Allosteric coupling from g protein to the agonist-binding pocket in gpcrs
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT G-protein-coupled receptors (GPCRs) remain the primary conduit by which cells detect environmental stimuli and communicate with each other1. Upon activation by extracellular
agonists, these seven-transmembrane-domain-containing receptors interact with heterotrimeric G proteins to regulate downstream second messenger and/or protein kinase cascades1.
Crystallographic evidence from a prototypic GPCR, the β2-adrenergic receptor (β2AR), in complex with its cognate G protein, Gs, has provided a model for how agonist binding promotes
conformational changes that propagate through the GPCR and into the nucleotide-binding pocket of the G protein α-subunit to catalyse GDP release, the key step required for GTP binding and
activation of G proteins2. The structure also offers hints about how G-protein binding may, in turn, allosterically influence ligand binding. Here we provide functional evidence that
G-protein coupling to the β2AR stabilizes a ‘closed’ receptor conformation characterized by restricted access to and egress from the hormone-binding site. Surprisingly, the effects of G
protein on the hormone-binding site can be observed in the absence of a bound agonist, where G-protein coupling driven by basal receptor activity impedes the association of agonists, partial
agonists, antagonists and inverse agonists. The ability of bound ligands to dissociate from the receptor is also hindered, providing a structural explanation for the G-protein-mediated
enhancement of agonist affinity, which has been observed for many GPCR–G-protein pairs. Our data also indicate that, in contrast to agonist binding alone, coupling of a G protein in the
absence of an agonist stabilizes large structural changes in a GPCR. The effects of nucleotide-free G protein on ligand-binding kinetics are shared by other members of the superfamily of
GPCRs, suggesting that a common mechanism may underlie G-protein-mediated enhancement of agonist affinity. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS MECHANISTIC INSIGHTS INTO G-PROTEIN
COUPLING WITH AN AGONIST-BOUND G-PROTEIN-COUPLED RECEPTOR Article 12 June 2024 STRUCTURES OF Β1-ADRENERGIC RECEPTOR IN COMPLEX WITH GS AND LIGANDS OF DIFFERENT EFFICACIES Article Open access
14 July 2022 AUTOREGULATION OF GPCR SIGNALLING THROUGH THE THIRD INTRACELLULAR LOOP Article Open access 08 March 2023 REFERENCES * Pierce, K. L., Premont, R. T. & Lefkowitz, R. J.
Seven-transmembrane receptors. _Nature Rev. Mol. Cell Biol._ 3, 639–650 (2002) Article CAS Google Scholar * Rasmussen, S. G. et al. Crystal structure of the β2 adrenergic receptor–Gs
protein complex. _Nature_ 477, 549–555 (2011) Article ADS CAS Google Scholar * Venter, J. C. et al. The sequence of the human genome. _Science_ 291, 1304–1351 (2001) Article ADS CAS
Google Scholar * Sprang, S. R. G protein mechanisms: insights from structural analysis. _Annu. Rev. Biochem._ 66, 639–678 (1997) Article CAS Google Scholar * Chung, K. Y. et al.
Conformational changes in the G protein Gs induced by the β2 adrenergic receptor. _Nature_ 477, 611–615 (2011) Article ADS CAS Google Scholar * Westfield, G. H. et al. Structural
flexibility of the Gαs α-helical domain in the β2-adrenoceptor Gs complex. _Proc. Natl Acad. Sci. USA_ 108, 16086–16091 (2011) Article ADS CAS Google Scholar * Maguire, M. E., Van
Arsdale, P. M. & Gilman, A. G. An agonist-specific effect of guanine nucleotides on binding to the beta adrenergic receptor. _Mol. Pharmacol._ 12, 335–339 (1976) CAS PubMed Google
Scholar * Ross, E. M., Maguire, M. E., Sturgill, T. W., Biltonen, R. L. & Gilman, A. G. Relationship between the β-adrenergic receptor and adenylate cyclase. _J. Biol. Chem._ 252,
5761–5775 (1977) Article CAS Google Scholar * De Lean, A., Stadel, J. M. & Lefkowitz, R. J. A ternary complex model explains the agonist-specific binding properties of the adenylate
cyclase-coupled β-adrenergic receptor. _J. Biol. Chem._ 255, 7108–7117 (1980) Article CAS Google Scholar * Yao, X. J. et al. The effect of ligand efficacy on the formation and stability
of a GPCR–G protein complex. _Proc. Natl Acad. Sci. USA_ 106, 9501–9506 (2009) Article ADS CAS Google Scholar * Rasmussen, S. G. et al. Structure of a nanobody-stabilized active state of
the β2 adrenoceptor. _Nature_ 469, 175–180 (2011) Article ADS CAS Google Scholar * Irannejad, R. et al. Conformational biosensors reveal GPCR signalling from endosomes. _Nature_ 495,
534–538 (2013) Article ADS CAS Google Scholar * Lefkowitz, R. J. & Williams, L. T. Catecholamine binding to the β-adrenergic receptor. _Proc. Natl Acad. Sci. USA_ 74, 515–519 (1977)
Article ADS CAS Google Scholar * Ring, A. M. et al. Adrenaline-activated structure of β2-adrenoceptor stabilized by an engineered nanobody. _Nature_ 502, 575–579 (2013) Article ADS CAS
Google Scholar * Bokoch, M. P. et al. Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. _Nature_ 463, 108–112 (2010) Article ADS CAS Google
Scholar * Kikkawa, H., Isogaya, M., Nagao, T. & Kurose, H. The role of the seventh transmembrane region in high affinity binding of a β2-selective agonist TA-2005. _Mol. Pharmacol._ 53,
128–134 (1998) Article CAS Google Scholar * Dror, R. O. et al. Pathway and mechanism of drug binding to G-protein-coupled receptors. _Proc. Natl Acad. Sci. USA_ 108, 13118–13123 (2011)
Article ADS CAS Google Scholar * Rosenbaum, D. M. et al. Structure and function of an irreversible agonist–β2 adrenoceptor complex. _Nature_ 469, 236–240 (2011) Article ADS CAS Google
Scholar * Warne, T. et al. The structural basis for agonist and partial agonist action on a β1-adrenergic receptor. _Nature_ 469, 241–244 (2011) Article ADS CAS Google Scholar *
Samama, P., Cotecchia, S., Costa, T. & Lefkowitz, R. J. A mutation-induced activated state of the β2-adrenergic receptor. Extending the ternary complex model. _J. Biol. Chem._ 268,
4625–4636 (1993) Article CAS Google Scholar * Weiss, J. M., Morgan, P. H., Lutz, M. W. & Kenakin, T. P. The cubic ternary complex receptor-occupancy model. I. Model description. _J.
Theor. Biol._ 178, 151–167 (1996) Article ADS CAS Google Scholar * Burgisser, E., De Lean, A. & Lefkowitz, R. J. Reciprocal modulation of agonist and antagonist binding to muscarinic
cholinergic receptor by guanine nucleotide. _Proc. Natl Acad. Sci. USA_ 79, 1732–1736 (1982) Article ADS CAS Google Scholar * Bylund, D. B., Gerety, M. E., Happe, H. K. & Murrin, L.
C. A robust GTP-induced shift in α2-adrenoceptor agonist affinity in tissue sections from rat brain. _J. Neurosci. Methods_ 105, 159–166 (2001) Article CAS Google Scholar * Prater, M.
R., Taylor, H., Munshi, R. & Linden, J. Indirect effect of guanine nucleotides on antagonist binding to A1 adenosine receptors: occupation of cryptic binding sites by endogenous
vesicular adenosine. _Mol. Pharmacol._ 42, 765–772 (1992) CAS PubMed Google Scholar * Werling, L. L., Puttfarcken, P. S. & Cox, B. M. Multiple agonist-affinity states of opioid
receptors: regulation of binding by guanyl nucleotides in guinea pig cortical, NG108-15, and 7315c cell membranes. _Mol. Pharmacol._ 33, 423–431 (1988) CAS PubMed Google Scholar * Haga,
K. et al. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. _Nature_ 482, 547–551 (2012) Article ADS CAS Google Scholar * Kruse, A. C. et al. Activation
and allosteric modulation of a muscarinic acetylcholine receptor. _Nature_ 504, 101–106 (2013) Article ADS CAS Google Scholar * Manglik, A. et al. Crystal structure of the μ-opioid
receptor bound to a morphinan antagonist. _Nature_ 485, 321–326 (2012) Article ADS CAS Google Scholar * Huang, W. et al. Structural insights into μ-opioid receptor activation. _Nature_
524, 315–321 (2015) Article ADS CAS Google Scholar * Katritch, V. et al. Allosteric sodium in class A GPCR signaling. _Trends Biochem. Sci._ 39, 233–244 (2014) Article CAS Google
Scholar * Kozasa, T. & Gilman, A. G. Purification of recombinant G proteins from Sf9 cells by hexahistidine tagging of associated subunits. Characterization of α12 and inhibition of
adenylyl cyclase by αz . _J. Biol. Chem._ 270, 1734–1741 (1995) Article CAS Google Scholar * Whorton, M. R. et al. A monomeric G protein-coupled receptor isolated in a high-density
lipoprotein particle efficiently activates its G protein. _Proc. Natl Acad. Sci. USA_ 104, 7682–7687 (2007) Article ADS CAS Google Scholar * White, J. F. et al. Structure of the
agonist-bound neurotensin receptor. _Nature_ 490, 508–513 (2012) Article ADS CAS Google Scholar * Krumm, B. E., White, J. F., Shah, P. & Grisshammer, R. Structural prerequisites for
G-protein activation by the neurotensin receptor. _Nature Commun._ 6, 7895–7895 (2015) Article ADS CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank T. S. Kobilka for
preparation of affinity chromatography reagents and F. S. Thian for help with cell culture. We thank J. Traynor and J. Tesmer for their support and use of their laboratory space for J.P.M.
This work was supported by the Lundbeck Foundation (Junior Group Leader Fellowship to S.G.F.R.); Fund for Scientific Research of Flanders (FWO-Vlaanderen) and the Institute for the
encouragement of Scientific Research and Innovation of Brussels (ISRIB) (E.P. and J.S.); National Institute of Neural Disorders and Stroke grant RO1-NS28471 (B.K.K.); the Mather Charitable
Foundation (B.K.K.); National Institute of General Medical Sciences grants RO1-GM083118 and U19-GM106990 (B.K.K. and R.K.S.) and RO1-GM068603 (R.K.S.); National Institutes of Drug Abuse
R21-031418 (B.K.K. and R.K.S.); Michigan Diabetes Research and Training Center Grant, National Institute of Diabetes and Digestive and Kidney Diseases, P60DK-20572 (R.K.S.); University of
Michigan Biological Sciences Scholars Program (R.K.S.) and the Rackham School of Graduate Studies (B.T.D.); Molecular Biophysics Training Grant T32GM008270 (B.T.D.); Cell and Molecular
Biology Training Grant T32GM007315 (G.A.V.-R.) and Pharmacological Sciences Training Program T32GM007767 (J.P.M.); and AHA Midwest Affiliate Predoctoral Fellowship 13PRE17110027 (J.P.M.).
AUTHOR INFORMATION Author notes * Brian T. DeVree and Jacob P. Mahoney: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of Pharmacology, University of
Michigan Medical School, Ann Arbor, 48109, Michigan, USA Brian T. DeVree, Jacob P. Mahoney, Gisselle A. Vélez-Ruiz, Adam J. Kuszak, Elin Edwald & Roger K. Sunahara * Department of
Cellular and Molecular Physiology, Stanford University, Palo Alto, 94305, California, USA Soren G. F. Rasmussen, Juan-Jose Fung, Aashish Manglik, Matthieu Masureel, Yang Du, Rachel A. Matt
& Brian K. Kobilka * Structural Biology Research Center, VIB, Vrije Universiteit Brussel (VUB), Pleinlaan 2, Brussels, 1050, Belgium Els Pardon * Structural Biology Brussels, Vrije
Universiteit Brussel (VUB), Pleinlaan 2, Brussels, 1050, Belgium Jan Steyaert * Department of Pharmacology, University of California San Diego School of Medicine, 9500 Gilman Drive, La
Jolla, 92093, California, USA Roger K. Sunahara Authors * Brian T. DeVree View author publications You can also search for this author inPubMed Google Scholar * Jacob P. Mahoney View author
publications You can also search for this author inPubMed Google Scholar * Gisselle A. Vélez-Ruiz View author publications You can also search for this author inPubMed Google Scholar * Soren
G. F. Rasmussen View author publications You can also search for this author inPubMed Google Scholar * Adam J. Kuszak View author publications You can also search for this author inPubMed
Google Scholar * Elin Edwald View author publications You can also search for this author inPubMed Google Scholar * Juan-Jose Fung View author publications You can also search for this
author inPubMed Google Scholar * Aashish Manglik View author publications You can also search for this author inPubMed Google Scholar * Matthieu Masureel View author publications You can
also search for this author inPubMed Google Scholar * Yang Du View author publications You can also search for this author inPubMed Google Scholar * Rachel A. Matt View author publications
You can also search for this author inPubMed Google Scholar * Els Pardon View author publications You can also search for this author inPubMed Google Scholar * Jan Steyaert View author
publications You can also search for this author inPubMed Google Scholar * Brian K. Kobilka View author publications You can also search for this author inPubMed Google Scholar * Roger K.
Sunahara View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS B.T.D., J.P.M., G.A.V.-R., B.K.K. and R.K.S. designed the experiments. B.T.D.,
J.P.M., G.A.V.-R. and A.J.K. performed research; S.G.F.R., E.E., J.-J.F., A.M., M.M., Y.D., R.A.M., E.P. and J.S. contributed valuable reagents/analytic tools; B.T.D., J.P.M., G.A.V.-R.,
B.K.K. and R.K.S. analysed data; and B.T.D., J.P.M., B.K.K. and R.K.S. wrote the paper. CORRESPONDING AUTHOR Correspondence to Roger K. Sunahara. ETHICS DECLARATIONS COMPETING INTERESTS The
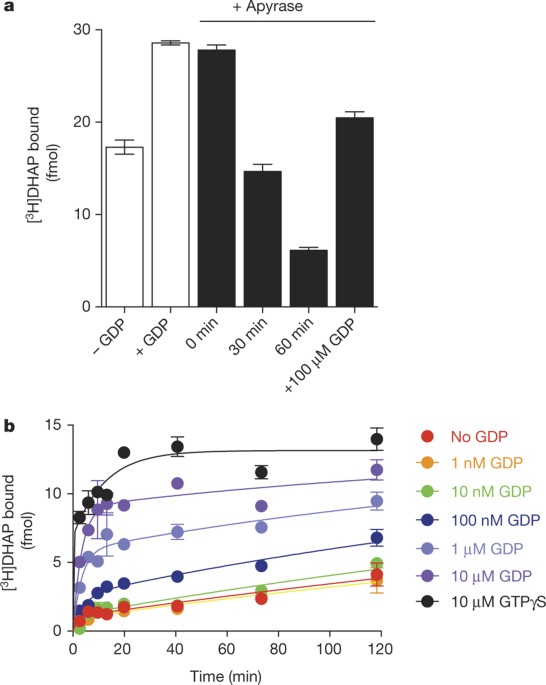
authors declare no competing financial interests. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIGURE 1 CONFIRMATION OF NUCLEOTIDE REMOVAL FROM Β2AR•GS BY APYRASE. Gs and Flag-tagged β2AR
were reconstituted in rHDL and treated with the non-specific nucleotide lyase, apyrase. Samples were applied to an anti-Flag affinity resin to remove products of the GDP degradation (GMP and
Pi). Samples were incubated with 100 nM [35S]GTPγS at room temperature. At various times, samples were subjected to rapid filtration through glass fibre filters (GF/B) followed by 10
volumes of ice-cold buffer washes containing 10 μM GDP. Filters were dried and subjected to liquid scintillation counting (Top-Count, Perkin-Elmer). To a first approximation, the rapid
binding event suggests that the complex is empty of nucleotide, based on the limited temporal resolution of this mixing and filtration technique. [3H]DHAP and [35S]GTPγS binding to the
reconstituted complex yields a final R:G ratio of 1:0.95, suggesting that up to 95% of the β2AR–rHDL particles contain a single functional G protein. This suggests that only those G proteins
associated with the β2AR will bind [35S]GTPγS within this time frame in the absence of receptor agonists. Data are shown as mean ± s.e.m. from _n_ = 3 independent experiments performed in
duplicate. EXTENDED DATA FIGURE 2 GDP ACCELERATES [3H]DHAP BINDING TO Β2AR•GS. A, Time course monitoring [3H]DHAP association to apyrase-treated β2AR•Gs complexes in the presence of varying
GDP concentrations. GDP increases both the observed association rate constant and the maximum binding of [3H]DHAP. B, Concentration–response curve showing enhancement of the observed
[3H]DHAP association rate constant by GDP (half-maximum effective concentration (EC50) = 181 ± 66 nM). All data are shown as mean ± s.e.m. from _n_ = 3 independent experiments performed in
duplicate. EXTENDED DATA FIGURE 3 EFFECT OF GUANINE NUCLEOTIDES ON [3H]DHAP BINDING TO Β2AR•GS. A, In saturation binding assays, addition of GTPγS to apyrase-treated β2AR•Gs complexes
increased the observed maximal binding, _B_max, for [3H]DHAP without significantly altering _K_d (control: _B_max = 5.5 ± 0.52 fmol, _K_d = 0.88 nM; +GTPγS: _B_max = 16.6 ± 1.9 fmol, _K_d =
0.56 nM). B, Both GDP and GTPγS could enhance maximal [3H]DHAP binding in a concentration-dependent manner (GDP log(EC50) = −6.42 ± 0.12, or EC50 ≈ 386 nM; GTPγS log(EC50) = −7.45 ± −0.16,
or EC50 ≈ 35 nM). All data are shown as mean ± s.e.m. from _n_ = 3 independent experiments performed in duplicate. EXTENDED DATA FIGURE 4 EFFECT OF NB80 ON ANTAGONIST BINDING TO THE Β2AR. A,
Association of [3H]DHAP is progressively slowed after pre-incubation of the β2AR with increasing concentrations of Nb80. B, If [3H]DHAP is allowed to first equilibrate with the β2AR, Nb80
slows [3H]DHAP dissociation from β2AR in a concentration-dependent manner. C, Owing to the dramatic slowing of [3H]DHAP binding kinetics, Nb80 (but not a control nanobody, Nb30, which has no
effect on agonist affinity for β2AR) seems competitive with [3H]DHAP if insufficient time is given to reach equilibrium. Data shown are from assays incubated for 90 min at room temperature.
All data are shown as mean ± s.e.m. from _n_ = 3 independent experiments performed in duplicate. EXTENDED DATA FIGURE 5 Y308A MUTATION ABOLISHES THE RATE-SLOWING EFFECTS OF NB80. A, B, Time
course of [3H]DHAP binding to wild-type (WT) β2AR (A) or β2AR(Y308A) (B) after pre-incubation of receptor with Nb80. Nb80 significantly slowed [3H]DHAP association to wild-type β2AR (−Nb80
observed rate constant, _k_obs = 0.45 ± 0.05 min−1 or association half-time, _t_½ = 1.5 ± 0.2 min, +Nb80 _k_obs = 0.20 ± 0.03 min−1 or _t_½ = 3.5 ± 0.5 min; _P_ = 0.011 by an unpaired
two-tailed _t_-test), but less effectively slowed [3H]DHAP association to β2AR(Y308A) (−Nb80 _k_obs = 0.50 ± 0.06 min−1 or _t_½ = 1.4 ± 0.2 min; +Nb80 _k_obs = 0.32 ± 0.01 min−1 or _t_½ =
2.2 ± 0.1 min; _P_ = 0.05 by an unpaired two-tailed _t_-test. All data are shown as mean ± s.e.m. from _n_ = 4 (−Nb80) or _n_ = 3 (+Nb80) independent experiments performed in duplicate. C,
D, Time course of [3H]formoterol binding to wild-type β2AR (C) or β2AR(Y308A) (D) after pre-incubation of receptor with Nb80. Nb80 slowed [3H]formoterol association to wild-type β2AR (0.1 μM
Nb80 _k_obs = 0.68 ± 0.13 min−1 or _t_½ = 1.0 ± 0.2 min, 10 μM Nb80 _k_obs = 0.27 ± 0.05 min−1 or _t_½ = 2.6 ± 0.5 min; _P_ = 0.031 by an unpaired two-tailed _t_-test). However, with
β2AR(Y308A), Nb80 had little effect on the observed association rate constant but enhanced the amount of [3H]formoterol bound (0.1 μM Nb80 _k_obs = 0.37 ± 0.11 min−1 or _t_½ = 1.9 ± 0.6 min
with a plateau of 10.1 ± 0.8 fmol, 10 μM Nb80 _k_obs = 0.53 ± 0.13 min−1 or _t_½ = 1.3 ± 0.4 min with a plateau of 21.3 ± 1.2 fmol; unpaired two-tailed _t_-test of the _k_obs values showed
_P_ = 0.4). All data are shown as mean ± s.e.m. from _n_ = 4 independent experiments performed in duplicate. EXTENDED DATA FIGURE 6 THE CLOSED CONFORMATION STABILIZED BY AGONIST AND G
PROTEIN (OR MIMIC). Illustrated are the crystal structures of agonist-versus inverse-agonist-bound β2AR (cyan) and β1AR (yellow), where only β2AR is bound to G protein. Similarly, the MOPr
(orange) adopts a closed conformation upon binding the G-protein surrogate, Nb39. β2AR, PDB accession 2RH1; β2AR•Gs, PDB accession 3SN6; β1AR, PDB accession 2YCW; β1AR-iso, PDB accession
2Y03; MOPr, PDB accession 4DKL; MOPr–Nb39, PDB accession 5C1M; M2R, PDB accession 3UON; M2R–Nb9-8, PDB accession 4MQS. EXTENDED DATA FIGURE 7 EFFECT OF GUANINE NUCLEOTIDES ON [3H]ANTAGONIST
BINDING ARE ALSO SEEN IN COMPETITION BINDING ASSAYS. A, Agonist (isoproterenol) competition binding using apyrase-treated β2AR•Gs complexes shows the characteristic G-protein-dependent shift
in agonist affinity, along with a dramatic increase in total [3H]DHAP binding, upon the addition of 10 μM GTPγS. B, Normalization of the data from A yields a plot representative of what is
commonly reported in the literature. C, Similar to the β2AR, agonist (morphine) competition binding using MOPr•Go complexes shows the characteristic G-protein-dependent shift in agonist
affinity, along with a dramatic increase in total [3H]DPN binding, upon the addition of 10 μM GTPγS. D, Normalization of the data from C. All data are shown as mean ± s.e.m. from _n_ = 3
independent experiments performed in duplicate. EXTENDED DATA FIGURE 8 THE MOPR AND M2R BEHAVE SIMILARLY TO THE Β2AR WHEN BOUND TO NUCLEOTIDE-FREE G PROTEIN OR AN ACTIVE-STATE-STABILIZING
NANOBODY. A, After apyrase treatment of M2R•Go complexes, addition of 10 μM GTPγS enhances association of [3H]N-methylscopolamine ([3H]NMS) to M2R (vehicle _k_obs = 0.32 ± 0.02 min−1 or _t_½
= 2.2 ± 0.1 min, +GTPγS _k_obs = 0.54 ± 0.02 min−1 or _t_½ = 1.3 ± 0.1 min; _P_ = 0.002 by an unpaired two-tailed _t-_test). Data are shown as mean ± s.e.m. from _n_ = 3 independent
experiments performed in duplicate. Addition of GDP was also able to increase the rate of [3H]NMS binding (inset; log (EC50) = 6.91 ± 0.18 or EC50 ≈ 123 nM; mean ± s.e.m. from _n_ = 2
independent experiments performed in duplicate). B, Pre-treatment of M2R with either 10 μM (black circles) or 100 μM (red squares) Nb9-8 (ref. 27) impairs association of [3H]iperoxo to M2R
(10 μM Nb9-8 _k_obs = 0.68 ± 0.09 min−1 or _t_½ = 1.0 ± 0.2 min, 100 μM Nb9-8 _k_obs = 0.25 ± 0.04 min−1 or _t_½ = 2.8 ± 0.5 min; _P_ = 0.04 by an unpaired two-tailed _t-_test). Data are
shown as mean ± s.e.m. from _n_ = 3 (10 μM Nb9-8) or _n_ = 2 (100 μM Nb9-8) independent experiments performed in duplicate. C, Addition of 10 μM GTPγS to apyrase-treated MOPr•Go complexes
hastened association of the antagonist [3H]diprenorphine ([3H]DPN) to MOPr (apyrase _k_obs = 0.06 ± 0.02 min−1 or _t_½ = 9.8 ± 1.3 min, +GTPγS _k_obs = 0.12 ± 0.01 min−1 or _t_½ = 5.6 ± 0.6
min; _P_ = 0.1 by an unpaired two-tailed _t-_test). The effect of nucleotide-free G protein was recapitulated by pre-incubating MOPr with Nb39 (ref. 28) (inset; control _k_obs = 0.13 ± 0.01
min−1, +100 μM Nb39 _k_obs = 0.07 ± 0.02 min−1). Data are shown as mean ± s.e.m. from _n_ = 2 (MOPr•Go) or _n_ = 3 (MOPr + Nb39) independent experiments performed in duplicate. EXTENDED DATA
FIGURE 9 THE EXTRACELLULAR REGIONS IN THE ACTIVE CONFORMATIONS OF PEPTIDE HORMONE/AGONIST RECEPTORS MOPR AND NTS-R1. Illustrated are the crystal structures of the inactive and active (or
partially active neurotensin receptor 1, NTS-R1) conformations of the MOPr and NTS-R1 from the top or extracellular view of the receptor. The surface rendering highlights residues or
structure on the extracellular face that change upon receptor activation (circled). The MOPr in its inactive conformation (purple) is compared to the Nb39-bound (G-protein mimic) form in
blue. Similarly, the inactive NTS-R1 (ref. 33) (green) is compared with a mutant NTS-R1 (ref. 34) that adopts a partially active conformation (orange). MOPr, PDB accession 4DKL; MOPr-Nb39,
PDB accession 5C1M; NTS-R1, PDB accession 4GRV; active-like NTS-R1, PDB accession 4XEE. EXTENDED DATA FIGURE 10 MODEL OF G-PROTEIN-DEPENDENT HIGH-AFFINITY AGONIST BINDING. A, B, As in Fig.
5, nucleotide-free G-protein-stabilized family A GPCRs experience alterations in the extracellular face of the receptor, thus affecting the orthosteric-binding site. In a monoamine receptor
such as the β2AR, G-protein binding and GDP loss accompanies the stabilization of a closed, active conformation of the receptor, as in A. B, For family members such as MOPr or NTS-R1, where
the peptide hormones/agonists are considerably larger, the influence of the G-protein-mediated changes in the extracellular domain structure result in similar effects on orthosteric ligand
dissociation. Rather than closing over the orthosteric site as with monoamine receptors as in A, the extracellular face may contain structures and residues that ‘pinch’ the larger ligands.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION This file contains Supplementary Methods, a Supplementary Discussion and additional references. (PDF 297 kb) ACTIVATION OF THE Β2AR Morph
of the β2AR in its inactive conformation bound to inverse agonist carazolol (PDB: 2RH1) and the β2AR in its active conformation bound to agonist BI-167607 and nanobody Nb80 (PDB: 3P0G). For
reference, epinephrine is modeled in the orthosteric binding site. Morphs were generated using Chimera (Pettersen, E,F., et al. UCSF Chimera--a visualization system for exploratory research
and analysis. _J Comput Chem_. 2004 25, 1605-12) and rendered with Pymol (The PyMOL Molecular Graphics System, Schrödinger, LLC). Highlighted are Phe193ECL2 and Tyr3087.35. (MOV 8205 kb)
COMPARISON OF Β1AR AND Β2AR The closed, active conformation is stabilized by the G protein. Morph of the β2AR in its inactive conformation bound to inverse agonist carazolol (cyan, PDB:
2RH1) and the β2AR in its active conformation bound to agonist BI-167607 and nanobody Nb80 (PDB: 3P0G). Superimposed on top is a similar morph transitioning between the
carazolol-boundββ1-adrenergic receptor (β1AR, lime green, PDB: 2YCW) and isoproterenol-bound (but not G protein- or Nb-bound, PDB: 2Y03). While the β2AR adopts a closed conformation
stabilized by agonist and G protein, the β1AR bound only to isoproterenol does not. For reference, epinephrine is modeled in the orthosteric binding site. Note that nanobody Nb80 has been
omitted from the animation for simplicity. Highlighted are Phe193ECL2 and Tyr3087.35 on β2AR and the conserved residues Phe201ECL2 and Phe3527.35 on β1AR. Morphs were generated using Chimera
(Pettersen, E,F., _et al_. UCSF Chimera--a visualization system for exploratory research and analysis. _J Comput Chem_. 2004 25, 1605-12) and rendered with Pymol (The PyMOL Molecular
Graphics System, Schrödinger, LLC). (MOV 3417 kb) ACTIVATION OF THE Μ-OPIOID RECEPTOR, MOPR The ribbon structure of the μ-opioid receptor (MOPr) in its inactive conformation bound to
β-funaltrexamine (cyan, PDB: 4DKL) and the MOPr in its active conformation bound to agonist BU72 and Nb39 (orange, PDB: 5C1M). For reference only BU72 is displayed as spheres. Note that Nb39
is not illustrated for simplicity. Morph between the inactive and active conformations was generated using Chimera (Pettersen, E,F., _et al_. UCSF Chimera--a visualization system for
exploratory research and analysis. _J Comput Chem_. 2004 25, 1605-12) and rendered with Pymol (The PyMOL Molecular Graphics System, Schrödinger, LLC). (MOV 5952 kb) ACTIVATION OF THE M2
MUSCARINIC RECEPTOR, M2R Top view of the ribbon structure of the M2 muscarinic receptor (M2R) in its inactive conformation bound to antagonist 3-quinuclidinyl benzilate, (QNB, PDB: 3UON) and
the M2R in its active conformation bound to agonist iperoxo and nanobody Nb9-8 (PDB: 4MQS). Illustrated are sidechain residues Y1043.33, Y4036.38 and Y4267.39 to highlight the ‘lid-like’
structure over the orthosteric site. Acetylcholine is modeled into the iperoxo binding site and illustrated in stick figure for reference purposes. Note that Nb9-8 is not depicted for
simplicity. Morphs were generated between the inactive and active conformations using Chimera (Pettersen, E,F., _et al_. UCSF Chimera--a visualization system for exploratory research and
analysis. _J Comput Chem_. 2004 25, 1605-12) and rendered with Pymol (The PyMOL Molecular Graphics System, Schrödinger, LLC). (MOV 2726 kb) ACTIVATION OF THE M2R SIDE VIEW Side view of the
ribbon structure of the M2R (above) to highlight the ‘lid-like’ structure over the orthosteric site. Illustrated are side chain residues Y4036.38 and Y4267.39 moving toward Y1043.33 during
the formation of the active conformation. Note that TM5 was removed from the rendering so that the tyrosine residues may be easily viewed. Morphs were generated between the inactive and
active conformations using Chimera (Pettersen, E,F., _et al_. UCSF Chimera--a visualization system for exploratory research and analysis. _J Comput Chem_. 2004 25, 1605-12) and rendered with
Pymol (The PyMOL Molecular Graphics System, Schrödinger, LLC). (MOV 2616 kb) STABILIZATION OF THE ACTIVE STATE OF RHODOPSIN BY G PROTEIN Top view of the ribbon structure of bovine rhodopsin
in its inactive conformation (PDB:1F88) (Palczewski, K. _et al_. Crystal structure of rhodopsin: A G protein-coupled receptor Science 289: 739-745 (2000)) and the photoactivated meta-stable
form of rhodopsin bound to the C-terminal fragment of the G protein alpha subunit, transducin (transducin not shown) (PDB:3PAR) (Choe H. W. _et al_. Crystal structure of metarhodopsin II.
_Nature_ 471, 651–655 (2011)). Note the pre-existing ‘lid-like’ structure over the orthosteric site formed by the ECL2 and N-terminus. Photoisomerization of 11-cis retinal is illustrated in
magenta. Morphs were generated between the inactive and active conformations using Chimera (Pettersen, E,F., _et al_. UCSF Chimera--a visualization system for exploratory research and
analysis. _J Comput Chem_. 2004 25, 1605-12) and rendered with Pymol (The PyMOL Molecular Graphics System, Schrödinger, LLC). (MOV 6005 kb) STABILIZATION OF THE ACTIVE STATE OF RHODOPSIN BY
ARRESTIN Top view of the ribbon structure of bovine rhodopsin in its inactive conformation (PDB:1F88) (Palczewski, K. _et al_. Crystal structure of rhodopsin: A G protein-coupled receptor
Science 289: 739-745 (2000)) and an active mutant of opsin bound to activated arrestin (arrestin not shown) (PDB:4ZWJ) (Kang, Y. _et al_. Crystal structure of rhodopsin bound to arrestin by
femtosecond X-ray laser. _Nature_ 523, 561-567 (2015)). Note the similarities in conformational changes as with metarhodopsin bound to the C-terminal helix of transducin. Photoisomerization
of 11-cis retinal, based on the metarhodopsin structures (Extended data: Movie SM6) has been modeled into the opsin-arrestin structure and is illustrated in magenta. Morphs were generated
between inactive and arrestin-bound conformations of rhodopsin using Chimera (Pettersen, E,F., _et al_. UCSF Chimera--a visualization system for exploratory research and analysis. _J Comput
Chem_. 2004 25, 1605-12.) and rendered with Pymol (The PyMOL Molecular Graphics System, Schrödinger, LLC). (MOV 5598 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR
FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE DeVree, B.,
Mahoney, J., Vélez-Ruiz, G. _et al._ Allosteric coupling from G protein to the agonist-binding pocket in GPCRs. _Nature_ 535, 182–186 (2016). https://doi.org/10.1038/nature18324 Download
citation * Received: 19 August 2015 * Accepted: 13 May 2016 * Published: 29 June 2016 * Issue Date: 07 July 2016 * DOI: https://doi.org/10.1038/nature18324 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative