Mechanism of silk processing in insects and spiders
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
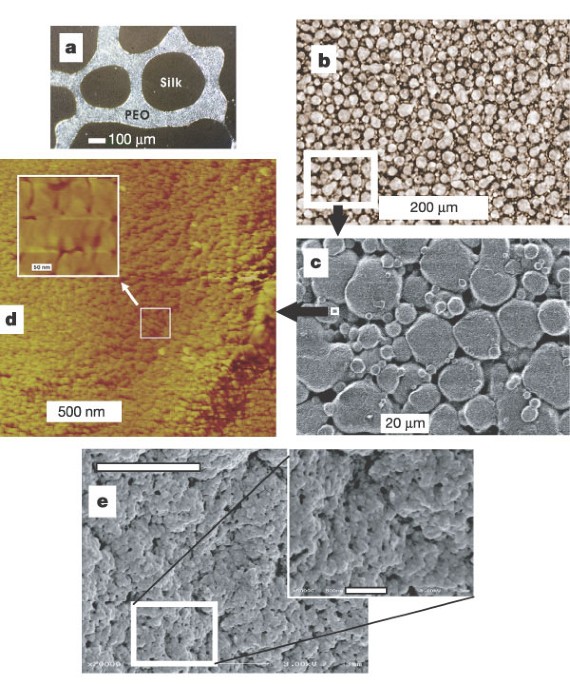
ABSTRACT Silk spinning by insects and spiders leads to the formation of fibres that exhibit high strength and toughness1. The lack of understanding of the protein processing in silk glands
has prevented the recapitulation of these properties _in vitro_ from reconstituted or genetically engineered silks. Here we report the identification of emulsion formation and micellar
structures from aqueous solutions of reconstituted silkworm silk fibroin as a first step in the process to control water and protein–protein interactions. The sizes (100–200 nm diameter) of
these structures could be predicted from hydrophobicity plots of silk protein primary sequence2. These micelles subsequently aggregated into larger ‘globules’ and gel‐like states as the
concentration of silk fibroin increased, while maintaining solubility owing to the hydrophilic regions of the protein interspersed among the larger hydrophobic regions. Upon physical
shearing or stretching structural transitions, increased birefringence and morphological alignment were demonstrated, indicating that this process mimics the behaviour of similar native silk
proteins _in vivo_. Final morphological features of these silk materials are similar to those observed in native silkworm fibres. Access through your institution Buy or subscribe This is a
preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per
year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during
checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS EXPLORING THE
SELF-ASSEMBLY OF SILK PROTEINS THROUGH LIQUID-LIQUID PHASE SEPARATION Article Open access 28 April 2025 MOLECULAR ORGANIZATION OF FIBROIN HEAVY CHAIN AND MECHANISM OF FIBRE FORMATION IN
_BOMBYX MORI_ Article Open access 29 June 2024 THE SILK OF GORSE SPIDER MITE _TETRANYCHUS LINTEARIUS_ REPRESENTS A NOVEL NATURAL SOURCE OF NANOPARTICLES AND BIOMATERIALS Article Open access
28 October 2020 REFERENCES * Vollrath, F. & Knight, D. P. Liquid crystalline spinning of spider silk. _Nature_ 410, 541–548 (2001) Article ADS CAS Google Scholar * Zhou, C. Z. et al.
Fine organization of _Bombyx mori_ fibroin heavy chain gene. _Nucleic Acids Res._ 28, 2413–2419 (2000) Article CAS Google Scholar * Altman, G. H. et al. Silk matrix for tissue engineered
anterior cruciate ligaments. _Biomaterials_ 23, 4131–4141 (2002) Article CAS Google Scholar * Mita, K., Ichimura, S. & James, T. C. Highly repetitive structure and its organization
of the silk fibroin gene. _J. Mol. Evol._ 38, 583–592 (1994) Article ADS CAS Google Scholar * Yamada, H., Nakao, H., Takasu, Y. & Tsubouchi, K. Preparation of undegraded native
molecular fibroin solution from silkworm cocoons. _Mater. Sci. Eng. C_ 14, 41–46 (2001) Article Google Scholar * Sofia, S., McCarthy, M. B., Gronowicz, G. & Kaplan, D. L.
Functionalized silk-based biomaterials for bone formation. _J. Biomed. Mater. Res._ 54, 139–148 (2001) Article CAS Google Scholar * Jin, H.-J., Fridrikh, S. V., Rutledge, G. C. &
Kaplan, D. L. Electrospinning _Bombyx mori_ silk with poly(ethylene oxide). _Biomacromolecules_ 3, 1233–1239 (2002) Article CAS Google Scholar * Roseman, M. A. Hydrophilicity of polar
amino-acid side-chain is markedly reduced by flanking peptide-bonds. _J. Mol. Biol._ 200, 513–522 (1988) Article CAS Google Scholar * Ochi, A., Hossain, K. S., Magoshi, J. & Nemoto,
N. Rheology and dynamic light scattering of silk fibroin solution extracted from the middle division of _Bombyx mori_ silkworm. _Biomacromolecules_ 3, 1187–1196 (2002) Article CAS Google
Scholar * Discher, D. E. & Eisenberg, A. Polymer vesicles. _Science_ 297, 967–973 (2002) Article ADS CAS Google Scholar * Malstom, M. & Lindman, B. Self-assembly in aqueous
block copolymer solutions. _Macromolecules_ 25, 5440–5445 (1992) Article ADS Google Scholar * Kwon, K. W., Park, M. J., Bae, Y. H., Kim, H. D. & Char, K. Gelation behavior of
PEO-PLGA-PEO triblock copolymers in water. _Polymer_ 43, 3353–3358 (2002) Article CAS Google Scholar * Magoshi, J., Mizuide, M. & Magoshi, Y. Physical properties and structure of
silk. VI. Conformational changes in silk fibroin induced by immersion in water at 2 to 130 °C. _J. Polym. Sci._ 17 (Polymer Physics Edition), 515–520 (1979) CAS Google Scholar * Ishida,
M., Asakura, T., Yoko, M. & Saito, H. Solvent- and mechanical-treatment-induced conformational transition of silk fibroins studied by high-resolution solid-state 13C NMR spectroscopy.
_Macromolecules_ 23, 88–94 (1990) Article ADS CAS Google Scholar * Seidel, A. et al. Regenerated spider silk: Processing, properties, and structure. _Macromolecules_ 33, 775–780 (2000)
Article ADS CAS Google Scholar * Valluzzi, R., Szela, S., Avtges, P., Kirschner, D. & Kaplan, D. L. Methionine redox controlled crystallization of biosynthetic silk spidroin. _J.
Phys. Chem. B_ 103, 11382–11392 (1999) Article CAS Google Scholar * Wilson, D., Valluzzi, R. & Kaplan, D. Conformational transitions in model silk peptides. _Biophys. J._ 78,
2690–2701 (2001) Article Google Scholar * Shen, Y., Johnson, M. A. & Martin, D. C. Microstructural characterization of _Bombyx mori_ silk fibers. _Macromolecules_ 31, 8857–8864 (1998)
Article ADS CAS Google Scholar * Asakura, T., Kuzuhara, A., Tabeta, R. & Saitô, H. Conformation characterization of _Bombyx mori_ silk fibroin in the solid state by high-frequency
13C cross polarization-magic angle spinning NMR, X-ray diffraction, and infra spectroscopy. _Macromolecules_ 18, 1841–1845 (1985) Article ADS CAS Google Scholar * Putthanarat, S.,
Stribeck, N., Fossey, S. A., Eby, R. K. & Adams, W. W. Investigation of the nanofibrils of silk fibers. _Polymer_ 41, 7735–7747 (2000) Article CAS Google Scholar * van Beek, J. D.,
Hess, S., Vollrath, F. & Meier, B. H. The molecular structure of spider dragline silk: Folding and orientation of the protein backbone. _Proc. Natl Acad. Sci. USA_ 99, 10266–10271 (2002)
Article ADS CAS Google Scholar * Perez-Rigueiro, J., Viney, C., Llorca, J. & Elices, M. Silkworm silk as an engineering material. _J. Appl. Polym. Sci._ 70, 2439–2447 (1998) Article
CAS Google Scholar * Poza, P., Pérez-Rigueiro, J., Elices, M. & Llorca, J. Fractographic analysis of silkworm and spider silk. _Eng. Fracture Mech._ 69, 1035–1048 (2002) Article
Google Scholar * Auvray, X. et al. Influence of solvent headgroup interactions on the formation of lyotropic liquid crystal phases of surfactants in water and nonaqueous protic and aprotic
solvents. _Langmuir_ 8, 2671–2679 (1992) Article CAS Google Scholar * Lele, A. K. et al. Deformation induced hydrophobicity: Implications in spider silk formation. _Chem. Eng. Sci._ 56,
5793–5800 (2001) Article CAS Google Scholar * Tanaka, T. et al. Phase separation structure in poly(vinyl alcohol) silk fibroin blend films. _Polym. Int._ 45, 175–184 (1998) Article CAS
Google Scholar * Knight, D. P. & Vollrath, F. Biological liquid crystal elastomers. _Phil. Trans. R. Soc. Lond. B_ 357, 155–163 (2002) Article CAS Google Scholar * Knight, D. P.
& Vollrath, F. Liquid crystals and flow elongation in a spider's silk production line. _Proc. R. Soc. Lond. B_ 266, 519–523 (1999) Article Google Scholar * Viney, C. Natural
silks: Archetypal supramolecular assembly of polymer fibres. _Supramol. Sci._ 4, 75–81 (1997) Article CAS Google Scholar * Minoura, N., Tsukada, M. & Nagura, M. Physico-chemical
properties of silk fibroin membrane as a biomaterial. _Biomaterials_ 11, 430–434 (1990) Article CAS Google Scholar * Altman, G. H. et al. Silk-based biomaterials. _Biomaterials_ 24,
401–416 (2003) Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank R. Valluzzi and J. Park for technical input. This work was supported by the NIH, the NSF and the
DoD (Air Force). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Departments of Chemical & Biological Engineering & Biomedical Engineering, Tufts University, Medford, Massachusetts,
02155, USA Hyoung-Joon Jin & David L. Kaplan Authors * Hyoung-Joon Jin View author publications You can also search for this author inPubMed Google Scholar * David L. Kaplan View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to David L. Kaplan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare
that they have no competing financial interests. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jin, HJ., Kaplan, D. Mechanism of silk processing in
insects and spiders. _Nature_ 424, 1057–1061 (2003). https://doi.org/10.1038/nature01809 Download citation * Received: 22 April 2003 * Accepted: 27 May 2003 * Issue Date: 28 August 2003 *
DOI: https://doi.org/10.1038/nature01809 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative