adult size and still within the exponential phase of growth19, perhaps indicating that the presence of radial
canals in the hindlimb bones reflects normal growth along a sigmoid curve of mass versus age as in other dinosaurs including _P. lujiatunensis_20. However, the fact that the radial canals
are limited to the hindlimb implies fast growth of the hindlimb relative to the forelimb at this stage of ontogeny, perhaps indicating a quadrupedal-to-bipedal postural shift like that
inferred for _P. lujiatunensis_ in the present study. However, radial canals were not observed in any of the hindlimb bones that we sectioned, suggesting that their occurrence in _P.
mongoliensis_ represents either a genuine difference in growth pattern between the two species or a result of pathology19. The largest _P. mongoliensis_ femur sampled was 210 mm long,
representing a 9-year-old animal that had still not reached maximal body size. By contrast, the largest known _P. lujiatunensis_ femur measures 202 mm (ref. 20), and must have been close to
final size because the largest femur sampled in the present study is only about 160 mm long but appears almost fully grown on the basis of histology. This femur represents an individual
(IVPP V12617) that we consider to be 10 years old on histological grounds, although it was previously interpreted as a 6 year old based on femur size alone20. Femur length comparisons
informed by histology suggest larger adult body size in _P. mongoliensis_, but inferred growth curves for the two species19,20 show a considerably higher mass for _P. lujiatunensis_ than for
_P. mongoliensis_ (38 kg in the former versus 25 kg in the latter). There are several possible solutions to this paradox, including the fact that the mass estimates used to construct the
growth curves were based on circumference rather than length measurements19,20 and could represent a difference in robustness between the two species, but distinguishing among the various
possibilities will require an analysis directly comparing the two species in terms of both histology and skeletal proportions. We note, however, that both logistic growth curves extrapolate
well beyond the available histological data, and we therefore prefer the direct histological and meristic evidence suggesting that _P. mongoliensis_ grew to a larger final size than _P.
lujiatunensis_. The greater inferred adult femur length of _P. mongoliensis_ presumably indicates that the body mass of mature individuals was also greater in this species than in _P.
lujiatunensis_, unless the two taxa were strikingly different in their proportions. We conclude that the histological evidence for a postural shift presented in this study for _P.
lujiatunensis_ is corroborated by histological evidence20 in _P. mongoliensis_. In both cases, histology shows a differential change in bone apposition at mid-shaft, with the hindlimb bones
speeding up growth relative to the forelimb bones. The specific changes in histology differ between the two species of _Psittacosaurus_, and the shift appears to have happened at a larger
body size in _P. mongoliensis_ than in _P. lujiatunensis_, consistent with the presumably larger final body size of the former. _Psittacosaurus_ has been generally regarded as a habitual or
obligate biped6,7,8,10,11,12, although the structure of the manus6,7 and a few other skeletal features21 have been interpreted as indicating some quadrupedal capability. A recent analysis of
the distribution of osteological correlates of quadrupedality among ornithischians strongly favoured the view that _Psittacosaurus_ was mainly bipedal22. Ornithischian quadrupeds typically
possess a large anterolateral process on the ulna, hoof-like manual unguals, an everted dorsal edge of the ilium, a reduced fourth trochanter, and a femur that is longer than the tibia. In
_P. lujiatunensis_ specimens of any age the proximal end of the ulna bears only a small, laterally directed bulge, the dorsal edge of the ilium is narrow and non-everted, the fourth
trochanter is large and pendant when preserved intact, and the tibia is slightly longer than the femur. Few well-preserved manual unguals are present in our sample. Available examples are
shorter relative to their width than the pedal unguals, but are pointed rather than hoof-like. This suite of characters, which is shared with other very basal ceratopsians22, strongly
suggests that _P. lujiatensis_ was bipedal. However, this interpretation may only apply to adults. Ontogenetic changes in the various indicators of quadrupedality have not been
investigated22, and it is uncertain how the features in question would be affected by an ontogenetic postural shift of the kind inferred for some dinosaurs. The forelimb-to-hindlimb ratio
has traditionally been used as a postural indicator in dinosaurs23, with high ratios indicating quadrupedality. However, the utility of this metric in distinguishing quadrupeds from bipeds
may be limited22. The ratio in subadult to adult _P. lujiatunensis_, as determined in this study (0.59–0.66), corresponds to the lower part of that reported by Maidment and Barrett22 for
eurypodan thyreophorans, even though other osteological indicators point clearly to bipedality for _Psittacosaurus_ and quadrupedality for eurypodans. Similarly, the ancestral value of 0.67
reconstructed by Maidment and Barrett22 for the unambiguously quadrupedal ceratopsids falls only just outside the range for subadult to adult _P. lujiatunensis_. There appears to be a range
of values consistent with either bipedality or quadrupedality. However, the forelimb-to-hindlimb ratio is extremely high (averaging 0.84) in hatchlings of _P. lujiatunensis_, far exceeding
values typical of eurypodans, ceratopsids and other quadrupedal dinosaurs22. The extreme values seen in hatchlings strongly suggest that they were essentially quadrupeds. This points to an
ontogenetic shift along the continuum from quadrupedality to bipedality in the ontogeny of _P. lujiatunensis_, although the shift was not necessarily between obligate versions of the former
and latter conditions. It is possible, for example, that hatchlings were primarily quadrupedal but resorted to bipedality at high speeds, whereas adults were quadrupedal only when moving
very slowly. However, the proportionally long forelimbs of hatchlings, combined with the skeletal evidence for bipedality in adults, clearly implies that at least a limited postural shift
took place. We envision the body of _P. lujiatunensis_ as fundamentally suited to bipedality22. Hatchlings, however, were equipped with long forelimbs that could have been placed on the
substrate when necessary to provide stability, particularly during slow locomotion. As an individual matured, its forelimb would have grown proportionally shorter, its balance would
presumably have improved, and the forelimb would have contacted the substrate less often. Histology contributes to understanding of the ontogeny of posture in _P. lujiatunensis_ in that
counting LAGs makes it possible to relate changes in forelimb-to-hindlimb ratio to age in years, demonstrating that very high values were confined to specimens with less than one LAG.
One-year-olds may have already been significantly less quadrupedal than hatchlings, and any subsequent changes were gradual. Growth patterns inferred from measurements of long bones can also
be compared with patterns of bone deposition inferred from histology. The two lines of evidence agree, and reinforce each other, to the extent that both suggest a shift from quadrupedality
to bipedality based on an increasing length discrepancy between the hindlimb and the forelimb over the course of ontogeny. However, histology also suggests that the shift occurred sometime
after completion of the third year of growth, when forelimb growth accelerated and hindlimb growth was retarded, while measurements of long bones imply that the shift was at least initiated
earlier based on rapid decrease in the forelimb-to-hindlimb ratio during early ontogeny. Furthermore, long-bone measurements do not appear to confirm the histological evidence for
particularly rapid growth of the forelimb bones during early ontogeny, and particularly rapid growth of the hindlimb bones during middle ontogeny. It is likely that these discrepancies
between histological evidence and limb bone measurements regarding growth in _P. lujiatunensis_ are at least partly the result of inadequate sample size, and random variation in the limb
proportions of the individuals in the data set. Although measurements of limb bones provide strong evidence for a shift towards greater bipedality, these data may be spread too thinly across
ontogeny to reliably pinpoint when and how the shift took place. However, the discrepancies between histological and measurement-based inferences about growth also suggest that the
relationship between vascular canal orientation and rate of increase in actual bone dimensions requires further investigation, and may be a particularly fruitful area for future research.
The negative forelimb allometry that evidently characterized the ontogeny of _P. lujiatunensis_ is comparable to that previously reported in the sauropodomorph dinosaur _Massospondylus_,
which is inferred based on patterns of skeletal allometry to have been quadrupedal as a hatchling but to have shifted to a bipedal posture later in ontogeny2,24. Even hatchlings of at least
some therizinosaurian theropods may have been preferentially quadrupedal, given the existence of embryos whose forelimbs are nearly equal in length to their hindlimbs25, although in the
absence of preserved hatchlings this inference must be considered uncertain. An ontogenetic shift from quadrupedality to bipedality has also been inferred in the ornithopod _Dryosaurus_,
based on changes in the femoral cross-sectional geometry during growth26. By contrast, the ornithopods _Maiasaura_ and _Iguanodon_ are inferred to have undergone a postural shift from
juvenile bipedality to adult quadrupedality3,4. Our findings add to a growing body of evidence suggesting that ontogenetic quadrupedal-to-bipedal postural shifts were widespread in
dinosaurs. Such shifts have now been inferred to have taken place in some members of at least two of the three major dinosaurian clades, Sauropodomorpha and Ornithischia. There is even a
possible example of such a shift within the third clade, Theropoda, based on the elongate forelimbs that have been reported in therizinosaurian embryos25. However, the humerus measures
55–65% of the length of the femur in some theropod embryos, including oviraptorid27 and troodontid28 specimens, in striking contrast to the near-equality in length between the humerus and
femur seen in the therizinosaurians25. It is clear that hatchlings were bipedal in some theropods, even if they were quadrupedal in at least some therizinosaurians. Selection acting on
juveniles must have favoured bipedal hatchlings in some theropods and ornithopods, but quadrupedal hatchlings in other theropods, some ornithischians, and at least some sauropodomorphs. The
possibility that basal members of all three major dinosaurian clades retained a quadrupedal hatchling stage is intriguing and cannot presently be excluded, but awaits further testing based
on future discoveries. Similarly, the quadrupedal-to-bipedal shifts that occur in at least some taxa may recapitulate the evolutionary transition from quadrupedal basal archosaurs to bipedal
avemetatarsalians that took place during the Early and Middle Triassic29,30, but this scenario will remain speculative until more information is available regarding the ontogeny of the
early avemetatarsalian bipeds themselves. Our study highlights the benefits of considering histology alongside limb proportions and qualitative morphological features in studying the
ontogeny of posture and locomotion in a fossil tetrapod. Counting LAGs makes it possible to roughly determine the age of each individual sampled, which provides a basis for determining the
timing of changes in both limb bone measurements and patterns of vascular canal orientation. Measurements of limb bones for individuals of known ages can be used to build up a direct but
discontinuous record of growth, defined as increase in bone dimensions, whereas the complementary record provided by vascular canal orientation and other histological features is indirect
but continuous. Quantitative analysis of the link between histological indicators of bone growth and changes in the actual dimensions of long bones remains as an intriguing avenue for future
research, and one that may eventually make it possible to use histology as a basis for more precise inferences about growth patterns and postural shifts. METHODS HISTOLOGICAL SECTIONING
Histological thin sections of long bones were made using standard techniques. Previous studies of dinosaur long-bone histology, as well as general principles of bone growth, indicate that a
section taken at the middle of the shaft of a long bone is optimal for obtaining a maximally complete growth record from that bone19,31,32. This arises from the predominantly appositional
growth of this part of the shaft, and the location of the neutral zone in this region32. Specimens were embedded in resin, and mid-shaft, diaphyseal transverse thin sections were cut using a
diamond circular saw fitted with a diamond-tipped wafering blade. One surface of each section was smoothed with a wheel grinder/polisher, and then ground manually using grinding powder (600
grit) to produce a smooth texture ideal for gluing to a glass slide. The section was then cut to a thickness of about 250 μm with a diamond circular saw before being ground further to the
desired final thickness of 50–80 μm, leaving the exposed surface of the section smooth. Each slide was then cleaned in a water-filled ultrasonic cleaner to remove microscopic grit, and
finally capped with a glass cover slip. The completed thin sections were studied in normal and polarized light. AGE DETERMINATION The histological sections made it possible to estimate the
age in years of each individual at the time of death, based on the typical pattern of formation of LAGs on an annual basis in dinosaur long bones. In sections that showed a small medullary
cavity, the number of visible LAGs was taken to correspond approximately to the individual’s age in years, although it is unlikely that each LAG was formed exactly on the anniversary of
hatching. Strictly speaking, the number of LAGs can be taken as a maximum bound on age in years, with a possible error of several months, but in this paper we use the phrase ‘_N_-year-old
individual’ to mean ‘individual in which _N_ LAGs had formed’. However, the phenomenon of enlargement of the medullary cavity during growth introduced a well-understood complication33, in
that such enlargement occurs through internal resorption of the bone cortex that may eliminate LAGs beginning with the innermost. To avoid underestimating ages as a result of this type of
erosion, we used smaller examples of the bone in question as a reference point. The section being evaluated was graphically superimposed on equivalently orientated sections from smaller
bones, and any LAG from the smaller bone that was entirely overlapped by the medullary cavity of the larger bone was assumed to be missing in the latter. For example, only two LAGs are
visible in the tibia of IVPP V14341.1, but superimposing the section of this tibia on tibial sections from smaller individuals indicated that the medullary cavity of IVPP V14341.1 was large
enough to have subsumed the first LAG formed during growth of the tibia (Supplementary Figs S3–4). Accordingly, the LAGs visible in this specimen were identified as the second and third,
rather than the first and second, and the specimen was identified as a 3 years old. Similarly, it can be inferred that the three innermost LAGs were obliterated by medullary cavity expansion
in the humerus of the adult specimen IVPP V12617 (Fig. 7). VASCULAR CANAL ORIENTATION We classified vascular canals visible in our thin sections as longitudinal, radial or reticular33, and
noted which canal types were present in each growth interval (that is, interval bounded by LAGs) in each section. The relative numbers of the different canal types present were visually
estimated. Provided that the medullary cavity had not expanded enough to destroy part of the record, a specimen with _N_ LAGs could provide information about the vascularity of bone formed
during the first year of growth (first-year bone), and bone formed during all subsequent years up to N+1. For example, a specimen with two LAGs could provide information about the
vascularity of first year, second year and third year bone. Given that reticular and particularly radial canals are associated with relatively rapid deposition of bone, based on evidence
from living birds34, their presence was taken as an indication of rapid deposition in _P. lujiatunensis_. LIMB BONE ALLOMETRY Coefficients of allometry were calculated for the lengths of the
humerus, radius, ulna, tibia and fibula relative to that of the femur, and for forelimb (humerus+radius) length relative to hindlimb (femur+tibia) length. The length measurements were
log-transformed and then saved as a.txt file that could be read by the computer program R (R Development Core Team, 2008). As in previous work24, the slope of the regression line on a
bivariate plot of the lengths of two bones or limbs was interpreted as the allometric coefficient of length for one bone or limb relative to the other. Linear regressions were carried out in
R, using the lm command. In all cases the 95% confidence interval for the slope excluded the isometric value of 1, indicating statistically significant negative (for coefficients <1) or
positive (for coefficients >1) allometry relative to the femur or hindlimb. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Zhao, Q. _et al._ Histology and postural change during the
growth of the ceratopsian dinosaur _Psittacosaurus lujiatunensis_. _Nat. Commun._ 4:2079 doi: 10.1038/ncomms3079 (2013). REFERENCES * Carrano, M. T. in_The Sauropods: Evolution and
Paleobiology_ eds Curry Rogers K. A., Wilson J. A. 229–251University of California Press (2005). * Reisz, R. R., Evans, D. C., Sues, H. D. & Scott, D. Embryonic skeletal anatomy of the
sauropodomorph dinosaur _Massospondylus_ from the Lower Jurassic of South Africa. _J. Vert. Paleontol._ 30, 1653–1665 (2010). Article Google Scholar * Dilkes, D. W. An ontogenetic
perspective on locomotion in the Late Cretaceous dinosaur _Maiasaura peeblesorum_ (Ornithischia: Hadrosauridae). _Can. J. Earth Sci._ 38, 1205–1227 (2001). Article ADS Google Scholar *
Norman, D. B. On the ornithischian dinosaur _Iguanodon bernissartensis_ from the Lower Cretaceous of Bernissart (Belgium). _Institut Royal des Sciences Naturelles de Belgique, Memoires_ 178,
1–103 (1980). Google Scholar * Sereno, P. C. in_New Perspectives on Horned Dinosaurs: The Royal Tyrrell Museum Ceratopsian Symposium_ eds Ryan M. J., Chinnery B. J., Eberth D. A.
21–58Indiana University Press (2010). * Sereno, P. C. in_The Encyclopedia of Dinosaurs_ eds Currie P. J., Padian K. P. 611–613Academic Press (1997). * Sereno, P. C. in_The Dinosauria_ eds
Weishanpel D. B., Dodson P., Osmlska H. 579–592University of California Press (1990). * Osborn, H. F. _Psittacosaurus_ and _Protiguanodon_: two Lower Cretaceous iguanodonts from Mongolia.
_Am. Mus. Novit._ 127, 1–16 (1924). Google Scholar * You, H. L. & Dodson, P. in_The Dinosauria_ eds Weishampel D. B., Dodson P., Osmolska H. 478–493Univeristy of California Press
(2004). * Chinnery, B. Morphometric analysis of evolutionary trends in the ceratopsian postcranial skeleton. _J. Vert. Paleontol._ 24, 591–609 (2004). Article Google Scholar * Chinnery, B.
J. & Horner, J. R. A new neoceratopsian dinosaur linking North American and Asian taxa. _J. Vert. Paleontol._ 27, 625–641 (2007). Article Google Scholar * Senter, P. Analysis of
forelimb function in basal ceratopsians. _J. Zool._ 273, 305–314 (2007). Article Google Scholar * Hutchinson, J. R. & Gatesy, S. M. Dinosaur locomotion - beyond the bones. _Nature_
440, 292–294 (2006). Article CAS ADS PubMed Google Scholar * Xu, X., Forster, C. A., Clark, J. M. & Mo, J. Y. A basal ceratopsian with transitional features from the Late Jurassic
of northwestern China. _Proc. R. Soc. B_ 273, 2135–2140 (2006). Article PubMed Google Scholar * Zhao, Q., Barrett, P. M. & Eberth, D. A. Social behaviour and mass mortality in the
basal ceratopsian dinosaur _Psittacosaurus_ (Early Cretaceous, People’s Republic of China). _Palaeontology_ 50, 1023–1029 (2007). Article Google Scholar * Zhou, C. F., Gao, K. Q., Fox, R.
C. & Chen, S. H. A new species of _Psittacosaurus_ (Dinosauria: Ceratopsia) from the Early Cretaceous Yixian Formation, Liaoning, China. _Palaeoworld_ 15, 100–114 (2006). Article Google
Scholar * You, H. L. & Xu, X. An adult specimen of _Hongshanosaurus houi_ (Dinosauria: Psittacosauridae) from the Lower Cretaceous of Western Liaoning Province, China. _Acta Geologica
Sinica (English Edition)_ 79, 168–173 (2005). Article Google Scholar * Zhou, C. F., Gao, K. Q., Du, K. X., Qi, W. W. & Zhang, S. Advances in the study of psittacosaurids and the
application of CT Scan. _Acta Scientiarum Naturalium Universitatis Pekinensis_ 42, 146–152 (2006). Google Scholar * Erickson, G. M. & Tumanova, T. A. Growth curve of _Psittacosaurus
mongoliensis_ Osborn (Ceratopsia: Psittacosauridae) inferred from long bone histology. _Zool. J. Linn. Soc._ 130, 551–566 (2000). Article Google Scholar * Erickson, G. M., Makovicky, P.
J., Inouye, B. D., Zhou, C. F. & Gao, K. Q. A life table for _Psittacosaurus lujiatunensis_: initial insights into ornithischian dinosaur population biology. _Anat. Rec._ 292, 1684–1684
(2009). Article Google Scholar * Maryanska, T. & Osmólska, H. On ornithischian phylogeny. _Acta Palaeontol. Pol._ 30, 137–150 (1985). Google Scholar * Maidment, S. C. R. &
Barrett, P. M. Osteological correlates for quadrupedality in ornithischian dinosaurs. _Acta Palaeontol. Pol_ In press. ( doi:10.4202/app.2012.0065). * Galton, P. M. The posture of
hadrosaurian dinosaurs. _J. Paleontol._ 44, 464–473 (1970). Google Scholar * Reisz, R. R., Scott, D., Sues, H. D., Evans, D. C. & Raath, M. A. Embryos of an Early Jurassic prosauropod
dinosaur and their evolutionary significance. _Science_ 309, 761–764 (2005). Article CAS ADS PubMed Google Scholar * Kundrat, M., Cruickshank, A. R. I., Manning, T. W. & Nudds, J.
Embryos of therizinosauroid theropods from the Upper Cretaceous of China: diagnosis and analysis of ossification patterns. _Acta Zoologica_ 89, 231–251 (2008). Article Google Scholar *
Heinrich, R. E., Ruff, C. B. & Weishampel, D. B. Femoral ontogeny and locomotor biomechanics of _Dryosaurus lettowvorbecki_ (Dinosauria, Iguanodontia). _Zool. J. Linn. Soc._ 108, 179–196
(1993). Article Google Scholar * Weishampel, D. B. et al. New oviraptorid embryos from Bugin-Tsav, Nemegt Formation (Upper Cretaceous), Mongolia, with insights into their habitat and
growth. _J. Vert. Paleontol._ 28, 1110–1119 (2008). Article Google Scholar * Varricchio, D. J., Horner, J. R. & Jackson, F. D. Embryos and eggs for the Cretaceous theropod dinosaur
_Troodon formosus_. _J. Vert. Paleontol._ 22, 564–576 (2002). Article Google Scholar * Sereno, P. C. Basal archosaurs: Phylogenetic relationships and functional implications. _J. Vert.
Paleontol._ 11, 1–53 (1991). Article Google Scholar * Brusatte, S. L., Niedzwiedzki, G. & Butler, R. J. Footprints pull origin and diversification of dinosaur stem lineage deep into
Early Triassic. _Proc. R. Soc. B_ 278, 1107–1113 (2011). Article PubMed Google Scholar * Horner, J. R., De Ricqles, A. & Padian, K. Long bone histology of the hadrosaurid dinosaur
_Maiasaura peeblesorum_: growth dynamics and physiology based on an ontogenetic series of skeletal elements. _J. Vert. Paleontol._ 20, 115–129 (2000). Article Google Scholar * Sander, P.
M. Longbone histology of the Tendaguru sauropods: implications for growth and biology. _Paleobiology_ 26, 466–488 (2000). Article Google Scholar * Chinsamy-Turan, A. _The Microstructure of
Dinosaur Bone: Deciphering Biology with Fine-Scale Techniques_ 195The Johns Hopkins University Press (2005). * de Margerie, E. et al. Assessing a relationship between bone microstructure
and growth rate: a fluorescent labelling study in the king penguin chick (_Aptenodytes patagonicus_). _J. Exp. Biol._ 207, 869–879 (2004). Article CAS PubMed Google Scholar Download
references ACKNOWLEDGEMENTS We thank K. Stein and S. Hayashi for help and training in thin section preparation, and S. Powell and Q. Shi for help in preparing illustrations. This study was
supported by a PhD grant to Q.Z. from BIS (Department for Business Innovation & Skills) and CSC (China Scholarship Council), as well as travel grants from the Bob Savage Memorial Fund
and LESV (Key Laboratory of Evolutionary Systematics of Vertebrates, Chinese Academy of Sciences). This study was also supported by grants from the National Natural Science Foundation of
China (presented to X.X.) and the German Research Foundation (to P.M.S.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, UK
Qi Zhao & Michael J. Benton * Key Laboratory of Vertebrate Evolution and Human Origin of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese
Academy of Sciences, Beijing, 100044, China Qi Zhao, Corwin Sullivan & Xing Xu * Division of Paleontology, Steinmann Institute, Rheinische Friedrich-Wilhelms-Universität Bonn, Nussallee
8, D-53115, Bonn, Germany P. Martin Sander Authors * Qi Zhao View author publications You can also search for this author inPubMed Google Scholar * Michael J. Benton View author publications
You can also search for this author inPubMed Google Scholar * Corwin Sullivan View author publications You can also search for this author inPubMed Google Scholar * P. Martin Sander View
author publications You can also search for this author inPubMed Google Scholar * Xing Xu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
X.X. and M.J.B. designed the project. Q.Z., M.J.B., X.X. and P.M.S. performed the research. Q.Z., M.J.B., X.X., P.M.S. and C.S. wrote the manuscript. CORRESPONDING AUTHOR Correspondence to
Qi Zhao. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figures S1-S4 and
Supplementary Tables S1-S2 (PDF 990 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhao, Q., Benton, M., Sullivan, C. _et al._ Histology and
postural change during the growth of the ceratopsian dinosaur _Psittacosaurus lujiatunensis_. _Nat Commun_ 4, 2079 (2013). https://doi.org/10.1038/ncomms3079 Download citation * Received: 17
March 2013 * Accepted: 30 May 2013 * Published: 28 June 2013 * DOI: https://doi.org/10.1038/ncomms3079 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative