Aggressive melanoma cells escape from bmp7-mediated autocrine growth inhibition through coordinated noggin upregulation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Bone morphogenetic proteins (BMPs) are members of the TGF-_β_ superfamily responsible for mediating a diverse array of cellular functions both during embryogenesis and in adult
life. Previously, we reported that upregulation of BMP7 in human melanoma correlates with tumor progression. However, melanoma cells are either inhibited by or become resistant to BMP7 as a
function of tumor progression, with normal melanocytes being most susceptible. Herein, real-time quantitative reverse transcriptase-polymerase chain reactions and western blotting revealed
that the expression of BMP antagonist, Noggin, correlates with resistance to BMP7 in advanced melanoma cells. To test the hypothesis that coordinated upregulation of Noggin protects advanced
melanoma cells from autocrine inhibition by BMP7, functional expression of Noggin in susceptible melanoma cells was achieved by adenoviral gene transfer. The Noggin-overexpressing cells
exhibited a growth advantage in response to subsequent BMP7 transduction _in vitro_ under anchorage-dependent and -independent conditions, in three-dimensional skin reconstructs, as well as
_in vivo_ in severe combined immunodeficient mice. In concordance, Noggin knockdown by lentiviral shRNA confers sensitivity to BMP7-induced growth inhibition in advanced melanoma cells. Our
findings suggest that, like TGF-_β_, BMP7 acts as an autocrine growth inhibitor in melanocytic cells, and that advanced melanoma cells may escape from BMP7-induced inhibition through
concomitant aberrant expression of Noggin. SIMILAR CONTENT BEING VIEWED BY OTHERS BONE MORPHOGENETIC PROTEIN INDUCES BONE INVASION OF MELANOMA BY EPITHELIAL–MESENCHYMAL TRANSITION VIA THE
SMAD1/5 SIGNALING PATHWAY Article 09 September 2021 LATE STAGE MELANOMA IS HALLMARKED BY LOW NLGN4X EXPRESSION LEADING TO HIF1A ACCUMULATION Article Open access 20 June 2024 TARGETING
NON-CANONICAL ACTIVATION OF GLI1 BY THE SOX2-BRD4 TRANSCRIPTIONAL COMPLEX IMPROVES THE EFFICACY OF HEDGEHOG PATHWAY INHIBITION IN MELANOMA Article Open access 06 May 2021 MAIN Bone
morphogenetic proteins (BMPs) are pleiotropic cytokines belonging to the TGF-_β_ superfamily. Over 20 members of BMPs have been identified in a wide variety of organisms ranging from insects
to mammals.1 Although BMPs were originally shown to induce endochondral bone formation, they are now considered as components of a highly conserved signaling pathway that controls cell
growth, differentiation, apoptosis, motility, angiogenesis, and matrix synthesis not only during embryogenesis but also in adult life.2, 3 Signaling by BMPs is mediated through both type I
and type II transmembrane serine-threonine kinase receptors. Upon ligand binding, the constitutive type II kinase activates the type I receptor and initiates the signal transduction cascade
by phosphorylating receptor-regulated ‘mother against decapentaplegic’ (R-Smad) proteins (eg, Smad 1, 5, and 8). Given the diversity of responses to BMP and the complexity of morphogenic
events, their activities are delicately regulated by secretory antagonists (such as Noggin, Chordin, Gremlin, Sclerostin, Follistatin, DAN/Cerberus, and Glypican-3 (GPC3)), signaling
inhibitors (including SnoN, Smurf 1 and 2, and Smad 6 and 7), and pseudoreceptor BAMBI (BMP and activin membrane-bound inhibitor).4 The discovery that perturbations in BMP pathways are
genetically responsible for certain hereditary cancer syndromes (such as familial juvenile polyposis and a subset of Cowden syndrome5, 6) has prompted the delineation of their significance
in carcinogenesis. Evidence now indicates that various sporadic human cancers also exhibit aberrations in BMP signaling, contributing to tumor development and progression.7, 8, 9, 10, 11 It
is now clear that the actions of BMPs are cell type specific, and that the roles of BMPs in carcinogenesis are quite complex, with divergent pro-tumor and anti-tumor effects resulting from
both autocrine and paracrine responses.4 However, relatively little is known about BMP signaling in melanoma. Recently, we4 and Rothhammer _et al_12 independently reported that multiple
BMPs, including BMP-2, -4, -6, -7, and -8, are upregulated in melanoma. The expression of BMP7 in particular correlates with tumor progression and disease recurrence,13 but overexpression of
BMP7 paradoxically inhibits cell growth to varying degrees through G0–G1 cell-cycle arrest and induction of apoptosis. Normal melanocytes are most susceptible to transduced BMP7, whereas
melanoma cells are increasingly resistant with tumor progression. The resistance of melanoma cells corresponds to the expression of BMP7 antagonist, Noggin. Using adenoviral transfer, we
obtained evidence that forced expression of Noggin in susceptible melanoma cells protects them from BMP7-induced growth inhibition. Furthermore, Noggin-overexpressing cells exhibit a growth
advantage in response to subsequent BMP7 transduction both _in vitro_ in soft agar and three-dimensional (3D) skin reconstructs, and _in vivo_ in severe combined immunodeficient (SCID) mice
as compared to control green fluorescent protein (GFP)-transduced counterparts. Consistent with these, lentiviral shRNA-mediated Noggin knockdown confers sensitivity to BMP7 in advanced
melanoma cells. Our findings suggest that, similar to TGF-_β_, BMP7 functions as an autocrine growth inhibitor in melanocytic cells, and that advanced melanoma cells may escape BMP7-induced
inhibition through coordinated upregulation of Noggin. MATERIALS AND METHODS CELL CULTURE The isolation and culture of normal human melanocytes was performed as previously described.14
Isogenic melanoma cell lines derived from the same patient at different disease stages were maintained as described.15, 16 These consist of primary vertical growth phase (VGP) melanoma cell
lines WM115 and WM983A, their lymph node metastatic counterparts WM239A and WM983C, respectively. In addition, metastatic/aggressive variants selected in an experimental metastasis model _in
vivo_, such as 1205Lu and C8161, and their parental cell lines WM793 (a VGP primary human melanoma cell line), and C81-61 (a metastatic human melanoma cell line), respectively, were also
included. Normal foreskin keratinocytes and fibroblasts were isolated and propagated as previously described.17, 18 IMMUNOHISTOCHEMISTRY Decoded formalin-fixed and paraffin-embedded melanoma
tissue sections were obtained from the archive of the Department of Pathology at the University of Iowa. The sections were dewaxed, quenched, and incubated with a mouse monoclonal antibody
raised against human BMP7 (MAB3541; R&D Systems, Minneapolis, MN, USA) at 25 _μ_g/ml overnight at 4°C. The sections were then washed to remove unbound antibodies and incubated with
horseradish peroxidase (HRP)-conjugated rabbit anti-mouse secondary antibodies (R0260; DakoCytomation, Carpinteria, CA, USA) for 30 min at room temperature. Visualization was performed using
a DAB HRP Substrate-Chromogen 2 liquid component kit (GTX 73338; GeneTex Inc., San Antonio, TX, USA) with hematoxylin counter stain. As a control, isotype-matched mouse immunoglobulin (Ig)
was used in place of the primary antibody. Experiments were repeated twice with consistency. SEMIQUANTITATIVE RT-PCR Total RNAs were extracted from cultured melanoma cells using
RNAqueous®-4PCR (Ambion, Austin, TX, USA) according to the manufacturer's instructions. First-strand cDNA was reverse transcribed from 0.1 _μ_g/ml of total RNA with RETROscript (Ambion)
using random decamers to prime the reactions. RCR was performed with SuperTaq (Ambion) under conditions described in the package inserts using S-15 as a loading control. The expected sizes
of the PCR products, the primer sequences, their annealing temperature, and number of cycles performed are summarized in Table 1. The number of cycles utilized for each set was determined to
be within the linear range of amplification of the particular product. Reaction products were analyzed by electrophoresis on 2% ethidium bromide gels and the bands captured by a UVP
Biochemi System (Upland, CA, USA) and the expression normalized to the S-15 control. REAL-TIME QUANTITATIVE RT-PCR Total RNA samples from subconfluent cultures of melanoma cells were
prepared using RNAqueous®-4PCR (Ambion) according to the manufacturer's protocol. For each cell line, 5 _μ_g of total RNA was reverse transcribed into cDNA in a final reaction mix of 25
_μ_l using SuperScript III First-Strand Synthesis System for RT–PCR (Invitrogen). All reagents and probes for real-time RT-PCR were obtained from Applied Biosystems (Foster City, CA, USA).
All the probes (except the Noggin probes) used span the intron splice sites, which only detect cDNA. Real-time quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) was
performed on a 7300 Real-Time PCR System (Applied Biosystems) in a 25 _μ_l reaction mix containing 1 _μ_l cDNA, 1 × TaqMan Universal PCR Mater Mix and 1 × BMP7 (Hs00233477_m1), Noggin
(Hs00271352_s1), Sclerostin (Hs00401764_m1), Gremlin (Hs00171951_m1), GPC3 (Hs00170471_m1), Chordin (Hs00415315_m1), BAMBI (Hs00180818_m1), Smurf2 (Hs00909284_m1), or GAPDH assay (4352339E).
Thermocycling was carried out at 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 15 s, and 60°C for 1 min. All samples were run in triplicate. The relative amounts of BMP
inhibitor transcripts were analyzed using the 2 -ΔΔ C T method.19 Experiments were repeated twice with consistency. ADENOVIRAL VECTORS The adenoviral vectors carrying the GFP (Ad/GFP), and
human BMP7 (Ad/BMP7) were obtained from the Vector Core, University of Pennsylvania (Philadelphia, PA, USA), and Dr R Franceschi at the University of Michigan (Ann Arbor, MI, USA),20
respectively. The recombinant Ad expressing mouse Noggin protein (Ad/Noggin) was constructed as follows. The mouse Noggin cDNA (GenBank accession no. MMU79163) was isolated from the plasmid
pMgB950 containing the sequence kindly provided by Dr RM Harland from University of California, Berkeley21, 22 using _Not_I and _Bam_HI digestion. The resulting mouse Noggin cDNA was
subcloned into the pacAd5CMVK-NpA recombinant adenoviral vector backbone23 (obtained from the Gene Therapy Center Vector Core at the University of Iowa) to generate a mouse Noggin proviral
plasmid pAd.CMV-mNoggin. Recombinant Ad was generated and amplified from this proviral plasmid as previously described23 and titered using Adeno-X® Rapid Titering Kit (Clontech Laboratories
Inc., Mountain View, CA, USA). ADENOVIRAL INFECTION OF MELANOMA CELLS Subconfluent melanoma cells were transduced with 10 PFU (plaque-forming unit) per cell of replication-deficient Ads for
2 h at 37°C in a minimum amount of serum-free Dulbecco's modified Eagle's medium sufficient to cover the culture vessels. The optimal PFU was previously determined as the minimum
amount of virus required to yield the highest overall gene transfer efficiency without apparent cytotoxicity.18, 24 Viral suspensions were then replaced by regular growth medium and cells
were incubated overnight. When indicated, subsequent infection with a second adenoviral vector may be performed. After viral infection, cells were allowed to recover for at least 16 h before
use. The high efficiency of gene transfer (over 95%)18, 24 eliminates the need for selection. ENZYME-LINKED IMMUNOSORBANT ASSAY At 24 h after viral transfection of melanocytic cells, the
growth medium was replaced with serum-free medium consisting of MCDB153/L15 (v/v: 4/1), CaCl2 (2 mmol/l), and insulin (5 _μ_g/ml) and incubated for 24 h. The supernatant was then collected,
volume-measured, and cleared by centrifugation. BMP7 in tissue culture supernatant was quantified in triplicate wells using the human BMP7 DuoSet ELISA Development kit (DY354; R&D
Systems) according to the manufacturer's protocol. For VEGF quantification, equal amount of cell lysate (refer to western blotting for cell lysate preparation) from each sample was
added in duplicate wells and enzyme-linked immunosorbant assay (ELISA) was performed using the human VEGF Quantikine kit (R&D Systems). The results (expressed as amount of secreted BMP7
per ml per 106 cells for 24 h and amount of VEGF per 100 _μ_g lysate, respectively) from one representative experiment were shown, however, assays were repeated twice with consistency.
WESTERN BLOTTING Subconfluent cultures were washed with phosphate-buffered saline (PBS), and extracted in lysis buffer containing 1% Triton X-100, 1% deoxycholic acid, 2 mmol/l CaCl2, and
protease inhibitors (10 _μ_g/ml leupeptin, 10 _μ_g/ml aprotinin, 1.8 mg/ml iodoacetamide, and 1 mmol/l phenylmethyl sulfonyl fluoride) in PBS. Cell lysates were quantified by a BCA protein
assay kit (Pierce, Rockford, IL, USA). An equal amount (50–100 _μ_g) of total protein from each sample was subjected to electrophoresis on NuPAGE 4–12% Bis-Tris gels (Invitrogen),
transblotted onto nitrocellulose membranes (Pierce), and probed with primary antibodies, such as anti-phospho Smad 1, 5, and 8 (Cell Signaling, Beverly, MA, USA), anti-Noggin (RP57-16),25,
26 anti-bFGF (Abcam, Cambridge, MA, USA), anti-Cripto-1 (R&D Systems), and anti-Nodal (Chemicon, Temecula, CA, USA) antibodies, followed by a peroxidase-conjugated secondary antibody
(Pierce). Immunoreactive bands were detected using SuperSignal West Femto Chemiluminescent Substrate (Pierce). Subsequent reprobing using anti-_β_-actin or anti-tubulin (Abcam) was also
performed for internal loading control. Experiments were performed at least twice with consistency. CONVENTIONAL ANCHORAGE-DEPENDENT GROWTH ASSAY Subconfluent cultures were trypsinized and
seeded in 35-mm wells at 1–4 × 105 cells per well. Cells were refed twice weekly. At given intervals, cells in quadruplicate wells were harvested and counted in a Coulter counter (Coulter
Electronics, Luton, UK). Statistical analyses were performed using the Mann–Whitney _U_-test. Experiments were repeated twice with similar results. SOFT AGAR ASSAY Melanoma cells were
suspended in MCDB153/L15 medium (v/v: 4/1) supplemented with 25 _μ_g/ml bovine pituitary extract, 2 ng/ml epidermal growth factor (EGF), 2 _μ_g/ml insulin, 4% fetal bovine serum, and 0.25%
agar and plated in triplicate at 6 × 104 cells per well in six-well plates. After 2 weeks, colonies were counted using an inverted microscope. Mann–Whitney _U_-test was used for statistical
analyses. Experiments were repeated twice and similar results were obtained. Data presented represent results from one experiment. CELL GROWTH AND INVASION IN 3D SKIN RECONSTRUCTS Skin
reconstructs were prepared as previously described.17, 18, 27 Briefly, the growth and invasion of melanoma cells was tested in artificial skin reconstructs, in which human foreskin dermal
fibroblasts in rat tail type I collagen were placed on a precast acellular collagen gel. After 6 days, the constricted collagen gels formed a concave surface, serving as a cradle for seeding
epidermal cells. Melanoma cells were then mixed with keratinocytes at a 1:5–10 ratio and seeded onto the dermal constructs. After 5 days, cultures were lifted to the air–liquid level for an
additional 10 days to allow stratification of epidermal keratinocytes. The reconstructs were then harvested, fixed in paraformaldehyde, embedded in paraffin, sectioned, and stained with
hematoxylin and eosin. Apoptosis was evaluated using the Apo-BrdU-IHC™ _In Situ_ DNA Fragmentation Assay Kit (BioVision Inc., Mountain View, CA, USA). For each condition, triplicate wells
were evaluated and experiments were repeated twice with similar results. CELL-CYCLE ANALYSIS At 24 h after viral infection, melanoma cells were maintained at less than 70% confluence for 3
days. Cells were then harvested and fixed with 70% ethanol at 4°C for 1 h. After washing with PBS, cells were stained with 50 _μ_g/ml of propidium iodide in PBS containing RNase A (0.5
mg/ml), Tris-HCl (0.5 mM), and NaCl (0.75 mM) for 30 min at 4°C in the dark, and analyzed on flow cytometer at the University of Iowa Flow Cytometry Core Facility. Data shown represent
results from one experiment, however, the assay was performed twice with similar results. APOPTOSIS DETECTION BY ANTI-PHOSPHO HISTONE H2B At 48 h after viral infection, melanoma cells were
harvested and stained for 40 min with 20 _μ_g/ml of anti-phospho histone H2B (Upstate, Lake Placid, NY, USA), an early marker of apoptosis, at 4°C, in triplicates. After removal of excessive
primary antibodies, the cells were incubated with Cy3-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) and then analyzed by
fluorescence-activated cell sorting (FACS) at the University of Iowa Flow Cytometry Core Facility. Statistical analyses were performed using Mann–Whitney _U_-test. Data shown represent
results from one experiment, however, the assay was performed twice with similar results. _IN VIVO_ TUMORIGENICITY Subconfluent melanoma cells were sequentially transduced with different
combinations of Ad/GFP, Ad/Nog, and Ad/BMP7 at 10 PFU per cell with a 24 h interval between infections. At 16 h after the second viral infection, cells were harvested and suspended in
serum-free medium at a density of 108 cells per ml. Cell suspension (100 _μ_l) were injected subcutaneously in the dorsal skin of each SCID mouse (seven mice per condition). Tumor volume was
monitored twice a week and determined as follows: (maximal dimension × minimal dimension)2/2. Statistical analyses were performed using ANOVA following log transformation. The mice were
killed 14 days after injection. Tumors were dissected, weighed, fixed in formalin, and subject to histopathologic examination. NOGGIN KNOCKDOWN IN MELANOMA CELLS BY LENTIVIRAL SHRNA
Recombinant lentiviral vectors were generated by co-transfecting pLKO.1-Noggin (Sigma), harboring shRNA against human Noggin, or nontarget control shRNA (Sigma) with packaging plasmids VSVg
and pCMV-ΔR8.2 (Sigma) into 293T packaging cells using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Culture supernatants containing recombinant
lentiviral particles were used to infect melanoma cells (1205Lu and C8161). Two days after infection, cells were selected with puromycin (1 _μ_g/ml) for a period of 7 days. RESULTS BMP7
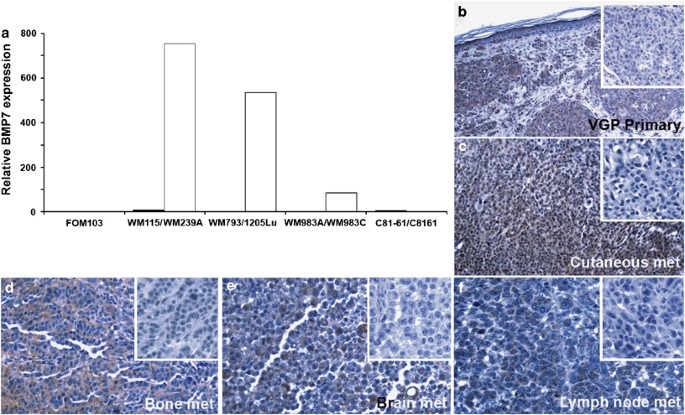
EXPRESSION IN MELANOMA CORRELATES WITH TUMOR PROGRESSION Taking advantage of the isogenic cell lines (Materials and Methods) derived from the same patient at different disease stages as well
as aggressive variants selected in an experimental metastasis model _in vivo_,15 BMP7 mRNA expression was found to correlate with tumor progression using real-time qRT-PCR (Figure 1a; a
normal melanocyte culture (FOM103) was included as baseline control). Cell lines derived from primary melanomas (WM115, WM793, and WM983A) exhibited very low copies of BMP7 transcripts,
whereas their metastatic counterparts (WM239A, 1205Lu, and WM983C) expressed abundant BMP7 mRNA (Figure 1a). One exceptional metastatic melanoma cell pair, C81-61/C8161, however, displayed
low levels of BMP7 transcripts (Figure 1a). Immunohistochemistry confirmed upregulation of BMP7 protein expression in human melanoma tissue (Figures 1b–f), using kidney sections as a
positive control, as collecting duct tubules have been shown previously to express BMP7.28 Immunoreactivity was detected in primary (Figure 1b) as well as metastatic melanoma samples,
including metastases to lymph node, cutaneous, brain, and bone (Figures 1c–f). Thus, BMP7 expression correlates with tumor progression and the observed upregulation in aggressive melanoma
cells _in vitro_ is biologically relevant and does not represent a tissue culture artifact. ADENOVIRAL GENE TRANSFER RESULTS IN FUNCTIONAL SECRETION OF BIOLOGICALLY ACTIVE BMP7 TRANSGENE
PRODUCT IN MELANOMA CELLS To investigate the biological consequences of BMP7 upregulation in melanoma progression, overexpression of the transgene in melanoma cell pairs (WM793/1205Lu and
C81-61/C8161) was achieved using recombinant Ad. Analysis of the culture supernatants using ELISA revealed that the BMP7-transduced cells produced approximately 1000 ng of BMP7 per ml per
106 cells for 24 h, whereas their mock- or GFP-transduced counterparts exhibited low/undetectable endogenous levels of BMP7 (Figure 2a). As expected, BMP7 overexpression resulted in
increased R-Smad phosphorylation/activation by western blotting, compared to the control GFP-transduced cells, except in the highly aggressive metastatic melanoma cell line C8161 (Figure
2b). Screening by semiquantitative RT-PCR demonstrated that melanoma cells express all six known BMP receptors and their downstream signaling machinery, such as Smads (data not shown). The
expression of BMPR IB (Alk6) and BMP RII at the protein level was also confirmed by western blotting (data not shown). These data suggest that virally induced BMP7 is biologically active and
functions as an autocrine activator of R-Smad phosphorylation in melanoma cells. AUTOCRINE EFFECTS OF BMP7 ON MELANOMA GROWTH, INVASION, AND MOTILITY Adenoviral gene transfer of BMP7 led to
differential growth inhibition in melanoma cells of different stages of tumor progression. Primary (WM793) and less-aggressive metastatic (C81-61) melanoma cells were susceptible with
greater than 70% growth inhibition (Figure 2c), whereas their metastatic (1205Lu) and highly aggressive (C8161) counterparts are relatively resistant (Figure 2c). C8161, in particular, was
completely resistant. To explore the underlying mechanisms of BMP7-mediated growth inhibition in melanoma cells, we performed cell-cycle analysis using propidium iodide. We found that BMP7
transduction results in G0–G1 cell-cycle arrest. Interestingly, the extent to which BMP7 induces G0–G1 cell-cycle arrest correlates with the resistance to growth inhibition by BMP7. In
BMP7-sensitive early/less-aggressive melanoma cells, such as WM793 and C81-61, the percentage of resting cell population raises drastically from ∼35% in GFP control construct-transduced
cells to 70% in BMP7-transduced cells, whereas the relatively resistant 1205Lu cells exhibit a modest increase (from ∼50 to ∼64%) and the resistant C8161 melanoma cells show no significant
difference (Figure 2d). In addition, when the cells were stained with an early marker for apoptosis, anti-phospho Histone2B, the majority of BMP7-transduced cells underwent apoptosis (Figure
2c). The degree of BMP7-induced apoptotic cell death also correlates with sensitivity to growth inhibition by BMP7. BMP7-sensitive early/less-aggressive variants (WM793 and C81-61) display
over 90% positive cells following BMP7 transduction, compared to ∼30% in GFP-transduced cells, whereas their relatively BMP7-resistant, more aggressive counterparts (1205Lu and C8161) show
only ∼40–55% positivity following BMP7 transduction, compared to ∼40% in GFP-transduced cells (Figure 2e). No changes in phospho Histone 2B expression were observed between the GFP- and
BMP7-transfected resistant cell line, C8161 (Figure 2e). As various BMPs have been shown to contribute to tumor progression through stimulating cell motility and invasion,7, 29, 30, 31 we
tested whether BMP7 enhances melanoma migration and invasion _in vitro_. Using an _in vtro_ scratch migration assay and time-lap video recording, we found no significant difference in cell
migration between Ad/BMP7- and Ad/GFP-transduced melanoma cells (data not shown). In addition, the cells behaved similarly in a modified Boyden chamber assay (data not shown).
CHARACTERIZATION OF BMP7-TRANSDUCED MELANOMA CELLS IN 3D SKIN RECONSTRUCTS To determine the biological consequences of BMP7 transduction in melanoma cells in an appropriate tissue context, a
3D skin reconstruct model was used to test the invasive capacity as well as the growth characteristics of these cells. This model consists of a dermal compartment containing fibroblasts in
a collagen gel separated from an epidermal compartment composed of melanocytic cells and keratinocytes by a naturally deposited basement membrane,27 enabling functional studies of individual
genes in a biologically relevant milieu. Ad/GFP-transduced VGP primary melanoma cells WM793 grow as nests and solitary units within the epidermis and occasionally in the superficial dermis
(Figure 3a), whereas their BMP7-transduced counterparts display only remnants of small clusters as well as single cells at the dermal–epidermal junction and superficial dermis. Free 3′-OH
ends resulting from DNA fragmentation and indicative of apoptotic cell death are detected in these cells using the Apo-BrdU-IHC™ _In Situ_ DNA Fragmentation Assay Kit (BioVision; Figure 3f).
Control GFP vector transduced aggressive 1205Lu melanoma cells traverse the basement membrane and grow deeply into the dermis, forming invasive tumor nests (Figure 3c), whereas, their
Ad/BMP7-infected counterparts show dermal tumor nesting with morphological evidence of apoptosis (Figures 3d and e), such as nuclear condensation and formation of apoptotic bodies. Similar
results were obtained using C81-61 metastatic melanoma cells (Figures 3g and h). However, when we incorporate highly aggressive C8161 metastatic melanoma cells, which are shown to be
resistant to BMP7-mediated autocrine inhibition in the traditional 2D culture (Figure 2c), both the control Ad/GFP- and Ad/BMP7-infected cells grow aggressively into the dermis and
eventually partially replace the epidermis (Figures 3i and j). These data suggested that consistent with the results obtained from the conventional monolayer culture, BMP7 is growth
inhibitory in melanoma cells and that advanced/aggressive melanoma cells are progressively resistant. ADVANCED/AGGRESSIVE MELANOMA CELLS ESCAPE GROWTH INHIBITION BY BMP7 THROUGH CONCOMITANT
UPREGULATION OF BMP ANTAGONIST, NOGGIN Using semiquantitative RT-PCR, initial screening indicates that the resistance to induced BMP7 in advanced/aggressive melanoma cells correlates with
upregulation of BMP antagonist, Noggin, but not Dan/Cerberus, Follistatin, Sclerostin, Gremlin, Chordin, GPC3, Smurf 1 and 2, SnoN, Smad6, Smad7, or BAMBI.4 Real-time qRT-PCR (Figure 4a) and
western blotting (Figure 4b) further confirmed these observations. It is worth noting that BMP7 transduction did not induce Smad phosphorylation in the highly aggressive metastatic melanoma
cell line C8161 (Figure 2b), which exhibited abundant Noggin transcripts (∼104-fold increase compared to its less-aggressive parental cell line C81-61; Figure 4a), consistent with the known
antagonist function of Noggin. To test the hypothesis that advanced/aggressive melanoma cells escape growth inhibition by BMP7 through coordinated upregulation of Noggin, we first
overexpressed Noggin in susceptible melanoma cells in an attempt to rescue them from BMP7-mediated growth inhibition. Forced functional expression of Noggin was achieved by adenoviral gene
transfer. Western blotting confirmed the presence of the transgene product at the protein level (Figure 4c) and the transduced Noggin was effective in blocking BMP7-induced Smad signaling
(Figure 4d). Conventional growth assays revealed that pre-infection with Ad/Nog protects susceptible melanoma cells from subsequently induced BMP7 (Figure 5a; Supplementary Figure 1A). In
soft agar assays, Noggin transduction in BMP7-susceptible melanoma cells restores colony formation (Figure 5b; Supplementary Figure 1B). In 3D skin reconstructs, Noggin transduction rescues
WM793 primary VGP melanoma cells from BMP7-induced apoptotic cell death, leading to tumor growth in the superficial dermis, at the dermal–epidermal junction, and within the epidermis (Figure
5c; Supplementary Figure 1C). Similar rescue is also observed in other melanoma cell lines (WM164, 1205Lu, and C81-61; data not shown). In addition, in tumorigenicity assays in SCID mice,
Noggin transduction, as expected, protects melanoma cells from BMP7-mediated growth inhibition (Figure 6; Supplementary Figure 2). At 17 days post-subcutaneous injection, the tumors derived
from Nog/BMP7-transduced 1205Lu melanoma cells measure four times larger in size and weigh twice as much as those from GFP- and BMP7 double-transduced counterparts (Figures 6a and b;
Supplementary Figures 2A and B). Routine histology examination of the tumors revealed that the Ad/GFP- and Ad/BMP7 double-infected cells induce ectopic bone formation at the periphery of the
tumors (Figure 6c and Supplementary Figure 2C, left panel), consistent with the known osteogenic function of BMP7, whereas the Nog/BMP7-infected cells grow as large, partially encapsulated,
subcutaneous nodules without evidence of heterotropic ossification (Figure 6c and Supplementary Figure 2C, right panel). To test the hypothesis that Noggin knockdown in advanced/resistant
melanoma cells confers sensitivity to BMP7-induced growth inhibition, we generated stable Noggin knockdown 1205Lu and C8161 cell lines using the lentiviral shRNA approach. As shown in Figure
7a by western blotting, over 75% knockdown efficiency was achieved (using cells infected by lentiviral vector containing control shRNA as control). In conventional monolayer growth assays,
both 1205Lu and C8161 Noggin knockdown variants exhibit increased sensitivity to BMP7, compared to their nontarget control shRNA counterparts (Figure 7b). To explore the possibility that
Noggin may restore growth in BMP7-transduced melanoma cells indirectly through induction of other growth factors, we examined the expression of potential melanoma growth-promoting factors,
such as bFGF, Nodal, Cripto-1 (by western blotting), and VEGF (by ELISA) following Noggin overexpression (Figure 8). We found that Noggin overexpression upregulates Nodal and VEGF in one
(WM793/1205Lu) but not the other isogenic melanoma cell pairs. This suggests that Noggin rescue of melanoma growth in response to BMP7 may in part be attributed to induction of Nodal and
VEGF in some but not all melanoma cell lines. DISCUSSION Although originally identified by their capacity to induce endochondral bone formation, BMP signaling pathways have now been shown to
be critically involved in a diverse array of nonosteogenic processes.4 The discovery that perturbations in BMP signaling are genetically responsible for certain familial cancer syndromes,
such as familial juvenile polyposis, has stimulated active interests in delineating the functional significance of BMPs in tumor development and progression. Consistent with this,
reactivation of developmental/morphogenetic signaling pathways such as Hedgehog, Wnt, and Notch, has also recently been implicated in tumorigenesis. It is postulated that such activations
may lead to or result from tumor dedifferentiation toward a stem cell-like phenotype.32 Despite the substantial progress achieved during the past years, relatively little is known about BMP
signaling in melanocytic cells. Using semiquantitative RT-PCR, we previously screened expression of BMPs in melanocytic cells4 and reported that the expression of BMP7, in particular,
correlates with tumor aggressiveness _in vitro_. We have now verified our previous observation by real-time qRT-PCR (Figure 1a). The biological relevance of BMP7 upregulation was further
confirmed _in situ_ by immunohistochemistry on melanoma tissue sections (Figures 1b–f). Aberrant BMP7 expression during tumor progression is not unique to melanoma. High levels of BMP7 have
also been detected in bone metastasis of prostate cancer.33 Studies have demonstrated that prostate carcinoma cells produce increasing amounts of BMPs as they progress to a more aggressive
phenotype and that the upregulation of BMP7 expression in metastatic cells is a critical component of developing osteoblastic lesions. In addition, BMP7 is also upregulated in breast
carcinoma and the expression paradoxically correlates with differentiation markers, such as estrogen and progesterone receptors.34 On the contrary, nephroblastoma cells exhibit
downregulation of BMP7.35 The apparent divergent regulation of BMP7 in different human cancers may reflect the cell type-specific actions of individual BMPs. BMP7-mediated growth regulation
has been extensively studied in carcinogenesis. The findings have been conflicting with divergent effects of both growth stimulation and inhibition.36, 37, 38 To investigate the biological
consequences of BMP7 in human melanoma, we overexpressed the transgene using adenoviral gene transfer, which led to differential growth inhibition in traditional monolayer culture (Figure
2c), as well as in 3D skin reconstructs (Figure 3). When comparing isogenic cell lines, biologically advanced, aggressive melanoma cells (1205Lu and C8161) are less responsive, whereas their
biologically early (WM793) or less-aggressive counterparts (C81-61) appear more sensitive. Although the level of transduced BMP7 by adenoviral vectors may be superphysiologic, it allows for
augmentation of the biological readout to gain insights into BMP7 function in melanoma as the actions of cytokines can be subtle and difficult to appreciate.39 Although not a main focus of
our study, we found expression of all six known BMP receptors as well as their signal transduction proteins in melanoma cells using semiquantitative RT-PCR. In addition, gene sequencing also
revealed no evidence of mutations or deletions in BMP receptors (data not shown). Taken together, these results indicate an intact and functional autocrine BMP pathway in melanoma cells,
with the manifestation of growth suppression in biologically early cells, but resistance in late-aggressive cells. We further explored the mechanisms through which BMP7 expression exerts its
growth inhibitory activities in susceptible melanoma cells. Cell-cycle analyses revealed that ectopic expression of BMP7 resulted in G0–G1 cell-cycle arrest (Figure 2d). In addition, a
significant portion of BMP7-transduced cells expressed phospho-Histone2B, an early marker for apoptotic cells, by flow cytometry (Figure 2e). Apoptotic death in BMP7-transduced cells was
further confirmed by a TUNEL stain (Figure 3f). Consistent with our findings, involvement of BMP7 in cell-cycle control and apoptosis regulation has been previously described. BMP7 has been
shown to induce apoptosis in myeloma cells36 and to inhibit proliferation of androgen-insensitive prostate cancer cells, eg PC-3 and DU-145, both _in vitro_ and _in vivo_ through G0–G1
cell-cycle arrest.40 In contrast, BMP7 promotes cell survival under serum deprivation in androgen-sensitive LNCaP and C4-2B prostate cancer cell lines.41 The seemingly disparate observations
in prostate cancer suggest that the biological responses to BMP7 in a given cell type may depend on cross talk with other signaling pathways.42 In contrast to what has been previously
reported, where BMP4 and BMP7 were shown to enhance cell invasion and migration in melanoma,12 and prostate as well as colon cancer cell lines,30, 31, 41 respectively, we did not observe
similar activities in BMP7-transduced melanoma cells in _in vitro_ scratch migration and modified Boyden chamber assays. Consistent with this, BMP7-transduced susceptible melanoma cells
retained their ability to invade the artificial dermis in our 3D skin reconstruct model even though the cells exhibited evidence of apoptosis (Figures 3b and d). This is not surprising given
that the biological responses of individual BMPs are cell- and tissue context specific.43 One possible mechanism through which melanoma cells may bypass the antiproliferative effect
elicited by BMP7 is the disruption of BMP signaling through BMP inhibitors. Using semiquantitative RT-PCR with confirmation by real-time qRT-PCR, it appears that the resistance to induced
BMP7 in advanced/aggressive melanoma correlates with upregulation of BMP antagonist, Noggin4 (Figure 4a). To test the hypothesis that concurrent upregulation of Noggin protects
advanced/aggressive melanoma cells from growth retardation by BMP7, we investigated the consequences of Noggin overexpression in susceptible melanoma cells, as well as those of Noggin
knockdown in resistant melanoma cells, in response to induced BMP7. We found that overexpression of Noggin conferred BMP7 resistance in susceptible melanoma cells not only _in vitro_ in
conventional monolayer growth assays (Figure 5a; Supplementary Figure 1A), soft agar clonogenicity assays (Figure 5b; Supplementary Figure 1B), and 3D skin reconstructs (Figure 5c;
Supplementary Figure 1C), but also _in vivo_ in experimental animals (Figure 6; Supplementary Figure 2). In conventional monolayer cultures, Noggin knockdown confers sensitivity to BMP7 in
resistant melanoma cells (Figure 7). Using western blotting and ELISA, we also found that Noggin upregulates melanoma growth-promoting factors, such as Nodal and VEGF in a subset of but not
all melanoma cell lines (Figure 8). These suggest that the observed restoration of growth by Noggin may in part be attributed to the indirect effect of Nodal and VEGF induction. There are
ample examples in which tumor cells harbor aberrant expression of BMP signaling inhibitors that contribute to tumorigenesis and progression. For instance, Chordin, which reduces the motility
of the tumor cells, is downregulated in ovarian cancer cells.44 In esophageal squamous cell carcinoma, Smurf2 expression correlates with poor prognosis.45 Loss of GPC3 was also noted in a
significant portion of ovarian and breast cancers.46 Furthermore, its restoration inhibited colony-forming potential suggesting that GPC3 acts as a negative growth regulator in these
tumors.47 In contrast, overexpression of GPC3 was demonstrated in embryonal tumors,48 colon cancer,49 hepatocellular carcinoma,50 and melanoma.51, 52 Analogous to Noggin counteracting the
autocrine inhibition of BMP7 in melanoma, upregulation of GPC3 in hepatocellular carcinoma has also been shown to modulate the growth inhibitory effect of BMP7.37 However, unlike Noggin,
GPC3 expression does not correlate with melanoma progression.51 In summary, two key events associated with BMP7 signaling take place during melanoma development and progression: (1) the
acquisition of the ability to express increased levels of BMP7 and (2) the development of resistance to the autocrine inhibition by BMP7 through concomitant upregulation of antagonist,
Noggin. Given that BMP7 is growth inhibitory in human melanoma, it remains puzzling as to why the malignant cells secrete such a factor without apparent autocrine benefits. There are a few
possible explanations. First, the degree of growth suppression by endogenous BMP7 may be moderate and thus easily overcome by other intrinsic/extrinsic pro-proliferative signals. Second,
interactions with other signaling pathways may modulate the growth inhibitory action of BMP7 in melanoma.53, 54, 55 Finally, BMP7 may offer paracrine stimulation for melanoma cells in the
tumor microenvironment. Indeed, recent studies indicated that BMPs may contribute to tumor progression through stromal induction, such as promoting angiogenesis.43, 56, 57 Thus, like
TGF-_β_, an accepted ‘double-edged sword’ in tumorigenesis, BMPs may function both as oncogenes and tumor suppressors depending on the relative dosage and disease stage.4 Moreover, it
remains to be determined whether inhibition of Noggin upregulation may unmask the antiproliferative effects of BMP7, a potentially novel translational strategy for melanoma therapy. It is
for this reason that further investigation is warranted to bridge the gap between our current knowledge of BMP7 signaling in melanoma and its potential as a therapeutic target. REFERENCES *
Balemans W, Van Hul W . Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. _Dev Biol_ 2002;250:231–250. Article CAS PubMed Google Scholar * Whitman M .
Smads and early developmental signaling by the TGFbeta superfamily. _Genes Dev_ 1998;12:2445–2462. Article CAS PubMed Google Scholar * Botchkarev VA . Bone morphogenetic proteins and
their antagonists in skin and hair follicle biology. _J Invest Dermatol_ 2003;120:36–47. Article CAS PubMed Google Scholar * Hsu MY, Rovinsky S, Penmatcha S, _et al_. Bone morphogenetic
proteins in melanoma: angel or devil? _Cancer Metastasis Rev_ 2005;24:251–263. Article CAS PubMed Google Scholar * Howe JR, Sayed MG, Ahmed AF, _et al_. The prevalence of MADH4 and
BMPR1A mutations in juvenile polyposis and absence of BMPR2, BMPR1B, and ACVR1 mutations. _J Med Genet_ 2004;41:484–491. Article CAS PubMed PubMed Central Google Scholar * Waite KA, Eng
C . From developmental disorder to heritable cancer: it's all in the BMP/TGF-beta family. _Nat Rev Genet_ 2003;4:763–773. Article CAS PubMed Google Scholar * Langenfeld EM, Calvano
SE, Abou-Nukta F, _et al_. The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cells. _Carcinogenesis_
2003;24:1445–1454. Article CAS PubMed Google Scholar * Kim IY, Lee DH, Lee DK, _et al_. Restoration of bone morphogenetic protein receptor type II expression leads to a decreased rate of
tumor growth in bladder transitional cell carcinoma cell line TSU-Pr1. _Cancer Res_ 2004;64:7355–7360. Article CAS PubMed Google Scholar * Horvath LG, Henshall SM, Kench JG, _et al_.
Loss of BMP2, Smad8, and Smad4 expression in prostate cancer progression. _Prostate_ 2004;59:234–242. Article CAS PubMed Google Scholar * Wen XZ, Miyake S, Akiyama Y, _et al_. BMP-2
modulates the proliferation and differentiation of normal and cancerous gastric cells. _Biochem Biophys Res Commun_ 2004;316:100–106. Article CAS PubMed Google Scholar * Helms MW,
Packeisen J, August C, _et al_. First evidence supporting a potential role for the BMP/SMAD pathway in the progression of oestrogen receptor-positive breast cancer. _J Pathol_
2005;206:366–376. Article CAS PubMed Google Scholar * Rothhammer T, Poser I, Soncin F, _et al_. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell
invasion and migration. _Cancer Res_ 2005;65:448–456. CAS PubMed Google Scholar * Rothhammer T, Wild PJ, Meyer S, _et al_. Bone morphogenetic protein 7 (BMP7) expression is a potential
noval prognostic marker for recurrence in patients with primary melanoma. _Cancer Biomarker_ 2007;3:111–117. Article CAS Google Scholar * Hsu M-Y, Li L, Herlyn M . Cultivation of normal
human epidermal melanocytes in the absence of phorbol esters. In: Picot J (ed). _Human Cell Culture Protocols_. Humana Press: Totowa, NJ, 2004, pp 13–28. Chapter Google Scholar * Hsu M-Y,
Elder DE, Herlyn M . Melanoma: the Wistar melanoma (WM) cell lines. In: Masters JRW, Palsson B (eds). _Human Cell Culture_, 1st edn. Kluwer Academic Publishers: Nowell, MA, 1999, pp 259–274.
Google Scholar * van der Schaft DW, Seftor RE, Seftor EA, _et al_. Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. _J Natl Cancer
Inst_ 2004;96:1473–1477. Article CAS PubMed Google Scholar * Hsu MY, Shih DT, Meier FE, _et al_. Adenoviral gene transfer of beta3 integrin subunit induces conversion from radial to
vertical growth phase in primary human melanoma. _Am J Pathol_ 1998;153:1435–1442. Article CAS PubMed PubMed Central Google Scholar * Hsu MY, Meier FE, Nesbit M, _et al_. E-cadherin
expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. _Am J Pathol_ 2000;156:1515–1525. Article
CAS PubMed PubMed Central Google Scholar * Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method.
_Methods_ 2001;25:402–408. Article CAS PubMed Google Scholar * Franceschi RT, Wang D, Krebsbach PH, _et al_. Gene therapy for bone formation: _in vitro_ and _in vivo_ osteogenic activity
of an adenovirus expressing BMP7. _J Cell Biochem_ 2000;78:476–486. Article CAS PubMed Google Scholar * Smith WC, Harland RM . Expression cloning of noggin, a new dorsalizing factor
localized to the Spemann organizer in Xenopus embryos. _Cell_ 1992;70:829–840. Article CAS PubMed Google Scholar * Zimmerman LB, De Jesus-Escobar JM, Harland RM . The Spemann organizer
signal noggin binds and inactivates bone morphogenetic protein 4. _Cell_ 1996;86:599–606. Article CAS PubMed Google Scholar * Anderson RD, Haskell RE, Xia H, _et al_. A simple method for
the rapid generation of recombinant adenovirus vectors. _Gene Therapy_ 2000;7:1034–1038. Article CAS PubMed Google Scholar * Hsu M, Andl T, Li G, _et al_. Cadherin repertoire determines
partner-specific gap junctional communication during melanoma progression. _J Cell Sci_ 2000;113:1535–1542. CAS PubMed Google Scholar * Valenzuela DM, Economides AN, Rojas E, _et al_.
Identification of mammalian noggin and its expression in the adult nervous system. _J Neurosci_ 1995;15:6077–6084. Article CAS PubMed PubMed Central Google Scholar * Marcelino J,
Sciortino CM, Romero MF, _et al_. Human disease-causing NOG missense mutations: effects on noggin secretion, dimer formation, and bone morphogenetic protein binding. _Proc Natl Acad Sci USA_
2001;98:11353–11358. Article CAS PubMed PubMed Central Google Scholar * Meier F, Nesbit M, Hsu MY, _et al_. Human melanoma progression in skin reconstructs: biological significance of
bFGF. _Am J Pathol_ 2000;156:193–200. Article CAS PubMed PubMed Central Google Scholar * Wang SN, Lapage J, Hirschberg R . Loss of tubular bone morphogenetic protein-7 in diabetic
nephropathy. _J Am Soc Nephrol_ 2001;12: 2392–2399. CAS PubMed Google Scholar * Scherberich A, Tucker RP, Degen M, _et al_. Tenascin-W is found in malignant mammary tumors, promotes
alpha8 integrin-dependent motility and requires p38MAPK activity for BMP-2 and TNF-alpha induced expression _in vitro_. _Oncogene_ 2005;24:1525–1532. Article CAS PubMed Google Scholar *
Ye L, Lewis-Russell JM, Kynaston H, _et al_. Endogenous bone morphogenetic protein-7 controls the motility of prostate cancer cells through regulation of bone morphogenetic protein
antagonists. _J Urol_ 2007;178:1086–1091. Article CAS PubMed Google Scholar * Grijelmo C, Rodrigue C, Svrcek M, _et al_. Proinvasive activity of BMP-7 through SMAD4/src-independent and
ERK/Rac/JNK-dependent signaling pathways in colon cancer cells. _Cell Signal_ 2007;19: 1722–1732. Article CAS PubMed Google Scholar * Taipale J, Beachy PA . The Hedgehog and Wnt
signalling pathways in cancer. _Nature_ 2001;411:349–354. Article CAS PubMed Google Scholar * Masuda H, Fukabori Y, Nakano K, _et al_. Increased expression of bone morphogenetic
protein-7 in bone metastatic prostate cancer. _Prostate_ 2003;54:268–274. Article CAS PubMed Google Scholar * Alarmo EL, Rauta J, Kauraniemi P, _et al_. Bone morphogenetic protein 7 is
widely overexpressed in primary breast cancer. _Genes Chromosomes Cancer_ 2006;45:411–419. Article CAS PubMed Google Scholar * Higinbotham KG, Karavanova ID, Diwan BA, _et al_. Deficient
expression of mRNA for the putative inductive factor bone morphogenetic protein-7 in chemically initiated rat nephroblastomas. _Mol Carcinog_ 1998;23:53–61. Article CAS PubMed Google
Scholar * Ro TB, Holt RU, Brenne AT, _et al_. Bone morphogenetic protein-5, -6 and -7 inhibit growth and induce apoptosis in human myeloma cells. _Oncogene_ 2004;23:3024–3032. Article
PubMed Google Scholar * Midorikawa Y, Ishikawa S, Iwanari H, _et al_. Glypican-3, overexpressed in hepatocellular carcinoma, modulates FGF2 and BMP-7 signaling. _Int J Cancer_
2003;103:455–465. Article CAS PubMed Google Scholar * Pouliot F, Blais A, Labrie C . Overexpression of a dominant negative type II bone morphogenetic protein receptor inhibits the growth
of human breast cancer cells. _Cancer Res_ 2003;63:277–281. CAS PubMed Google Scholar * Valtieri M, Tocci A, Gabbianelli M, _et al_. Enforced TAL-1 expression stimulates primitive,
erythroid and megakaryocytic progenitors but blocks the granulopoeitic differentiation program. _Cancer Res_ 1998;58:562–569. CAS PubMed Google Scholar * Miyazaki H, Watabe T, Kitamura T,
_et al_. BMP signals inhibit proliferation and _in vivo_ tumor growth of androgen-insensitive prostate carcinoma cells. _Oncogene_ 2004;23:9326–9335. Article CAS PubMed Google Scholar *
Yang S, Zhong C, Frenkel B, _et al_. Diverse biological effect and Smad signaling of bone morphogenetic protein 7 in prostate tumor cells. _Cancer Res_ 2005;65:5769–5777. Article CAS
PubMed Google Scholar * Ide H, Yoshida T, Matsumoto N, _et al_. Growth regulation of human prostate cancer cells by bone morphogenetic protein-2. _Cancer Res_ 1997;57:5022–5027. CAS
PubMed Google Scholar * Langenfeld EM, Langenfeld J . Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. _Mol Cancer Res_ 2004;2:141–149. CAS PubMed Google
Scholar * Moll F, Millet C, Noel D, _et al_. Chordin is underexpressed in ovarian tumors and reduces tumor cell motility. _FASEB J_ 2006;20:240–250. Article CAS PubMed Google Scholar *
Fukuchi M, Fukai Y, Masuda N, _et al_. High-level expression of the Smad ubiquitin ligase Smurf2 correlates with poor prognosis in patients with esophageal squamous cell carcinoma. _Cancer
Res_ 2002;62:7162–7165. CAS PubMed Google Scholar * Xiang YY, Ladeda V, Filmus J . Glypican-3 expression is silenced in human breast cancer. _Oncogene_ 2001;20:7408–7412. Article CAS
PubMed Google Scholar * Lin H, Huber R, Schlessinger D, _et al_. Frequent silencing of the GPC3 gene in ovarian cancer cell lines. _Cancer Res_ 1999;59:807–810. CAS PubMed Google Scholar
* Saikali Z, Sinnett D . Expression of glypican 3 (GPC3) in embryonal tumors. _Int J Cancer_ 2000;89:418–422. Article CAS PubMed Google Scholar * Lage H, Dietel M, Froschle G, _et al_.
Expression of the novel mitoxantrone resistance associated gene MXR7 in colorectal malignancies. _Int J Clin Pharmacol Ther_ 1998;36:58–60. CAS PubMed Google Scholar * Man XB, Tang L,
Zhang BH, _et al_. Upregulation of Glypican-3 expression in hepatocellular carcinoma but downregulation in cholangiocarcinoma indicates its differential diagnosis value in primary liver
cancers. _Liver Int_ 2005;25:962–966. Article CAS PubMed Google Scholar * Nakatsura T, Kageshita T, Ito S, _et al_. Identification of glypican-3 as a novel tumor marker for melanoma.
_Clin Cancer Res_ 2004;10: 6612–6621. Article CAS PubMed Google Scholar * Motomura Y, Senju S, Nakatsura T, _et al_. Embryonic stem cell-derived dendritic cells expressing glypican-3, a
recently identified oncofetal antigen, induce protective immunity against highly metastatic mouse melanoma, B16-F10. _Cancer Res_ 2006;66:2414–2422. Article CAS PubMed Google Scholar *
Kraunz KS, Nelson HH, Liu M, _et al_. Interaction between the bone morphogenetic proteins and Ras/MAP-kinase signalling pathways in lung cancer. _Br J Cancer_ 2005;93:949–952. Article CAS
PubMed PubMed Central Google Scholar * Nakashima A, Katagiri T, Tamura M . Cross-talk between Wnt and bone morphogenetic protein 2 (BMP-2) signaling in differentiation pathway of C2C12
myoblasts. _J Biol Chem_ 2005;280:37660–37668. Article CAS PubMed Google Scholar * Litsiou A, Hanson S, Streit A . A balance of FGF, BMP and WNT signalling positions the future placode
territory in the head. _Development_ 2005;132:4051–4062. Article CAS PubMed Google Scholar * Dai J, Kitagawa Y, Zhang J, _et al_. Vascular endothelial growth factor contributes to the
prostate cancer-induced osteoblast differentiation mediated by bone morphogenetic protein. _Cancer Res_ 2004;64: 994–999. Article CAS PubMed Google Scholar * Raida M, Clement JH, Leek
RD, _et al_. Bone morphogenetic protein 2 (BMP-2) and induction of tumor angiogenesis. _J Cancer Res Clin Oncol_ 2005;131:741–750. Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS We thank Dr Meenhard Herlyn (Wistar Institute, Phliadelphia, PA, USA) and Dr Mary JC Hendrix (Northwestern University, Chicago, IL, USA) for their generous gifts of melanoma
cell lines. Dr Renny T Franceschi (University of Michigan, Ann Arbor, MI, USA) is acknowledged for providing Ad/BMP7. This work was supported by start-up funds from Department of Pathology
and the Holden Comprehensive Cancer Center, University of Iowa, Iowa City, IA, USA; Department of Pathology, Brigham and Women's Hospital, Boston, MA, USA (M-YH); and NIH Grants
DK047967 (JFE), HL084815, CA93683, and AR42689 (GFM). AUTHOR INFORMATION Author notes * Sherry A Rovinsky and Chiou-Yan Lai: These authors contributed equally to this work. AUTHORS AND
AFFILIATIONS * Department of Pathology, Program in Dermatopathology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA Mei-Yu Hsu, Chiou-Yan Lai, Joan How &
George F Murphy * Department of Pathology, University of Iowa Carver College of Medicine, Iowa City, IA, USA Mei-Yu Hsu, Sherry A Rovinsky & Shadi Qasem * Department of Anatomy and Cell
Biology, University of Iowa Carver College of Medicine, Iowa City, IA, USA Xiaoming Liu & John F Engelhardt Authors * Mei-Yu Hsu View author publications You can also search for this
author inPubMed Google Scholar * Sherry A Rovinsky View author publications You can also search for this author inPubMed Google Scholar * Chiou-Yan Lai View author publications You can also
search for this author inPubMed Google Scholar * Shadi Qasem View author publications You can also search for this author inPubMed Google Scholar * Xiaoming Liu View author publications You
can also search for this author inPubMed Google Scholar * Joan How View author publications You can also search for this author inPubMed Google Scholar * John F Engelhardt View author
publications You can also search for this author inPubMed Google Scholar * George F Murphy View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING
AUTHOR Correspondence to Mei-Yu Hsu. ADDITIONAL INFORMATION DISCLOSURE/DUALITY OF INTEREST The authors have no duality of interest to declare. Supplementary Information accompanies the paper
on the Laboratory Investigation website (http://www.laboratoryinvestigation.org) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hsu, MY., Rovinsky, S.,
Lai, CY. _et al._ Aggressive melanoma cells escape from BMP7-mediated autocrine growth inhibition through coordinated Noggin upregulation. _Lab Invest_ 88, 842–855 (2008).
https://doi.org/10.1038/labinvest.2008.55 Download citation * Received: 29 October 2007 * Revised: 11 April 2008 * Accepted: 16 April 2008 * Published: 16 June 2008 * Issue Date: August 2008
* DOI: https://doi.org/10.1038/labinvest.2008.55 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * adenoviral gene transfer * BMP antagonist * BMP
inhibitor * skin reconstruct * TGF-_β_ * tumor progression