A role for ccl28–ccr3 in t-cell homing to the human upper airway mucosa
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Lymphocyte recruitment to peripheral tissues is fundamental for immune surveillance and homeostasis, but the chemokines and chemokine receptors responsible for tissue-specific
homing of T cells to the upper airway mucosa have not been determined. To address this, we analyzed the chemokines expressed in the normal human nasal mucosa and found that CCL28 is
preferentially expressed at a high level on the lumenal face of vascular endothelial cells in the mucosa. Analysis of the cognate chemokine receptors revealed that close to 50% of the CD4+ T
cells in the human nasal mucosa expressed the CCL28 receptor CCR3, whereas CCR3 was hardly detectable on T cells in the small intestine and skin. In the circulation, CCR3+ T cells comprised
a small subset that did not express homing receptors to the intestine or skin. Moreover, depletion of CCR3+CD4+ T cells abrogated the proliferative response of human blood CD4+ T cells
against the opportunistic nasopharyngeal pathogen _Haemophilus influenzae,_ indicating that the CCR3+CD4+ T-cell subset in the circulation contains antigen specificities relevant for the
upper airways. Together, these findings indicate that CCL28–CCR3 interactions are involved in the homeostatic trafficking of CD4+ T cells to the upper airways. SIMILAR CONTENT BEING VIEWED
BY OTHERS T CELL RECEPTOR SIGNALING STRENGTH ESTABLISHES THE CHEMOTACTIC PROPERTIES OF EFFECTOR CD8+ T CELLS THAT CONTROL TISSUE-RESIDENCY Article Open access 04 July 2023 ILC2-DERIVED LIF
LICENCES PROGRESS FROM TISSUE TO SYSTEMIC IMMUNITY Article Open access 07 August 2024 SPECIALIZED TRANSENDOTHELIAL DENDRITIC CELLS MEDIATE THYMIC T-CELL SELECTION AGAINST BLOOD-BORNE
MACROMOLECULES Article Open access 28 October 2021 INTRODUCTION Memory T cells are abundant within and beneath the epithelium in the airway mucosa.1 The concept of homeostatic
tissue-specific homing to non-lymphoid tissues, in which memory lymphocytes preferentially traffic to the anatomical region where they first encountered their specific antigen, is well
established.2 The tissue-tropic potential of lymphocytes appears to be imprinted during priming in regional lymphoid tissues,3 and the trafficking is mainly regulated at the level of cell
recruitment from the blood and into the tissues. Lymphocyte recruitment to tissues involves a sequential process including chemokine-mediated control of intravascular lymphocyte adhesion.
Studies have identified several constitutively expressed chemokines that control targeting of memory T cells to specific tissues.4 There is ample evidence to suggest that tissue-specific
lymphocyte homing also occurs in the respiratory tract. This is particularly evident from numerous studies showing that plasma-cell precursors primed in the upper respiratory tract
selectively migrate to the non-intestinal mucosa.5, 6 Moreover, recent animal studies have indicated that intranasal immunization induces T cells that are recruited to the airways under
steady-state conditions.7, 8 The best defined memory T-cell subsets targeted to specific extralymphoid sites are the skin-homing population, defined by expression of the cutaneous lymphocyte
antigen (CLA), and the gut-homing population, defined by the high-level expression of the α4β7 integrin.9 Furthermore, it is well established that homing of CLA+ T cells to cutaneous sites
is mediated by CCL17–CCR4,10 a subset of which appear to also require CCL27–CCR10,11 whereas homing of α4β7hi T cells to intestinal sites depend on CCL25–CCR9.12 Studies have also shown that
memory T cells specific for pathogens exhibiting cutaneous or intestinal tropism are found within the circulating skin- and gut-homing populations, respectively.13, 14 By analogy,
non-typeable _Haemophilus influenzae_ (NTHi) is an opportunistic pathogen normally colonizing the upper respiratory tract of children and adults that often cause infections such as otitis
media, sinusitis, and tonsillitis.15, 16 NTHi-specific memory T cells circulate at low frequencies in the peripheral blood of healthy donors.17 The selective tissue localization of NTHi
suggests that the immune responses to NTHi are associated with the generation of memory T cells trafficking to the upper airways. The molecular mechanisms regulating the homeostatic
migration of T cells to the human upper airway mucosa are not fully explored. In particular, identification of chemokines and the corresponding receptors controlling vascular recruitment is
lacking. However, several reports have shown that CCL28 is preferentially expressed in various mucosal tissues of the upper aerodigestive tract.18, 19 In fact, CCL28 may be assigned to a
subfamily of evolutionary conserved chemokines, including CCL25 and CCL27, which are constitutively expressed at epithelial barrier surfaces and are implicated in steady-state lymphocyte
homing to these sites.6, 18 In this study, we found evidence that the CCL28 receptor CCR3 is involved in the steady-state trafficking of CD4+ T cells to the human upper airway mucosa.
RESULTS TISSUE CHEMOKINE EXPRESSION PROFILES AT STEADY STATE To quantify the tissue expression levels of chemokines in the nasal mucosa compared with the small intestinal mucosa and skin, we
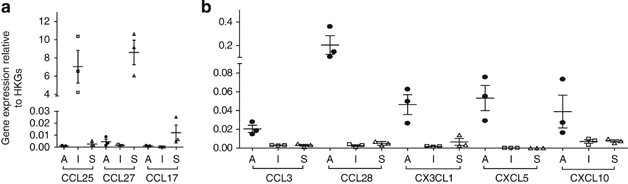
applied a real-time PCR array covering 39 different chemokines (Supplementary Table S1 online). To validate our approach, we first analyzed the expression of CCL25 as well as CCL27 and
CCL17, which are constitutively expressed in the small intestinal mucosa and the skin, respectively3 (Figure 1a). Indeed, CCL25 and CCL27 were preferentially expressed at high levels in the
small intestinal mucosa and the skin, respectively. Moreover, CCL17 transcripts were only detected in the skin samples. When comparing all chemokine transcripts expressed in the nasal mucosa
with the skin and the intestine, we identified seven chemokines preferentially expressed in the nasal mucosa (Figure 1b and Supplementary Table S1). Among these, CCL28 and CXCL5 were found
to exhibit the highest expression levels, and were subjected to further analysis. VASCULAR LOCALIZATION OF CCL28 IN THE NASAL MUCOSA To determine the protein expression and localization of
chemokines in the nasal mucosa, we performed multicolor immunofluorescence stainings. Interestingly, CCL28 revealed a striking co-localization with the endothelial marker vWf in the lamina
propria (Figure 2). In fact, the vast majority (>80%) of vWf+ vessels in the lamina propria coexpressed CCL28. Strong expression of CCL28 was detected on the luminal surface of the
endothelial cells, as well as in perivascular cells, with an apparent gradual decrease in staining intensity within the surrounding tissue. Epithelial cells, particularly in the glands, were
also strongly positive (data not shown). Immunostaining with a goat polyclonal anti-CCL28 antibody revealed a similar staining pattern (data not shown). CXCL5 appeared quite ubiquitously
expressed within the lamina propria of the nasal mucosa (Figure 2). Although we found CXCL5 localized to regions surrounding vascular cells, the luminal enrichment of this chemokine was less
apparent. CCL28 RECEPTOR EXPRESSION ON NASAL MUCOSA-DERIVED T CELLS Next we wanted to examine whether T cells in the nasal mucosa expressed the receptors for CCL28, CCR10, and CCR3.18, 20
Tissue specimens of nasal mucosa were enzymatically digested, and single cell suspensions were analyzed by FACS. We found a distinct population of viable CD3+CD4+ T cells that was readily
detected among the dispersed cells (Figure 3a). Strikingly, whereas CCR10 expression was hardly detectable, approximately 50% of these nasal mucosa-derived CD4+ T cells expressed CCR3
(Figure 3b). This strongly contrasted peripheral blood, in which CCR3 was expressed on a highly limited number of CD4+ T cells (<1%). Furthermore, the CD8+ T-cell fraction from nasal
mucosa contained approximately 20% CCR3+ cells, (Figure 3c), and was also negative for CCR10. However, CCR10 expression was clearly detectable on a population of CD138+ plasma cells from the
nasal mucosa (Supplementary Figure S1), as previously reported.5 A small percentage of the nasal mucosa-derived CD4+ T cells expressed the CXCL5 receptor CXCR1 (Figure 3d), but the level
was similar to the reported expression level in peripheral blood.21 Expression of the closely related receptor CXCR2 was only observed on a minor fraction (Figure 3d). Interestingly, similar
analysis performed on cells isolated from the skin and the small intestinal mucosa revealed very low numbers of CCR3+CD4+T cells (Figure 3f). CD4+ T cells expressing the homeostatic
chemokine receptor CCR6 were generally scarce in the nasal mucosa (Supplementary Figure S3a). Moreover, mucosal T cells were mostly CCR7− (Supplementary Figure S3a), thus likely representing
effector T cells with limited lymph node trafficking capacity. In the circulation, CCR10 is expressed on a limited number of CD4+ T cells confined to a subpopulation of skin-homing CLA+ T
cells.11 Conversely, we found that CCR3+CD4+ T cells in peripheral blood (<0.5%) were virtually negative for CLA (Figure 3g). Moreover, CCR3+CD4+ T cells appeared enriched within the
α4β7lo population, together indicating that these cells do not traffic to the skin or small intestine. DEPLETION OF CCR3+CD4+ T CELLS ABROGATES THE PROLIFERATIVE CD4+T-CELL RESPONSE TO THE
NTHI-DERIVED OUTER MEMBRANE PROTEIN P6 The highly conserved outer membrane protein P6 from a range of NTHi isolates has been shown to induce T-cell responses.22 To assess the presence of T
cells specific for NTHi within the CCR3+CD4+ T-cell population, we determined the P6-specific proliferative response in purified peripheral blood CD4+ T cells undepleted or depleted for
CCR3+ cells. To enable sufficient separation between the rare proliferating T cells and the non-responding population, CFSE dilution in T cells was assessed after 6 days of stimulation.
Analysis revealed that the subjects mounted a response to P6 following stimulation with antigen (Figure 4a), as published by others.17, 22 Strikingly, when CD4+ T cells were depleted for
CCR3+ cells with FACS sorting, the proliferative response was abrogated and not different from unstimulated control cells. Similar results were also obtained by depleting CD4+CCR3+ cells
with magnetic beads prior to antigen stimulation (data not shown), thus showing that the reduced proliferation was not an artifact introduced by cell passage through the FACS. On the
contrary, depletion of CD4+CCR3+ cells in donors vaccinated with tetanus toxoid affected the tetanus toxoid memory response to a low extent (Figure 4a). To assess whether cytokine production
by P6-stimulated T cells was altered by depletion of CCR3+CD4+ T cells, supernatants from the P6-stimulated cultures were analyzed. We found a significant reduction in the interleukin
(IL)-2 levels following CCR3 depletion in the donors exhibiting proliferative responses (Figure 4c). However, levels of other cytokines analyzed, including IL-4, IL-5, IL-10, IL-13, and
IFN-γ, were not significantly altered by depleting the CCR3+CD4+ population (data not shown). However, when directly analyzing IFN-γ production in P6-stimulated CD4+ T cells, we found that
the vast majority of proliferating T cells were IFN-γ+ (Figure 4d). To determine whether the P6-specific CD4+ T-cell response was HLA class II restricted, PBMCs were stimulated with P6 for 6
days, with or without the addition of an anti-HLA class II antibody. Analysis of cultures from responding donors revealed that such treatment consistently reduced the proliferation to
background levels observed in non-stimulated PBMCs (Figure 4e), thus strongly supporting that the P6-specific response is HLA class II dependent. To analyze the stimulatory effect of P6
directly on CD4+CCR3+ T cells, efforts were made to purify this subset through repeated rounds of positive selection. However, the low yield and reduced viability of the positively selected
cell population precluded further analysis. DISCUSSION CCL28 belongs to a subfamily of evolutionary conserved chemokines, including CCL25 and CCL27, which are constitutively expressed at
epithelial barrier surfaces and are implicated in steady-state lymphocyte homing to these sites.6, 18 Our observation of high constitutive expression of CCL28 in the steady-state nasal
mucosa is consistent with several other reports showing CCL28 expression in various mucosal tissues of the upper aerodigestive tract.18, 19 In the nasal mucosa, CCL28 protein was readily
detected on the luminal face of blood endothelial cells, which may indicate that endothelial cells produce this chemokine. However, it is well established that endothelial cells may
constitutively transcytose extravascular chemokines to their lumen,23, 24 thus the CCL28 may also be derived from other cells. CCL28 is a ligand for CCR10 and CCR3,18, 20 and CCL28–CCR10
interactions have been implicated in the dissemination of antibody-secreting cells to the upper airway mucosa. In fact, plasmablasts induced in the secondary lymphoid organs of the upper
airways express high levels of CCR10.5, 25 However, we found that T cells residing in the nasal mucosa did not express CCR10. This receptor therefore appears to be restricted to plasma cells
at this site.5 Strikingly, ∼50% of CD4+ T cells from healthy nasal mucosa express the CCL28 receptor CCR3. This translates into a ∼100-fold enrichment of this population compared with CD4+
T cells in blood. No enrichment of CCR3+CD4+ T cells was observed in the small intestinal mucosa or the skin. Furthermore, CCR3+CD4+ T cells in peripheral blood exhibited low expression
levels of the α4β7 integrin and CLA, indicating that circulating CCR3+CD4+ T cells do not home to the skin or the intestine. CCR3 has several chemokine ligands20 that may potentially account
for the tissue accumulation of this T-cell subset. The classical CCR3-ligand is CCL11, but we found this to be expressed at very low levels in the steady-state nasal mucosa (Supplementary
Table S1). CCL5 mRNA was significantly expressed in our tissue material, but subsequent immunostainings did not reveal any vascular localization of the chemokine (data not shown). Moreover,
transcripts for CCL7, CCL8, CCL13, and CCL15 were undetectable in the nasal mucosa. However, studies have shown that CCL28 is chemotactic for human eosinophils expressing CCR3,18 and
CCL28–CCR3 interactions were found to regulate eosinophil recruitment independently of CCL11 to the airways in a mouse model of allergic airway inflammation.26 Unfortunately, the low
frequency of CCR3+ T cells in peripheral blood precludes chemotaxis analysis of primary T cells with standard assays. However, we were able to detect rapid intracellular Ca flux specifically
in CCR3+ T cells following CCL28 stimulation (Supplementary Figure S2). Several studies have analyzed the chemokine expression in the upper airways during inflammation, and a role for the
CCR4 ligands CCL17 and CCL22 appears important for T-cell recruitment, as both of these chemokines are strongly upregulated following allergen challenge in atopic subjects.27, 28
Furthermore, a study revealed an increase of CCR4+ T cells in allergen-induced inflammation of the nasal mucosa in allergic rhinitis patients.29 However, we found that CCL17 and CCL22
(Figure 1) were expressed at very low levels at this site in the absence of inflammation, and CCR4+ cells constitute a minor T-cell fraction in healthy nasal mucosa (Supplementary Figure
S3a) as described by others.29 We did find that CXCL5 was expressed in the nasal mucosa at steady state, but not in the skin or intestinal mucosa. Studies have suggested a role for the CXCL5
receptor CXCR1 in T-cell trafficking to the nasal mucosa.21 However, we found no increase in the proportion of nasal mucosa-derived CD4+ T cells expressing CXCR1 compared with peripheral
blood. Accumulating evidence supports the idea that T cells acquire trafficking potentials to peripheral tissues following activation in the regional lymphoid tissues, thus ensuring homing
of memory T cells with specificities for antigens encountered in that anatomical region.3 This concept likely extends to the lower airways, as it was recently shown that human lung tissue
contain large numbers of influenza-specific memory T cells.30 Moreover, influenza-specific T cells exhibited a much reduced frequency in the circulation, and were undetectable in the skin.
In the upper airways, NTHi likely represents a frequently encountered pathogen, colonizing up to 75% of normal adults.15 Although direct demonstration of NTHi-specific T cells residing in
tissues is lacking, it is reasonable to assume that the specific memory T cells would recirculate primarily through the upper airways, including the nasal mucosa. Here, we show that the
P6-dependent proliferation of peripheral blood CD4+ T cells was completely abrogated by depleting CCR3+ cells. This finding indicates that the NTHi-reactive CD4+ T cells are confined to the
CCR3-expressing subset and adds further support to the notion that CCR3 is involved in memory T-cell trafficking to the upper airway mucosa. Moreover, our study argues that intranasal
vaccine for NTHi could be an attractive approach to induce protective immunity. Interestingly, tissue-resident T cells isolated from the lung were reportedly negative for CCR3,30, 31
indicating that CCR3 targets T-cell trafficking to the upper airways rather than the lung parenchyma. CCR3 expression on CD4+ T cells has been suggested to be a marker of Th2-polarized
memory T cells, being preferentially expressed on Th2 lines generated under specific culture conditions.32 However, analysis of freshly isolated T cells revealed that the CCR3+ population
contains about equal frequencies of Th1 and Th2 cells, and CCR3 is expressed by less than 5% of committed Th2 cells in the circulation.33 We found that CCR3+CD4+ T cells were homogenously
positive for the transcription factor T-bet (Supplementary Figure S3b), which is critical for Th1-cell differentiation, and a large fraction of the CCR3+ cells also coexpressed CXCR3, which
has been associated with Th1 cells. In fact, our preliminary studies of cytokine production by nasal mucosal T cells revealed that the vast majority of T cells were Th1, or to a lesser
extent Th17 polarized, whereas Th2 cells were hardly detectable at steady state (Hoffmann _et al._ in preparation). Moreover, the association between CCR3+ T cells and human allergic disease
is controversial; analysis of lung T cells from asthmatics revealed no difference in CCR3 expression compared with healthy controls.31, 34 However, one study reported increased numbers of
CD4+ T cells expressing CCR3 in the peripheral blood of allergic rhinitis patients,35 which was reduced following allergen immunotherapy. Furthermore, stimulation of CCR3-depleted CD4+ T
cells from these patients with allergen resulted in reduced IL-5-production, thus lending further support to the notion that the CCR3+ T-cell population contains antigen specificities
relevant to the upper airways. In conclusion, our findings suggest that CCL28, produced in the nasal mucosa, is presented by the local vascular endothelium and participate in the
steady-state homing of CCR3+CD4+ T cells. This suggests that CCR3 may serve as a candidate molecule to target CD4+ T cells operating in the upper airway mucosa. METHODS SUBJECTS. Nasal
mucosa prepared for flow cytometry and immunohistochemistry was obtained from the lower edge of the inferior turbinate during surgery for septum deviation. In all cases, the mucosa appeared
macroscopically normal and none of the donors had any history of allergic disease (_n_=38, age 19–51 years old, 16 women). Control tissue from duodenal mucosa was obtained from patients
(_n_=3, age 21, 50, and 75 years, all women) during gastroendoscopic examination for undefined stomach pain. Clinical workup and histopathological examination revealed no signs of
gastrointestinal disease in any of the patients. Macroscopically, normal skin samples were obtained by means of punch biopsies (2 mm) from healthy individuals (_n_=3, age 57, 63, and 70, all
women) undergoing plastic surgery for cosmetic reasons. Peripheral blood mononuclear cells (PBMCs) were obtained from healthy blood donors. All patients gave their written informed consent,
and the study was approved by the Norwegian Regional Committee for Medical Research Ethics. TISSUE PREPARATION AND GENE EXPRESSION ANALYSIS. Total RNA was extracted with TRI Reagent
(Sigma-Aldrich, St Louis, MO) and an RNeasy mini kit (Qiagen, Hilden, Germany). Reverse transcription of RNA was performed with an RT2 First Strand Kit (SABiosciences, Frederick, MD). For
analysis of chemokine transcripts, RT2 Profiler PCR arrays (all from SABiosciences) were designed to cover most characterized human chemokines (Supplementary Table S1). PCR was performed
according to the manufacturer’s protocol, and normalized threshold cycle (_C_t) value for each gene, (Δ_C_t), was determined by subtracting the mean _C_t value of the housekeeping genes from
the individual or mean _C_t value of the gene of interest. Normalized mRNA expression level was calculated as 2(−Δ_C_t). IMMUNOHISTOCHEMISTRY. Tissue preservation and processing was
performed essentially as described.5 To detect CCL28, we utilized clone 3B1 (mouse IgG1, AbD Serotec, Oxford, UK) or goat IgG (AF717, R&D Systems, Oxon, UK). CXCL5 was detected using
clone S5 (mouse IgG1, ABGENT, San Diego, CA). To detect von Willebrand factor (vWf), rabbit IgG (A0082, DakoCytomation, Glostrup, Denmark) was applied. Irrelevant isotype- and
concentration-matched primary antibodies served as negative controls, and Alexa-conjugated secondary antibodies were from Life Technologies (Paisley, UK). Microscopy was performed with an
Olympus FV1000 confocal microscope (Olympus, Hamburg, Germany). FACS ANALYSIS. Surgical specimens from healthy donors were finely minced with scissors and incubated with 1 mg ml−1
collagenase (Sigma-Aldrich). Identically treated PBMCs and mechanically disrupted tissue specimens served as controls for the collagenase sensitivity of epitopes. Dispersed cells were
stained with the following antibody conjugates: CCR3-APC (clone 61828, R&D Systems), CCR10-APC (clone 314305, R&D Systems), CD3-FITC (clone UCHT1, Diatec, Oslo, Norway),
CD4-PacificBlue (clone OKT4, eBioscience, San Diego, CA), CLA-FITC (clone HECA-452, BD Bioscience, Franklin Lakes, NJ), CXCR1-PE (clone 5A12, BD Bioscience), and CXCR2-PE (clone 6C6, BD
Bioscience). Unconjugated anti-α4β7 integrin (clone ACT-1) was generously provided by Andrew Lazarovits, and detected with anti-mouse IgG1-PE (Southern Biotech, Birmingham, AL). Propidium
iodide was used to exclude dead cells. Fluorescence-activated cell sorting (FACS) was performed on a BD LSR II (BD Biosciences), and analyzed using FlowJo 7.6.3 software (Three Star,
Ashland, OR). T-CELL PROLIFERATION EXPERIMENTS. CD4+ T cells were purified from PBMCs by negative isolation with Dynabeads followed by labeling with 1.5 μM carboxyfluorescein succinimidyl
ester (CFSE; both from Life Technologies). After staining with anti-CCR3-APC (clone 61828, R&D Systems), CCR3+CD4+ T cells were depleted by FACS sorting with a BD FACSAria (BD
Biosciences), after which CCR3-staining of CD4+ T cells was indistinguishable from isotype control staining (not shown). CCR3-depleted and undepleted CD4+ T cells were incubated for 6 days
with 50 μg ml−1 _H. influenzae_ outer membrane protein P636 or 30 μg ml−1 Tetanus toxoid (Calbiochem, Darmstadt, Germany) as control antigen. To ensure that T-cell proliferation was not
affected by inadvertent depletion of minor populations of antigen presenting cells expressing CCR3, T-cell fractions were cultured with irradiated autologous PBMCs labeled with CellTracker
Violet (Life Technologies) as antigen presenting cells. Cytokines secreted in the supernatants were measured with a Bio-Plex Human Cytokine Assay (Bio-Rad Laboratories, Hercules, CA) as
described by the manufacturer. Six independent donors were enrolled in the study and four of them exhibited a proliferative response to P6 stimulation. For the tetanus toxoid experiments,
three donors with known vaccination history were recruited. To examine cytokine production in T cells, CFSE-labeled PBMCs from donors with previously established P6-response (_n_=3) were
stimulated for 6 days with 50 μg ml−1 P6 protein, and then stimulated for 3.5 h with 1.5 ng ml−1 phorbol 12-myristate 13-acetate and 1 μg ml−1 ionomycin, with 10 μg ml−1 Brefeldin A (all
from Sigma-Aldrich) added after 1 h of stimulation. Cells were stained with Fixable Viability Dye eFluor 450 (eBioscience), followed by surface staining for CD3 and CD4. To detect
intracellular cytokines, cells were treated with cytofix/cytoperm (BD Biosciences) and stained with anti-IFN-γ-BV510 (Biolegend, San Diego, CA). To assess whether T-cell proliferation was
human leukocyte antigen (HLA) class II dependant, CFSE-labeled PBMCs from seven individual donors (of which five exhibited a proliferative response to P6 stimulation) were stimulated for 6
days with 50 μg ml−1 P6 protein together with a blocking monoclonal antibody to human HLA-DR, DP and DQ (Tu39, BD Biosciences) or a control mouse IgG2a antibody (UPC10, Sigma-Aldrich).
Antibodies were refreshed after 3 days of culture. STATISTICAL ANALYSIS. _P_-values were calculated by the GraphPad Prism 4 software (GraphPad Software, La Jolla, CA) using unpaired
_t_-tests. An asterisk in the figures represents _P_⩽0.05. REFERENCES * Jahnsen, F.L., Farstad, I.N., Aanesen, J.P. & Brandtzaeg, P. Phenotypic distribution of T cells in human nasal
mucosa differs from that in the gut. _Am. J. Respir. Cell Mol. Biol_ 18, 392–401 (1998). Article CAS Google Scholar * Masopust, D. & Schenkel, J.M. The integration of T cell
migration, differentiation and function. _Nat. Rev. Immunol._ 13, 309–320 (2013). Article CAS Google Scholar * Sigmundsdottir, H. & Butcher, E.C. Environmental cues, dendritic cells
and the programming of tissue-selective lymphocyte trafficking. _Nat. Immunol._ 9, 981–987 (2008). Article CAS PubMed Central Google Scholar * Bromley, S.K., Mempel, T.R. & Luster,
A.D. Orchestrating the orchestrators: chemokines in control of T cell traffic. _Nat. Immunol._ 9, 970–980 (2008). Article CAS Google Scholar * Johansen, F.E. _et al_. Regional induction
of adhesion molecules and chemokine receptors explains disparate homing of human B cells to systemic and mucosal effector sites: dispersion from tonsils. _Blood_ 106, 593–600 (2005). Article
CAS Google Scholar * Kunkel, E.J. & Butcher, E.C. Plasma-cell homing. _Nat. Rev. Immunol._ 3, 822–829 (2003). Article CAS Google Scholar * Cuburu, N. _et al_. Sublingual
immunization induces broad-based systemic and mucosal immune responses in mice. _Vaccine_ 25, 8598–8610 (2007). Article CAS PubMed Central Google Scholar * Takamura, S. _et al_. The
route of priming influences the ability of respiratory virus-specific memory CD8+ T cells to be activated by residual antigen. _J. Exp. Med._ 207, 1153–1160 (2010). Article CAS PubMed
Central Google Scholar * Campbell, J.J. & Butcher, E.C. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. _Curr.Opin.Immunol._ 12, 336–341 (2000). Article
CAS Google Scholar * Campbell, J.J. _et al_. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. _Nature_ 400, 776–780 (1999). Article
CAS Google Scholar * Soler, D., Humphreys, T.L., Spinola, S.M. & Campbell, J.J. CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. _Blood_ 101, 1677–1682 (2003). Article
CAS Google Scholar * Kunkel, E.J. _et al_. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune
compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. _J. Exp. Med._ 192, 761–768 (2000). Article CAS PubMed Central Google
Scholar * Koelle, D.M. _et al_. Expression of cutaneous lymphocyte-associated antigen by CD8(+) T cells specific for a skin-tropic virus. _J. Clin. Invest_ 110, 537–548 (2002). Article CAS
PubMed Central Google Scholar * Rott, L.S. _et al_. Expression of mucosal homing receptor alpha4beta7 by circulating CD4+ cells with memory for intestinal rotavirus. _J. Clin. Invest._
100, 1204–1208 (1997). Article CAS PubMed Central Google Scholar * Erwin, A.L. & Smith, A.L. Nontypeable _Haemophilus influenzae_: understanding virulence and commensal behavior.
_Trends Microbiol._ 15, 355–362 (2007). Article CAS Google Scholar * Murphy, T.F., Bakaletz, L.O. & Smeesters, P.R. Microbial interactions in the respiratory tract. _Pediatr. Infect.
Dis. J._ 28, S121–S126 (2009). Article Google Scholar * de Bree, G.J. _et al_. Characterization of CD4+ memory T cell responses directed against common respiratory pathogens in peripheral
blood and lung. _J. Infect. Dis._ 195, 1718–1725 (2007). Article Google Scholar * Pan, J. _et al_. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal
tissues. _J. Immunol._ 165, 2943–2949 (2000). Article CAS Google Scholar * Wang, W. _et al_. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2). _J. Biol. Chem._ 275,
22313–22323 (2000). Article CAS Google Scholar * Zlotnik, A. & Yoshie, O. The chemokine superfamily revisited. _Immunity_ 36, 705–716 (2012). Article CAS PubMed Central Google
Scholar * Francis, J.N. _et al_. CXCR1+CD4+ T cells in human allergic disease. _J. Immunol._ 172, 268–273 (2004). Article CAS Google Scholar * Epton, M.J., Hales, B.J., Thompson, P.J.
& Thomas, W.R. T cell cytokine responses to outer membrane proteins of _Haemophilus influenzae_ and the house dust mite allergens Der p 1 in allergic and non-allergic subjects. _Clin.
Exp. Allergy_ 32, 1589–1595 (2002). Article CAS Google Scholar * Baekkevold, E.S. _et al_. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell
recruitment. _J. Exp. Med._ 193, 1105–1112 (2001). Article CAS PubMed Central Google Scholar * Carlsen, H.S., Haraldsen, G., Brandtzaeg, P. & Baekkevold, E.S. Disparate lymphoid
chemokine expression in mice and men: no evidence of CCL21 synthesis by human high endothelial venules. _Blood_ 106, 444–446 (2005). Article CAS Google Scholar * Kunkel, E.J. _et al_.
CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. _J. Clin. Invest._ 111, 1001–1010 (2003). Article CAS PubMed Central Google
Scholar * John, A.E., Thomas, M.S., Berlin, A.A. & Lukacs, N.W. Temporal production of CCL28 corresponds to eosinophil accumulation and airway hyperreactivity in allergic airway
inflammation. _Am. J. Pathol._ 166, 345–353 (2005). Article CAS PubMed Central Google Scholar * Panina-Bordignon, P. _et al_. The C-C chemokine receptors CCR4 and CCR8 identify airway T
cells of allergen-challenged atopic asthmatics. _J. Clin. Invest._ 107, 1357–1364 (2001). Article CAS PubMed Central Google Scholar * Pilette, C., Francis, J.N., Till, S.J. & Durham,
S.R. CCR4 ligands are up-regulated in the airways of atopic asthmatics after segmental allergen challenge. _Eur. Respir. J._ 23, 876–884 (2004). Article CAS Google Scholar * Banfield, G.
_et al_. CC chemokine receptor 4 (CCR4) in human allergen-induced late nasal responses. _Allergy_ 65, 1126–1133 (2010). CAS PubMed PubMed Central Google Scholar * Purwar, R. _et al_.
Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. _PLoS.One_ 6, e16245 (2011). Article CAS PubMed Central Google Scholar *
Campbell, J.J. _et al_. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. _J. Immunol._ 166, 2842–2848 (2001). Article CAS Google Scholar * Sallusto,
F., Mackay, C.R. & Lanzavecchia, A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. _Science_ 277, 2005–2007 (1997). Article CAS Google Scholar * Kim,
C.H. _et al_. Rules of chemokine receptor association with T cell polarization _in vivo_. _J. Clin. Invest._ 108, 1331–1339 (2001). Article CAS PubMed Central Google Scholar * Morgan,
A.J. _et al_. IL-4-expressing bronchoalveolar T cells from asthmatic and healthy subjects preferentially express CCR 3 and CCR 4. _J. Allergy Clin. Immunol._ 116, 594–600 (2005). Article
CAS Google Scholar * Francis, J.N., Lloyd, C.M., Sabroe, I., Durham, S.R. & Till, S.J. T lymphocytes expressing CCR3 are increased in allergic rhinitis compared with non-allergic
controls and following allergen immunotherapy. _Allergy_ 62, 59–65 (2007). Article CAS PubMed Central Google Scholar * Hales, B.J. _et al_. Antibacterial antibody responses associated
with the development of asthma in house dust mite-sensitised and non-sensitised children. _Thorax_ 67, 321–327 (2012). Article Google Scholar Download references ACKNOWLEDGEMENTS We thank
Kathrine Hagelsteen, Aaste Aursjø and Lee Hazell for their excellent technical assistance. This study was supported by grants from the South-Eastern Norway Regional Health Authority and the
Norwegian Research Council through its Centres of Excellence funding scheme, project number 179573. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pathology and Centre for
Immune Regulation (CIR), Oslo University Hospital—Rikshospitalet and University of Oslo, Oslo, Norway E Danilova, I Skrindo, F E Johansen, F L Jahnsen & E S Baekkevold * Department of
Otorhinolaryngology, Akershus University Hospital, Nordbyhagen, Norway I Skrindo * Department of Otolaryngology, Lovisenberg Diakonale Hospital, Oslo, Norway E Gran * Centre for Child Health
Research, University of Western Australia, Telethon Institute for Child Health Research, Subiaco, Western Australia, Australia B J Hales & W A Smith * Department of Gastroenterology,
Akershus University Hospital, Nordbyhagen, Norway J Jahnsen * Department of Biosciences, University of Oslo, Oslo, Norway F E Johansen Authors * E Danilova View author publications You can
also search for this author inPubMed Google Scholar * I Skrindo View author publications You can also search for this author inPubMed Google Scholar * E Gran View author publications You can
also search for this author inPubMed Google Scholar * B J Hales View author publications You can also search for this author inPubMed Google Scholar * W A Smith View author publications You
can also search for this author inPubMed Google Scholar * J Jahnsen View author publications You can also search for this author inPubMed Google Scholar * F E Johansen View author
publications You can also search for this author inPubMed Google Scholar * F L Jahnsen View author publications You can also search for this author inPubMed Google Scholar * E S Baekkevold
View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to E S Baekkevold. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Material is linked to the online version of the paper SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 (JPG 56
KB) SUPPLEMENTARY FIGURE 2 (JPG 75 KB) SUPPLEMENTARY FIGURE 3 (JPG 135 KB) SUPPLEMENTARY FIGURE LEGENDS (DOC 44 KB) SUPPLEMENTARY TABLE (DOC 80 KB) POWERPOINT SLIDES POWERPOINT SLIDE FOR
FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Danilova, E.,
Skrindo, I., Gran, E. _et al._ A role for CCL28–CCR3 in T-cell homing to the human upper airway mucosa. _Mucosal Immunol_ 8, 107–114 (2015). https://doi.org/10.1038/mi.2014.46 Download
citation * Received: 08 October 2013 * Accepted: 11 May 2014 * Published: 11 June 2014 * Issue Date: January 2015 * DOI: https://doi.org/10.1038/mi.2014.46 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative