Tudor domains track down dna breaks
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
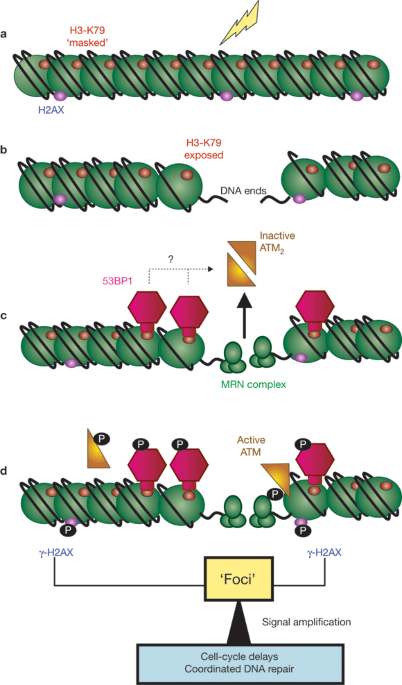
How do cells track down the occasional lesion among the billons of base pairs of chromosomal DNA? A surprising discovery unravels a potential new mechanism: the Tudor domains of the
DNA-damage response factor 53BP1 interact with methylated histones that are likely to become exposed during local chromatin relaxation at sites of DNA double-strand breaks. Access through
your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12
print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be
subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
REFERENCES * Celeste, A. et al. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. _Nature Cell Biol._ 5, 675–679 (2003). Article CAS Google Scholar *
Huyen, Y. et al. Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. _Nature_ 432, 406–411 (2004). Article CAS Google Scholar * Charier, G. et al. The Tudor
tandem of 53BP1: a new structural motif involved in DNA and RG-rich peptide binding. _Structure (Camb.)_ 12, 1551–1562 (2004). Article CAS Google Scholar * Bakkenist, C. J. & Kastan,
M. B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. _Nature_ 421, 499–506 (2003). Article CAS Google Scholar * Wang, B., Matsuoka, S.,
Carpenter, P. B. & Elledge, S. J. 53BP1, a mediator of the DNA damage checkpoint. _Science_ 298, 1435–1438 (2002). Article CAS Google Scholar * DiTullio, R. A., Jr. et al. 53BP1
functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. _Nature Cell Biol._ 4, 998–1002 (2002). Article CAS Google Scholar *
Fernandez-Capetillo, O. et al. DNA damage-induced G2–M checkpoint activation by histone H2AX and 53BP1. _Nature Cell Biol._ 4, 993–997 (2002). Article CAS Google Scholar * Mochan, T. A.,
Venere, M., DiTullio, R. A., Jr., & Halazonetis, T. D. 53BP1 and NFBD1/MDC1-Nbs1 function in parallel interacting pathways activating ataxia-telangiectasia mutated (ATM) in response to
DNA damage. _Cancer Res._ 63, 8586–8591 (2003). CAS PubMed Google Scholar * Uziel, T. et al. Requirement of the MRN complex for ATM activation by DNA damage. _EMBO J._ 22, 5612–5621
(2003). Article CAS Google Scholar * de Jager, M. et al. Human Rad50/Mre11 is a flexible complex that can tether DNA ends. _Mol. Cell_ 8, 1129–1135 (2001). Article CAS Google Scholar *
Game, J. C., Williamson, M. S. & Baccari, C. X-ray survival characteristics and genetic analysis for nine _Saccharomyces_ deletion mutants that affect radiation sensitivity. _Genetics_
DOI: 10.1534/genetics.104.028613 (2004). * Sanders, S. L. et al. Methylation of histone H4 lysine 20 controls recruitment of Crb2 to sites of DNA damage. _Cell_ DOI:
10.1016/S0092867404010530 (2004). Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * the The Wellcome Trust/Cancer Research UK Gurdon Institute, The Henry Wellcome Building of
Cancer and Developmental Biology, Tennis Court Road, Cambridge, CB2 1QN, UK Manuel Stucki & Stephen P. Jackson Authors * Manuel Stucki View author publications You can also search for
this author inPubMed Google Scholar * Stephen P. Jackson View author publications You can also search for this author inPubMed Google Scholar RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Stucki, M., Jackson, S. Tudor domains track down DNA breaks. _Nat Cell Biol_ 6, 1150–1152 (2004). https://doi.org/10.1038/ncb1204-1150 Download citation
* Issue Date: 01 December 2004 * DOI: https://doi.org/10.1038/ncb1204-1150 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative