Interactions between twist and other core epithelial–mesenchymal transition factors are controlled by gsk3-mediated phosphorylation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT A subset of transcription factors classified as neural crest ‘specifiers’ are also core epithelial–mesenchymal transition regulatory factors, both in the neural crest and in tumour
progression. The bHLH factor Twist is among the least well studied of these factors. Here we demonstrate that Twist is required for cranial neural crest formation and fate determination in
_Xenopus_. We further show that Twist function in the neural crest is dependent upon its carboxy-terminal WR domain. The WR domain mediates physical interactions between Twist and other core
epithelial–mesenchymal transition factors, including Snail1 and Snail2, which are essential for proper function. Interaction with Snail1/2, and Twist function more generally, is regulated
by GSK-3-β-mediated phosphorylation of conserved sites in the WR domain. Together, these findings elucidate a mechanism for coordinated control of a group of structurally diverse factors
that function as a regulatory unit in both developmental and pathological epithelial–mesenchymal transitions. You have full access to this article via your institution. Download PDF SIMILAR
CONTENT BEING VIEWED BY OTHERS SOX2 LEVELS REGULATE THE CHROMATIN OCCUPANCY OF WNT MEDIATORS IN EPIBLAST PROGENITORS RESPONSIBLE FOR VERTEBRATE BODY FORMATION Article Open access 12 May 2022
EPITHELIAL CELL PLASTICITY DRIVES ENDODERM FORMATION DURING GASTRULATION Article Open access 24 June 2021 COORDINATED REGULATION OF REL EXPRESSION BY MAP3K4, CBP, AND HDAC6 CONTROLS
PHENOTYPIC SWITCHING Article Open access 28 August 2020 INTRODUCTION The neural crest (NC) is a proliferative, multipotent stem cell population that arises at the neural plate border (NPB)
during mid-gastrulation, and ultimately gives rise to diverse derivatives that include neurons and glia of the peripheral nervous system, facial cartilage/bone and melanocytes1,2. NC cells
undergo an epithelial–mesenchymal transition (EMT), delaminate from the neuroepithelium and migrate to diverse sites throughout the embryo where they will differentiate1,2,3,4,5. NC cells
retain multipotency until early migratory stages via a mechanism dependent upon c-myc and Id36,7, before becoming competent to respond to signals that will induce differentiation. NC
formation is one of the few examples during embryonic development where a newly induced cell type exhibits greater developmental potential than the cells from which it was derived, making
the NC a fascinating model for asking questions about the molecular underpinnings of ‘stemness’ and its relationship to the capacity for migratory/invasive cell behaviour. In response to
NC-inducing signals, cells at the NPB initiate expression of NC ‘specifier’ genes, including Snail family members _Snail1_ and _Snail2_ (also known as _Slug_), SoxE factors (_Sox8_, _9_ and
_10_), the WH factor _Foxd3_ and the bHLH factor _Twist_3,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22. Collectively, these diverse factors constitute a gene regulatory network (GRN) that
governs formation and maintenance of the NC precursor population17,18,19. A subset of the proteins that control formation of NC stem cells are used reiteratively to control the subsequent
EMT and migratory behaviour of NC cells19,23. Importantly, these same factors, including Snail1, Snail2 and Twist, are core EMT regulatory factors and are also deployed during tumour
progression, and in other developmental contexts, to control this complex cellular transition5,19,20,21,24. Snail1 and Snail2 are the best-studied transcriptional regulators involved in both
NC specification and EMT2,17,18,19,20,21,23,24. These zinc-finger repressors are essential for formation of NC stem cells as well as for the onset of NC migration, and can directly
downregulate genes involved in cell adhesion and junctions22,25,26,27,28. Importantly, while Snail proteins can potently induce EMTs, their ability to drive this transition is highly context
dependent. For example, Snail factors direct formation of NC stem cells many hours before those cells will become migratory23. Similarly, Snail factors are expressed at the NPB in
non-vertebrate chordates in cells that never become migratory29. Thus, cellular context dictates when Snail proteins promote ‘stemness’ versus migratory/invasive behaviour. Recent work has
indicated that cellular levels of Snail proteins are one key determinant of their functional output during NC cell development30. Snail1/2 protein levels are regulated by the
ubiquitin–proteasome system (UPS), and these proteins are targeted for proteasomal degradation by the F-box protein Partner of paired (Ppa, also known as FBXL14). Stabilized Snail proteins
that cannot be targeted by Ppa induce premature NC migration30, demonstrating the necessity of tightly regulating the threshold levels of these factors present in cells. It is likely,
however, that additional mechanisms also contribute to controlling Snail protein function in a context-dependent manner. The bHLH factor Twist has been classified as a NC specifier17,18,19,
although it does not appear to have this role in amniotes. Importantly, like Snail1/2, Twist also functions as a core EMT regulatory factor in both developmental and pathological contexts31.
Twist possesses a basic domain that can interact with core Ebox sequence ‘CANNTG,’ a helix–loop–helix (HLH) domain that mediates homodimerization or dimerization with E12/E47, and a highly
conserved C-terminal domain, the WR domain or Twist box32. The WR domain has been shown to physically interact with another non-bHLH transcription factor, Runx2, to inhibit
osteoblast-specific gene expression33, and recently has been shown to similarly inhibit Sox9 activity during chondrogenesis34. It has also been suggested that the WR domain can function as
an activation domain for Twist-E12 dimers35. More recently, it has been shown that Twist, like Snail family proteins, is targeted for UPS-mediated degradation by the F-box protein Ppa, and
this regulation is dependent upon the WR domain36. Twist is implicated in the EMT/progression of multiple epithelial cancers, and its expression correlates with invasiveness and poor
outcome5,37,38,39,40. Twist can also promote increased cell proliferation and the ability to evade apoptosis in aggressive tumour cells37,41,42. Expression of this factor in primary tumour
cells has been shown to override oncogene-induced cellular senescence and apoptosis43,44,45, and has been linked to the maintenance of a ‘cancer stem cell’ state46,47,48. Twist is
distinguished from other NC specifiers by the restriction of its expression to cranial regions. This localization suggests that Twist might have a role in endowing cranial NC precursors with
the ability to give rise to mesectodermal derivatives, such as cartilage and bone. A better understanding of the function and regulation of Twist is essential to understanding NC stem cell
formation and the EMT/migration of these cells, and will shed important light on Twist’s role in regulating related states during tumour formation and metastasis. Here, using _Xenopus_ as a
model, we examine the expression and function of Twist in NC crest formation in cranial regions. We find that both gain and loss of Twist expression is incompatible with normal NC
development in _Xenopus_, indicating that correct levels of Twist expression are key to its function, and suggesting that this factor may be regulated, at least in part, by protein–protein
interactions. Consistent with such a model, we show that Twist physically interacts with core EMT factors Snail1 and Snail2 through its conserved WR domain, and inhibits the NC-inducing
activity of these factors. Finally, we identify multiple GSK3-β phosphorylation sites in the Twist C-terminus, and show that phosphorylation of these sites is essential for Twist function
and for its inhibitory interactions with Snail proteins. Our results lend important regulatory insights into a factor that has key roles in both development and cancer. RESULTS TWIST IS
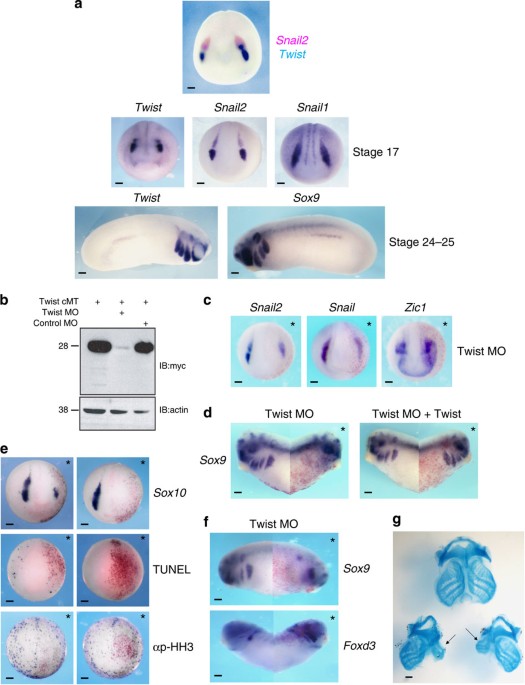
EXPRESSED IN PREMIGRATORY AND MIGRATORY NC CELLS In _Xenopus_, the expression of a number of NC ‘specifiers’, including _Snail1_, _Snail2_, _Sox8_, _Sox9_ and _Foxd3_, can be detected at the
NPB by late gastrula stages (Nieuwkoop and Faber stages 11.5–12). These factors are expressed throughout all NC precursors regardless of axial level19,21. _Twist_ expression is distinct
from other NC specifiers both temporally and spatially. _Twist_ expression is first detectable in NC precursor cells at stage 14, considerably later than several other NC specifiers,
including _Snail1_, _Snail2_, _Sox9_ and _Foxd3_, indicating that it is unlikely to be a regulatory input into the initial expression of these factors. _Twist_ expression initiates in an
anterior to posterior progression, beginning in the presumptive mandibular crest (Fig. 1a) and subsequently expanding to the hyoid and then branchial NC segments. Importantly, unlike other
NC specifiers, _Twist_ expression remains restricted to cranial regions and is not found in NC cells posterior to the branchial NC segment. _Twist_ is maintained in cranial NC cells as they
commence migration ventrally into the pharyngeal pouches, a period when they retain stem cell attributes, and is maintained in NC in the branchial arches through post-migratory stages
(Supplementary Fig. S1). TWIST IS REQUIRED FOR NORMAL NC DEVELOPMENT As _Twist_ is expressed in NC precursors and has been categorized in the context of the NC-GRN as a ‘neural crest
specifier’, we investigated the consequences of loss of Twist function for NC development. A translation blocking morpholino (MO) that can deplete Twist protein from early embryos (Fig. 1b)
was injected, together with β-galactosidase as a lineage tracer, in one cell at the 8-cell stage to target NC and avoid effects on the mesoderm (where _Twist_ is also expressed). Embryos
were cultured to early neurula stages and examined for expression of components of the NC-GRN (Fig. 1c). NC-GRN factors whose expression precedes that of _Twist_ in NC cells, such as
_Snail1_, _Snail2_ (Fig. 1c), were inhibited but less affected by Twist depletion than was _Sox10_, which has a later onset of expression (Fig. 1e). In contrast to the loss of NC specifier
expression, we found that expression domain of _Zic1_, a NPB specifier17,18,19, was expanded in Twist-depleted embryos, suggesting that the cells that did not express NC markers were stalled
in a NPB state and/or adopted alternative fates, such as placodes (Fig. 1c). Importantly, the effects of Twist depletion can be rescued by a form of Twist that cannot be targeted by the MO
(Fig. 1d). As the expression of a number of NC specifiers was diminished following Twist depletion, we examined whether their loss could be attributed to an increase in the number of
apoptotic cells. Embryos injected with Twist MO were allowed to develop to mid-neurula stages (stage 17) when apoptosis was assessed by TdT-mediated dUTP nick end labelling (TUNEL) staining.
Only a few TUNEL-positive nuclei were observed on both Twist-depleted and control sides of these embryos (Fig. 1e), strongly suggesting that the loss of gene expression reflects altered
cell specification as opposed to the death of specific cell populations. Similarly, we asked if observed changes in gene expression could be a consequence of altered cell proliferation/cell
cycle progression by examining the numbers of cells immunoreactive for phosphohistone H3. Again, no difference was noted in numbers of mitotic cells on the Twist-depleted versus control
sides of the embryos. LOSS OF TWIST ALTERS NC FATE DIVERSIFICATION Given that not all NC gene expression was lost in Twist-depleted embryos, we examined the consequences of Twist depletion
for formation of cranial NC derivatives to gain insights into the role of Twist in NC fate diversification in _Xenopus_. Accordingly, embryos injected with Twist MO at the 8-cell stage were
cultured to stages where effects on NC derivatives could be evaluated. At stage 28, injected embryos exhibited reduced expression of _Sox9_ in the branchial arches, where it marks developing
chondrocytes (Fig. 1f) and by stage 43 profound defects in cartilage formation and morphogenesis were observed (Fig. 1g). Conversely, _Foxd3_, which is expressed in presumptive cranial
glial cells at stage 28, was increased (Fig. 1f), suggesting that in the absence of Twist, cranial NC cells that would normally give rise to cartilage might instead adopt glial fates. TWIST
MISEXPRESSION INTERFERES WITH NCC DEVELOPMENT We further probed the function of Twist in NC cells through gain-of-function experiments. Twist misexpression led to increased _Snail1_
expression, whereas expression of _Snail2_ and _Sox10_ was diminished (Fig. 2a). These changes in NC gene expression were not owing to changes in proliferation or the numbers of apoptotic
cells (Fig. 2b). As Twist depletion in cranial NC precursors had profound consequences for NC fate diversification, we asked if Twist misexpression would as well. Significantly, Twist
misexpression caused defects opposite to those observed in Twist-depleted embryos. Expression of _Sox9_ in the branchial arches was enhanced, whereas expression of _Foxd3_ was greatly
diminished (Fig. 2c). TWIST INTERACTS WITH CORE EMT FACTORS SNAIL1 AND SNAIL2 It was notable that Twist depletion and misexpression had similar consequences for the expression of some early
NC factors (for example, _Snail2_ and _Sox10_). Similar phenotypes in gain- and loss-of-function experiments can indicate functional dependence on protein–protein interactions, where proper
stoichiometry is essential. As Twist helps maintain osteoblast precursors in an undifferentiated state by binding and inhibiting Runx2 (ref. 33), we asked if Twist might bind to, and
modulate the activity of, other NC regulatory factors. Embryos co-expressing myc-tagged Twist protein and flag-tagged forms of NC regulatory proteins Snail1, Snail2, Ppa and LMO4 were
cultured to late blastula stages when putative interacting factors were immunoprecipitated and their ability to bind Twist evaluated by western blot. Twist displayed robust interactions with
both Snail1 and Snail2 (Fig. 3a), indicating that its function in cranial NC cells may be at least partially dependent upon its ability to interact with other core EMT factors. By contrast,
Twist was unable to interact with LMO4, a Snail1/2-binding LIM adaptor protein essential for NC formation49. Interaction between Snail2 and Twist was found to depend mainly upon the Snail2
C-terminus, and does not require the SNAG domain (Fig. 3b). TWIST FUNCTION REQUIRES THE C-TERMINAL WR DOMAIN In osteoblasts, Twist’s interaction with Runx2 is dependent upon its C-terminal
WR domain. We therefore asked if Twist function in the cranial NC required this domain. We generated a Twist isoform with the WR domain deleted (Twist-ΔWR, Fig. 3c). Co-immunoprecipitation
(co-IP) assays comparing Snail binding to WT Twist or Twist-ΔWR demonstrated that the WR domain is necessary for interaction with Snail (Fig. 3d). Glutathione _S_-transferase (GST)-pulldown
assays indicate that the interaction between Twist and Snail2 is direct (Supplementary Fig. S1B). Consistent with its interaction with the Snail2 C-terminus, Twist diminishes recruitment of
Snail2 to chromatin, whereas Twist-ΔWR does not (Supplementary Fig. S2C,D). To determine if the WR domain is sufficient to mediate Snail interaction, this peptide was fused to the C-terminus
of bHLH protein E12 (Fig. 3c), which cannot itself interact with Snail. The E12–WR fusion protein was able to bind Snail, indicating that the WR domain is sufficient to mediate this
interaction (Fig. 3d). We further asked if the phenotypic consequences of Twist expression in the cranial NC were dependent upon the WR domain. At neural plate stages, Twist-ΔWR expression
resulted in loss of _Snail1_, _Snail2_ and _Sox10_ (Fig. 3e), similar to what is observed following Twist depletion (Fig. 1c). We also examined the effects of Twist-ΔWR on cranial NC cell
fate diversification. Embryos injected with Twist-ΔWR displayed decreased _Sox9_ expression in the branchial arches (Fig. 3f). Conversely, Twist-ΔWR-expressing embryos showed increased
expression of both _Sox10_ and _Foxd3_ in presumptive cranial glia (Fig. 3f). These results phenocopy the effects of Twist depletion (Fig. 1f), suggesting that deletion of the WR domain
creates a dominant inhibitory form of Twist, at least with respect to roles in NC cell fate diversification. Importantly, Twist-ΔWR does not act as a general inhibitor of bHLH protein
function; for example, it does not interfere with the ability of neurogenin to induce ectopic neurogenesis nor does it block Mitf activation of the Dct promoter (Supplementary Fig. S2A,B).
WR DOMAIN MUTATIONS ALTER TWIST BINDING TO SNAIL1/2 A previously characterized mouse Twist mutation known as ‘Charlie Chaplin’ promotes premature osteoblast differentiation50 and inhibits
Twist interaction with Runx2 (ref. 33). The causal mutation is a proline substitution in the WR domain. In the WR domain of _Xenopus_ Twist (amino acids (aa) 143–166), this mutation
corresponds to TwistS152P. We generated Twist mutants in which serine 152 was substituted either with proline to mimic the ‘Charlie Chaplin’ mutation, or with alanine. In co-IP assays, the
proline substitution enhanced interaction with Snail2 whereas the alanine mutation frequently diminished interaction but sometimes had no effect (Fig. 4a), suggesting that this site has a
role in the interaction but is not the main regulatory site (Fig. 4a). Importantly, the sequence proximal to serine 152 includes a serine residue four aa upstream, a spacing characteristic
of target sites for GSK-3β, which shows sequence preference for SxxxS* (where ‘x’ is any aa and S* represents a ‘priming’ phosphorylation) (Fig. 4b). To determine if serine 148 regulates
interaction between Twist and other core EMT factors, this residue was mutated to alanine or aspartic acid. TwistS148A blocked interaction between Twist and Snail2, whereas TwistS148D
strongly enhanced interaction (Fig. 4c) consistent with a model whereby phosphorylation of Twist at serine 148 regulates protein–protein interactions with the EMT factors Snail1/2. To
determine if serine 148 is essential for Twist function during NC fate diversification, embryos expressing TwistS148A or TwistS148D were examined by _in situ_ hybridization for formation of
NC derivatives. Embryos expressing TwistS148A showed decreased expression of _Sox9_ in the developing branchial arches at stage 28 (Fig. 4e), and decreased and malformed facial cartilages at
stage 43 (Supplementary Fig. S3). Conversely, TwistS148D-expressing embryos showed increased _Sox9_ expression, similar to wild-type Twist (Fig. 2c). TwistS148A-expressing embryos showed
increased expression of both _Sox10_ and _Foxd3_ in presumptive cranial glia whereas TwistS148D had the opposite effect. Together, these findings suggest that phosphorylation of Twist at
serine 148 is required for normal NC fate diversification. SNAIL CO-EXPRESSION MODULATES TWIST STABILITY Physical interaction between Snail1/2 and Twist could have many potential functional
consequences significant to the regulation of both NC development and developmental/pathological EMTs. As Twist stability is regulated by the UPS and is controlled in part by the WR domain,
we asked if co-expression of Snail1 or Snail2 altered Twist stability. Embryos expressing Twist alone, or co-expressing Snail1, were cultured over developmental time and collected at set
time intervals for western analysis. Co-expression of Snail1 was found to stabilize Twist (Supplementary Fig. S3B). A potential mechanism for this stabilization is provided by the finding
that co-expressing Snail2 interferes with the interaction between Twist and Ppa (Fig. 4d), suggesting that Snail2 may have greater affinity for Ppa than does Twist. TWIST IS A SUBSTRATE FOR
GSK-3Β-MEDIATED PHOSPHORYLATION As serine 148 resembles a GSK-3β site, we used immune-complex kinase assays to determine if Twist could be phosphorylated by GSK-3β _in vitro_. We noted that
there were two additional SxxxS motifs in the Twist C-terminus, up and downstream of serine 148/152, and generated serine to alanine mutations in each of them (Fig. 5a). Embryos expressing
WT Twist, or Twist carrying four or six C-terminal S to A mutations were cultured to blastula stages when the expressed proteins were immunoprecipitated and used as substrates in kinase
assays with recombinant GSK-3β. WT Twist was robustly phosphorylated by GSK-3β in these assays, whereas the Twist C-terminus carrying 6SA mutations showed greatly diminished phosphorylation
(Fig. 5b). Together, these data demonstrate that serines in the Twist C-terminus can serve as GSK-3β substrates _in vitro_. To determine if Twist phosphorylation in embryo extracts requires
GSK-3β activity, we asked if this phosphorylation was sensitive to LiCl, a known GSK-3β inhibitor51. For these assays, Twist was expressed in embryos, immunoprecipitated at stage 8 and
immobilized immune complexes incubated with either untreated embryo lysates or lysates treated with 100 mM LiCl. Treatment with LiCl substantially reduced Twist phosphorylation (Fig. 5d).
Moreover, the ability of LiCl treatment to inhibit phosphorylation of the Twist C-terminus was even more pronounced, and mutating the six serine residues in the C-terminus largely abolished
phosphorylation (Fig. 5f). Together, these findings demonstrate that the Twist C-terminus is a bonafide GSK-3β substrate. PHOSPHORYLATED TWIST INHIBITS SNAIL FUNCTION We next examined the
functional consequences of GSK-3β phosphorylation of the Twist C-terminus. We found that co-expression with GSK-3β renders Twist less stable (Fig. 6a), presumably owing to enhanced Ppa
binding. Co-expression of Snail1 protected Twist from destabilization and preventing phosphorylation of the six C-terminal serines blocked association of Twist with Snail factors as well as
destabilization (Fig. 6b). Interestingly, co-expression of Wnt8, which downregulates GSK-3β, led to decreased interaction between Twist and Snail2 (Fig. 6c). Collectively, our data suggested
a model in which Twist binds to and inhibits the activity of Snail proteins, and GSK-3β-mediated phosphorylation of the Twist C-terminus serves to promote this function. We therefore
hypothesized that unphosphorylated Twist would be a less effective Snail1/2 inhibitor. To test this hypothesis, embryos expressing TwistS148A or TwistS148D were examined for the effects on
Snail2-mediated NC precursor formation. Snail2 expression induces ectopic NC formation in this assay, and while TwistS148D potently blocked its effects, TwistS148A did not (Fig. 6d). These
findings support a model in which GSK-3β-mediated Twist phosphorylation regulates the functional inhibition of Snail family EMT regulatory factors. DISCUSSION A GRN describing the formation,
migration and differentiation of NC cells is beginning to be delineated17,18,19. A central challenge to understanding complex developmental processes such as NC development on a systems
level is determining how the function of proteins in the network are controlled individually and coordinately. This is particularly true for proteins, such as Twist, Snail1 and Snail2, which
also function as core EMT regulatory factors. Twist is a particularly interesting component of this network. While _Twist_ is expressed in cranial NC precursors in both _Xenopus_ and
zebrafish15,52, its early NC expression appears to have been lost in the mouse16,53, suggesting that Twist regulatory functions at these stages have been replaced by other factors in
mammals. In the mouse, as in _Xenopus_, _Twist_ is expressed in gastrula stage and presomitic mesoderm, and is also expressed in cranial and limb bud mesenchyme16,53. Heterozygous mutant
Twist mice are viable but display abnormal craniofacial structures, while twist−/− mice have severe defects in cephalic neural tube closure and malformed branchial arches and facial
primordium, showing that this protein is also essential for normal NC development in the mouse16,53. The co-expression of Twist and Snail1/2 in cranial NC precursors in _Xenopus_ makes this
an advantageous system to study functional interactions between these regulatory proteins, and such studies are important beyond the NC, because these factors also co-regulate other
developmental events as well as tumour progression. We recently demonstrated that despite their structural diversity, Twist, a bHLH factor, and the zinc-finger transcriptional repressors
Snail1/Snail2, are coordinately regulated. These factors, together with another core EMT factor Sip1, are targeted to the UPS by the same F-box protein, Ppa36. The functions of numerous
developmental regulatory proteins are regulated, at least in part, by the threshold concentration of protein allowed to accumulate in cells. It is highly significant, however, that a common
targeting mechanism has evolved to control the activity of a core group of functionally linked but structurally diverse factors. This suggests a need to control the activity of these factors
as a unit as they direct complex developmental events and cellular behaviours. The uncovering of one shared mechanism for regulating the function of core EMT regulatory factors Twist and
Snail1/2 raised the possibility that additional mechanisms exist for coordinately regulating these proteins. In the current study, we provide evidence for two further means by which Twist
function can be regulated in concert with Snail1/Snail2. The observation that gain and loss of Twist function had similar consequences for some aspects of NC development suggested a possible
functional dependence on protein–protein interaction, where proper stoichiometry is essential. Twist had been previously shown to maintain osteoblast precursors in an undifferentiated state
via a mechanism involving binding and inhibiting Runx2 (ref. 33). This suggested that Twist might function, in part, by binding to and modulating the activity of other NC regulatory
factors, and indeed we find strong DNA-independent interactions between Twist and the core EMT regulatory proteins Snail1/Snail2 (Slug). Interaction with Snail1/Snail2 did not interfere with
the ability of Twist to bind DNA or dimerize (R Lander, unpublished data). Co-expression of Snail1 or Snail2 rendered Twist protein more stable, however, in part owing to competition for
Ppa binding by Snail2 (Fig. 4d). Interaction between Twist and Snail1/2 is mediated by Twist’s C-terminal WR domain and by the C-terminal zinc fingers of Snail proteins, and diminishes
recruitment of Snail2 to Ebox sequences in chromatin immunoprecipitation (ChIP) assays (Supplementary Fig. S2). Interestingly, the Twist WR domain contains a serine residue previously shown
to be important for Twist function (serine 152) that could represent a priming phosphorylation site for the GSK3-β regulation of serine 148. Two additional conserved SxxxS sites lie up and
downstream of serine 148/152, and the clustering of such sites is a hallmark of canonical GSK-3β substrates, such as β-catenin and Ci66,67. We demonstrate using immune-complex kinase assays
that GSK-3β can phosphorylate the Twist C terminus in a manner dependent on these sites. Moreover, phosphorylation of Twist by endogenous kinases in _Xenopus_ embryo lysates displays strong
sensitivity to LiCl, a known GSK-3β inhibitor, providing further evidence that Twist is a physiological target of GSK-3β phosphorylation. In the future, it will be important to investigate
when and where Twist becomes phosphorylated by GSK-3β. Interestingly, mammalian Snail1 has also been shown to be a GSK-3β substrate, targeting it for beta-TrCP-mediated proteasomal
degradation54,55,56. Although this regulation is not conserved in Snail2 or in amniote Snail1 proteins34, this nonetheless implicates GSK-3β phosphorylation as an additional regulatory
mechanism common to these divergent core EMT factors. Multiple levels of shared regulation compellingly suggests that the activity of the core EMT factors must be controlled in concert for
correct execution of their shared functions. Moreover, our findings suggest that an important role of phosphorylated Twist is to hold Snail1/2 activity in check, and that this function is
regulated by GSK-3β. Further elucidation of the dynamic and coordinated regulation of these core EMT proteins as a functional unit will be an important area of future study. METHODS DNA
CONSTRUCTS Epitope-tagged versions of all complementary DNAs were generated by amplifying the coding and inserting them into pCS2-MycC or pCS2-FlagC vectors. _Xenopus_ Twist deletion mutants
were generated using the following primers: Twist Nterm sense: 5′-ATGATGCAGGAA-3′, antisense: 5′-TCTCAAGGACGA-3′; Twist Cterm sense: 5′-ATGGCGAGCAGCACC-3′, antisense: 5′-GTGAGATGCAGA-3′;
Twist ΔWR sense: 5′-ATGATGCAGGAA-3′, antisense: 5′-CACATAACTGCAGCTGGC-3′. The E12–WR domain fusion construct was generated by inserting the WR domain sequence
(5′-GCCCATGAGAGGCTCAGCTATGCCTTCTCCGTGTGGAGGATGGAGGGAGC CTGGTCCATGTCTGCATCTCAC-3′) into the _EcoRI_ site of _Xenopus_ E12 in pCS2-MycC vector. All constructs were confirmed by sequencing.
EMBRYOLOGICAL METHODS AND CARTILAGE STAINING All results shown are representative of at least three independent experiments. RNA for injection was produced _in vitro_ from linearized plasmid
templates using the Message Machine kit (Ambion). Embryos were injected at the 2-cell or 8-cell stage as noted and collected at the indicated stage. _In situ_ hybridization was performed
using digoxigenin-labelled RNA probes using the standard protocol6 and developed using BM Purple substrate (Roche). Embryo images were collected on an Olympus dissecting microscope fitted
with a × 10 objective and an Olympus QColor5 digital camera. Composite images were assembled using Adobe Photoshop. The Twist MO sequence is: 5′-CGGCACAATAAGGAGAAGGTCCCG -3′. For luciferase
assays, firefly luciferase and Renilla constructs (DNA) were injected alone or in combination with Mitf and/or TwistΔWR RNA into both cells of a 2-cell _Xenopus_ embryo. Embryos were
cultured until stage 17, collected in 10-embryo sets and lysed in 500 μl of passive lysis buffer using the reporter assay system kit (Dual-Luciferase; Promega). The _Dct_-luciferase reporter
contains the ~3.2-kb mouse _Dct_ promoter. For cartilage staining, embryos were fixed in formaldehyde at stage 46 and stained overnight in 0.2% alcian blue/30% acetic acid in EtOH. Embryos
were washed through a glycerol series into 80% glycerol/20% KOH before manual dissection of cartilages. PROLIFERATION AND TUNEL ASSAYS For phosphohistone H3 detection, Twist MO-injected or
Twist messenger RNA-injected embryos were fixed in formaldehyde at stage 17 and processed for β-galactosidase activity. α-Phosphohistone H3 antibody (Upstate Biotechnology) was used at a
concentration of 5 μg ml−1; α-rabbit IgG conjugated with alkaline phosphatase (Roche) was used at 1:1,000 and detected with BM Purple. For TUNEL assays, embryos injected with Twist MO or
mRNA-encoding Twist were allowed to develop until stage 17. TUNEL staining was carried out as described previously6. Briefly, fixed embryos were rehydrated in PBT and washed in TdT buffer
(Invitrogen) for 30 min. End labelling was carried out at room temperature overnight in TdT buffer containing 0.5 μM digoxigenin-dUTP (Roche) and 150 U ml−1 TdT (Invitrogen). Embryos were
washed at 65 °C in PBS/1 mM EDTA and detection of the digoxigenin epitope was carried out as for _in situ_ hybridization. IPS, WESTERN BLOTS AND STABILITY ASSAYS For IPs, embryos were
collected at stage 10, lysed in PBS+ 1% NP40 containing a protease inhibitor cocktail (Roche), and incubated with the indicated antibody (0.2 μg α-Myc (9E10, Santa Cruz) or 0.2 μg α-FlagM2
affinity purified (Sigma)) for 2 h on ice, followed by a 2 h incubation with protein A Sepharose beads. IPs were washed with RIPA buffer and resolved by SDS–polyacrylamide gel
electrophoresis (PAGE). Immunoblotting was performed using α-Myc (1:2,000), affinity purified α-FlagM2 (1:3,000) or α-actin ((1:1,000), Sigma) antibody as indicated. Labelled proteins were
detected using HRP-conjugated secondary antibodies and enhanced chemiluminescence (Amersham). PURIFICATION OF GST PROTEINS AND GST PULL-DOWN ASSAYS GST proteins were expressed in BL21 stain
of _E. coli_, sonicated and purified with glutathione-agarose (Sigma-Aldrich). Protein induction and bead attachment were verified by SDS–PAGE and Coomassie staining. Twist proteins were
transcribed and translated _in vitro_ using the quick coupled transcription/translation system (TNT) in the presence of [35S]methionine. Eight percent of the reaction mixture was kept as the
input. The remainder was incubated with glutathione bead-bound GST fusion proteins for 2 h at 4 °C in lysis buffer in a 500-μl volume. Glutathione-agarose was washed four times with RIPA
buffer, and bound proteins were released by boiling in SDS sample buffer, analysed by SDS–PAGE and imaged using autoradiography. CHIP AND QUANTITATIVE PCR (QPCR) ChIP was performed with 50
embryos per IP and fold enrichment of transcription factor was quantified using SYBR green qPCR. Embryos were injected at 2-cell stage with RNA for myc-tagged Snail2 and/or Flag-tagged Twist
at concentrations that correlate with endogenous levels. Expressed proteins levels were quantified using Odyssey licor scanner using infrared secondary antibody (Rockland #610-132-121).
Embryos were harvested for ChIP at stage 17. IP for myc-tagged proteins were performed using anti-Myc epitope (Sigma #C3956) on Protein G magnetic beads (Dynabeads, Invitrogen #100-04D).
qPCR was performed using primers for proximal promoters of Epidermal Keritin (Fwd: 5′-CCTGGAGCAAGGAGAGAGTG-3′; Rev: 5′-CGTAGCCTCAGGGTGTTTGT-3′) and, as a control, eEF1α (Fwd:
5′-TGCATGAAGCACAGCAGAAT-3′; Rev: 5′-CGGGTGAGGAAGAGAGGATT-3′) with SYBR Premix (Clontech #RR820W). Fold enrichment of Snail2 at the proximal promoters of Epidermal keratin and eEF1α was
calculated using ΔΔCT method and represented as mean from three separate biological replicates with error bars representing s.e.m. _IN VITRO_ AND _IN VIVO_ KINASE ASSAYS For _in vitro_
kinase assays, embryos were injected with RNA encoding Twist mutants (WR 6SA, WR 4SA and Cterm) at the 2-cell stage and collected at stage 8. Proteins were immunoprecipitated in the presence
of 20 mM β-glycerol phosphate from embryo lysates as detailed above. Following RIPA washes, the immune complexes were washed two times in PBS and four times in 1 × GSK3 kinase buffer (NEB)
and incubated with 1.5 μl 10 × GSK3 buffer, 0.5 μl 0.5 mM ATP (NEB), 0.5 μl 1M β-glycerol phosphate, 1 μl glycogen synthase kinase 3 (GSK-3) (NEB), 2 μl [γ-32P]ATP and 9.5 μl H2O for 20 min
at 30 °C. Reactions were stopped with 1 μl of 5 mM EDTA. Immune complexes were then washed four times with PBS+20 mM EDTA, and resolved by SDS–PAGE and visualized by autoradiography. For _in
vivo_ kinase assays, Twist proteins were immunoprecipitated, and Twist-bound PAS beads were washed four times in X1 Extraction Buffer (XB) then incubated with 15 μl _Xenopus_ embryo extract
(see Method below), 0.5 μl [γ-32P]ATP, 100 mM LiCl (or H2O control) for 30 min at room temperature. Reactions were stopped with 1 μl of 5 mM EDTA and immune complexes were washed four times
with PBS+20 mM EDTA, and samples were resolved by SDS–PAGE and visualized by autoradiography. _XENOPUS_ EMBRYO EXTRACT PREPARATION Extract preparation methods were adapted from Kim _et
al_.57 Fertilized _Xenopus_ embryos were collected at stage 9 and washed four times with 1 × Extraction Buffer (XB) plus 20 mM β-glycerol phosphate and protease inhibitors. Embryos were
incubated with 500 μl of 1 × XB (plus phosphatase/protease inhibitors and 10 μg ml−1 cytochalasin B) on ice for 3 min and then packed at a low speed (<100_g_ for 30 s). Excess liquid was
removed and eggs were crushed at 21,000_g_ for 5 min at 4 °C. The clear, cytoplasmic middle layer was transferred to a new, chilled tube. Protease inhibitors and cytochalasin B were added to
the extract. Four more rounds of centrifugation were then performed to obtain clear egg extracts. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Lander, R. _et al_. Interactions between
Twist and other core epithelial–mesenchymal transition factors are controlled by GSK3-mediated phosphorylation. _Nat. Commun._ 4:1542 doi: 10.1038/ncomms2543 (2013). REFERENCES * Le Douarin,
N. & Kalcheim, C. . _The Neural Crest._ 2nd edn Cambridge University Press (1999). * LaBonne, C. & Bronner-Fraser, M. . Molecular mechanisms of neural crest formation. _Annu. Rev.
Cell Dev. Biol._ 15, 81–112 (1999). Article CAS Google Scholar * Duband, J. L. . Neural crest delamination and migration: integrating regulations of cell interactions, locomotion,
survival and fate. _Adv. Exp. Med. Biol._ 589, 45–77 (2006). Article CAS Google Scholar * Thiery, J. P. & Sleeman, J. P. . Complex networks orchestrate epithelial-mesenchymal
transitions. _Nat. Rev. Mol. Cell Biol._ 7, 131–142 (2006). Article CAS Google Scholar * Yang, J. & Weinberg, R. A. . Epithelial-mesenchymal transition: at the crossroads of
development and tumor metastasis. _Dev. Cell_ 14, 818–829 (2008). Article CAS Google Scholar * Bellmeyer, A., Krase, J., Lindgren, J. & LaBonne, C. . The protooncogene c-Myc is an
essential regulator of neural crest formation in _Xenopus_. _Dev. Cell_ 4, 827–839 (2003). Article CAS Google Scholar * Light, W., Vernon, A. E., Lasorella, A., Iavarone, A. &
LaBonne, C. . _Xenopus_ Id3 is required downstream of Myc for the formation of multipotent neural crest progenitor cells. _Development_ 132, 1831–1841 (2005). Article CAS Google Scholar *
Kelsh, R. N. . Sorting out Sox10 functions in neural crest development. _Bioessays_ 28, 788–798 (2006). Article Google Scholar * Spokony, R. F., Aoki, Y., Saint-Germain, N., Magner-Fink,
E. & Saint-Jeannet, J. P. . The transcription factor Sox9 is required for cranial neural crest development in _Xenopus_. _Development_ 129, 421–432 (2002). CAS PubMed Google Scholar *
Aoki, Y. et al. Sox10 regulates the development of neural crest-derived melanocytes in _Xenopus_. _Dev. Biol._ 259, 19–33 (2003). Article CAS Google Scholar * Kellerer, S. et al.
Replacement of the Sox10 transcription factor by Sox8 reveals incomplete functional equivalence. _Development_ 133, 2875–2886 (2006). Article CAS Google Scholar * O’Donnell, M., Hong, C.
S., Huang, X., Delnicki, R. J. & Saint-Jeannet, J. P. . Functional analysis of Sox8 during neural crest development in _Xenopus_. _Development_ 133, 3817–3826 (2006). Article Google
Scholar * Lister, J. A. et al. Zebrafish Foxd3 is required for development of a subset of neural crest derivatives. _Dev. Biol._ 290, 92–104 (2006). Article CAS Google Scholar * Teng,
L., Mundell, N. A., Frist, A. Y., Wang, Q. & Labosky, P. A. . Requirement for Foxd3 in the maintenance of neural crest progenitors. _Development_ 135, 1615–1624 (2008). Article CAS
Google Scholar * Hopwood, N. D., Pluck, A. & Gurdon, J. B. . A _Xenopus_ mRNA related to _Drosophila_ twist is expressed in response to induction in the mesoderm and the neural crest.
_Cell_ 59, 893–903 (1989). Article CAS Google Scholar * Soo, K. et al. Twist function is required for the morphogenesis of the cephalic neural tube and the differentiation of the cranial
neural crest cells in the mouse embryo. _Dev. Biol._ 247, 251–270 (2002). Article CAS Google Scholar * Sauka-Spengler, T. & Bronner-Fraser, M. . A gene regulatory network orchestrates
neural crest formation. _Nat. Rev. Mol. Cell Biol._ 9, 557–568 (2008). Article CAS Google Scholar * Betancur, P., Bronner-Fraser, M. & Sauka-Spengler, T. . Assembling neural crest
regulatory circuits into a gene regulatory network. _Annu. Rev. Cell Dev. Biol._ 26, 581–603 (2010). Article CAS Google Scholar * Prasad, M. S., Sauka-Spengler, T. & Labonne, C. .
Induction of the neural crest state: control of stem cell attributes by gene regulatory, post-transcriptional and epigenetic interactions. _Dev. Biol._ 366, 10–21 (2012). Article CAS
Google Scholar * Wu, Y. & Zhou, B. P. . Snail: more than EMT. _Cell Adh. Migr._ 4, 199–203 (2010). Article Google Scholar * Taylor, K. M. & LaBonne, C. . Modulating the activity
of neural crest regulatory factors. _Curr. Opin. Genet. Dev._ 17, 326–331 (2007). Article CAS Google Scholar * Hajra, K. M., Chen, D. Y. & Fearon, E. R. . The SLUG zinc-finger protein
represses E-cadherin in breast cancer. _Cancer Res._ 62, 1613–1618 (2002). CAS PubMed Google Scholar * LaBonne, C. & Bronner-Fraser, M. . Snail-related transcriptional repressors are
required in _Xenopus_ for both the induction of the neural crest and its subsequent migration. _Dev. Biol._ 221, 195–205 (2000). Article CAS Google Scholar * Nieto, M. A. . The snail
superfamily of zinc-finger transcription factors. _Nat. Rev. Mol. Cell Biol._ 3, 155–166 (2002). Article CAS Google Scholar * Cano, A. et al. The transcription factor snail controls
epithelial-mesenchymal transitions by repressing E-cadherin expression. _Nat. Cell Biol._ 2, 76–83 (2000). Article CAS Google Scholar * Batlle, E. et al. The transcription factor snail is
a repressor of E-cadherin gene expression in epithelial tumour cells. _Nat. Cell Biol._ 2, 84–89 (2000). Article CAS Google Scholar * Taneyhill, L. A., Coles, E. G. & Bronner-Fraser,
M. . Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. _Development_ 134, 1481–1490 (2007). Article CAS Google Scholar * Ikenouchi,
J., Matsuda, M., Furuse, M. & Tsukita, S. . Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by
Snail. _J. Cell Sci._ 116, 1959–1967 (2003). Article CAS Google Scholar * Langeland, J. A., Tomsa, J. M., Jackman, W. R. Jr & Kimmel, C. B. . An amphioxus snail gene: expression in
paraxial mesoderm and neural plate suggests a conserved role in patterning the chordate embryo. _Dev. Genes Evol._ 208, 569–577 (1998). Article CAS Google Scholar * Vernon, A. E. &
LaBonne, C. . Slug stability is dynamically regulated during neural crest development by the F-box protein Ppa. _Development_ 133, 3359–3370 (2006). Article CAS Google Scholar * Vernon,
A. E. & LaBonne, C. . Tumor metastasis: a new twist on epithelial-mesenchymal transitions. _Curr. Biol._ 14, R719–R721 (2004). Article CAS Google Scholar * Castanon, I. & Baylies,
M. K. . A Twist in fate: evolutionary comparison of Twist structure and function. _Gene_ 287, 11–22 (2002). Article CAS Google Scholar * Bialek, P. et al. A twist code determines the
onset of osteoblast differentiation. _Dev. Cell_ 6, 423–435 (2004). Article CAS Google Scholar * Gu, S., Boyer, T. G. & Naski, M. C. . Basic helix-loop-helix transcription factor
Twist1 inhibits transactivator function of master chondrogenic regulator Sox9. _J. Biol. Chem._ 287, 21082–21092 (2012). Article CAS Google Scholar * Laursen, K. B., Mielke, E.,
Iannaccone, P. & Fuchtbauer, E. M. . Mechanism of transcriptional activation by the proto-oncogene Twist1. _J. Biol. Chem._ 282, 34623–34633 (2007). Article CAS Google Scholar *
Lander, R., Nordin, K. & LaBonne, C. . The F-box protein Ppa is a common regulator of core EMT factors Twist, Snail, Slug, and Sip1. _J. Cell Biol._ 194, 17–25 (2011). Article CAS
Google Scholar * Yang, J. et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. _Cell_ 117, 927–939 (2004). Article CAS Google Scholar * Kwok,
W. K. et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. _Cancer Res._ 65, 5153–5162 (2005). Article CAS Google Scholar * Zhang, Z. et al.
Significance of TWIST expression and its association with E-cadherin in bladder cancer. _Hum. Pathol._ 38, 598–606 (2007). Article CAS Google Scholar * Yang, M. H. et al. Comprehensive
analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. _Hepatology_ 50, 1464–1474 (2009). Article CAS Google Scholar * Feng, M. Y. et
al. Metastasis-induction and apoptosis-protection by TWIST in gastric cancer cells. _Clin. Exp. Metastasis_ 26, 1013–1023 (2009). Article CAS Google Scholar * Puisieux, A.,
Valsesia-Wittmann, S. & Ansieau, S. . A twist for survival and cancer progression. _Br. J. Cancer_ 94, 13–17 (2006). Article CAS Google Scholar * Maestro, R. et al. Twist is a
potential oncogene that inhibits apoptosis. _Genes Dev._ 13, 2207–2217 (1999). Article CAS Google Scholar * Valsesia-Wittmann, S. et al. Oncogenic cooperation between H-Twist and N-Myc
overrides failsafe programs in cancer cells. _Cancer Cell_ 6, 625–630 (2004). Article CAS Google Scholar * Ansieau, S. et al. Induction of EMT by twist proteins as a collateral effect of
tumor-promoting inactivation of premature senescence. _Cancer Cell_ 14, 79–89 (2008). Article CAS Google Scholar * Mani, S. A. et al. The epithelial-mesenchymal transition generates cells
with properties of stem cells. _Cell_ 133, 704–715 (2008). Article CAS Google Scholar * Battula, V. L. et al. Epithelial-mesenchymal transition-derived cells exhibit multilineage
differentiation potential similar to mesenchymal stem cells. _Stem Cells_ 28, 1435–1445 (2010). Article CAS Google Scholar * Vesuna, F., Lisok, A., Kimble, B. & Raman, V. . Twist
modulates breast cancer stem cells by transcriptional regulation of CD24 expression. _Neoplasia_ 11, 1318–1328 (2009). Article CAS Google Scholar * Ochoa, S. D., Salvador, S. &
Labonne, C. . The LIM adaptor protein LMO4 is an essential regulator of neural crest development. _Dev. Biol._ 361, 313–325 (2011). Article Google Scholar * Justice, M. J. . Capitalizing
on large-scale mouse mutagenesis screens. _Nat. Rev. Genet._ 1, 109–115 (2000). Article CAS Google Scholar * Klein, P. S. & Melton, D. A. . A molecular mechanism for the effect of
lithium on development. _Proc. Natl Acad. Sci. USA_ 93, 8455–8459 (1996). Article CAS ADS Google Scholar * Yeo, G. H., Cheah, F. S., Winkler, C., Jabs, E. W., Venkatesh, B. & Chong,
S. S. . Phylogenetic and evolutionary relationships and developmental expression patterns of the zebrafish twist gene family. _Dev. Genes Evol._ 219, 289–300 (2009). Article CAS Google
Scholar * Chen, Z. F. & Behringer, R. R. . Twist is required in head mesenchyme for cranial neural tube morphogenesis. _Genes Dev._ 9, 686–699 (1995). Article CAS Google Scholar *
Yook, J. I., Li, X. Y., Ota, I., Fearon, E. R. & Weiss, S. J. . Wnt-dependent regulation of the E-cadherin repressor snail. _J. Biol. Chem._ 280, 11740–11748 (2005). Article CAS Google
Scholar * Yook, J. I. et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. _Nat. Cell Biol._ 8, 1398–1406 (2006). Article CAS Google Scholar * Zhou, B.
P. et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. _Nat. Cell Biol._ 6, 931–940 (2004). Article CAS Google Scholar *
Kim, N. G., Xu, C. & Gumbiner, B. M. . Identification of targets of the Wnt pathway destruction complex in addition to beta-catenin. _Proc. Natl Acad. Sci. USA_ 106, 5165–5170 (2009).
Article CAS ADS Google Scholar Download references ACKNOWLEDGEMENTS We thank Joe Nguyen and Stephen Bock for technical assistance. R.L. was supported by an American Heart Association
predoctoral fellowship (0910066G) and was a Malkin Scholar of the RHLCCC. This work was supported by NIH R01CA114058 to C.L. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Molecular Biosciences, Northwestern University, Evanston, 60208, IL, USA Rachel Lander, Talia Nasr, Stacy D. Ochoa, Kara Nordin, Maneeshi S. Prasad & Carole LaBonne * Robert H. Lurie
Comprehensive Cancer Center, Northwestern University, Chicago, 60611, IL, USA Carole LaBonne Authors * Rachel Lander View author publications You can also search for this author inPubMed
Google Scholar * Talia Nasr View author publications You can also search for this author inPubMed Google Scholar * Stacy D. Ochoa View author publications You can also search for this author
inPubMed Google Scholar * Kara Nordin View author publications You can also search for this author inPubMed Google Scholar * Maneeshi S. Prasad View author publications You can also search
for this author inPubMed Google Scholar * Carole LaBonne View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS R.L. carried out the experiments,
with contributions of data by T.N., S.D.O., M.S.P. and K.N. R.L. and C.L. designed and interpreted the experiments and wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Carole
LaBonne. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figures S1-S3 (PDF
185 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lander, R., Nasr, T., Ochoa, S. _et al._ Interactions between Twist and other core
epithelial–mesenchymal transition factors are controlled by GSK3-mediated phosphorylation. _Nat Commun_ 4, 1542 (2013). https://doi.org/10.1038/ncomms2543 Download citation * Received: 25
June 2012 * Accepted: 24 January 2013 * Published: 26 February 2013 * DOI: https://doi.org/10.1038/ncomms2543 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative