Synthesis and antibacterial activity of 4″ or 6″-alkanoylamino derivatives of arbekacin
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Arbekacin, an aminoglycoside antibiotic, is an important drug because it shows a potent efficacy against methicillin-resistant _Staphylococcus aureus_. However, resistance to
arbekacin, which is caused mainly by the bifunctional aminoglycoside-modifying enzyme, has been observed, becoming a serious problem in medical practice. To create new arbekacin derivatives
active against resistant bacteria, we modified the C-4″ and 6″ positions of its 3-aminosugar portion. Regioselective amination of the 6″-position gave 6″-amino-6″-deoxyarbekacin (1), and it
was converted to a variety of 6″-_N_-alkanoyl derivatives (6a−z). Furthermore, regioselective modifications of the 4″-hydroxyl group were performed to give 4″-deoxy-4″-epiaminoarbekacin (2)
and its 4″-_N_-alkanoyl derivatives (12 and 13). Their antibacterial activity against _S. aureus_, including arbekacin-resistant bacteria, was evaluated. It was observed that
6″-amino-6″-_N_-[(_S_)-4-amino-2-hydroxybutyryl]-6″-deoxyarbekacin (6o) showed excellent antibacterial activity, even better than arbekacin. SIMILAR CONTENT BEING VIEWED BY OTHERS
SEMI-SYNTHESIS AND STRUCTURE-ACTIVITY RELATIONSHIP STUDY YIELD ANTIBACTERIAL VICENISTATIN DERIVATIVES WITH LOW CYTOTOXICITY Article 16 January 2024 DESIGN, SYNTHESIS AND OPTIMIZATION OF TARO
INHIBITORS AS MULTIFUNCTIONAL ANTIBIOTICS AGAINST METHICILLIN-RESISTANT _STAPHYLOCOCCUS AUREUS_ Article Open access 12 April 2025 DESIGN AND SYNTHESIS OF
4-[4-FORMYL-3-(2-NAPHTHYL)PYRAZOL-1-YL]BENZOIC ACID DERIVATIVES AS POTENT GROWTH INHIBITORS OF DRUG-RESISTANT _STAPHYLOCOCCUS AUREUS_ Article 29 June 2020 INTRODUCTION Aminoglycoside
antibiotics have been widely used in the treatment of serious infections caused by Gram-positive and/or Gram-negative bacteria. They interfere with bacterial protein synthesis by binding to
the A-site decoding region of 16S rRNA in the 30S ribosomal subunit.1, 2, 3, 4, 5 Arbekacin (ABK), which was synthesized from dibekacin by the attachment of the (_S_)-4-amino-2-hydoxybutyryl
(AHB)6 residue at the _N_-1 position, is efficacious for methicillin-resistant _Staphylococcus aureus_ (MRSA), and is used for the treatment of infected patients.7, 8 However, the emergence
of ABK-resistant MRSA, caused by bifunctional aminoglycoside-modifying enzyme AAC (6′)-APH (2″), has been observed, and causes a serious problem.9, 10, 11 Moreover, the appearance of
aminoglycoside-resistant _Pseudomonas aeruginosa_ and _Acinetobacter_ has recently been reported.12 Therefore, development of a new type of ABK derivative active against resistant bacteria
is urgently required to fight against the diseases caused by the pathogens. Deoxygenation, epimerization and/or deoxyamination have frequently strengthened the antibacterial activity of
aminoglycoside antibiotics.13, 14, 15 For example, 5-deoxy-5-epi substitution16 and 5, 4″-diepimerization17 enhanced the antibacterial activity of ABK, including the activity against
resistant bacteria. Also, it is well known that the introduction of the amino acid side-chain to the 1-amino group brings an improvement in antimicrobial activity.18, 19, 20, 21 Furthermore,
their combined modification is also thought to be an attractive approach. 6″-Amino-6″-deoxy-arbekacin (1)22 and 4″-deoxy-4″-epiamino- arbekacin (2),17 which introduced an amino group in the
4″ or 6″-position of its 3-aminosugar portion, were reported to preserve the antibacterial activity. In addition, the specific mechanism of the binding affinity of the hydroxyl groups at
these positions to the bacterial 16S rRNA has not been determined. These phenomena suggest that there is great potential for improvement of activity by chemical modification in this area.
Therefore, we focused on the 3-aminosugar portion bearing a novel amino group, to create new ABK derivatives active against resistant bacteria, and planned a structure–activity relationship
study on the _N_-acyl derivatives of the novel amino group. In this paper, we describe the synthesis of 4″- alkanoylamino-4″-epi or 6″-alkanoylamino derivatives of ABK by regioselective
amination and their antibacterial activities against _S. aureus_ including ABK-resistant MRSA. RESULTS AND DISCUSSION SYNTHESIS AND ANTIBACTERIAL ACTIVITY OF 6″-ALKANOYLAMINO DERIVATIVES OF
ABK Synthesis of 1 and its 6″-_N_-alkanoyl derivatives is shown in Scheme 1. Penta-_N_-Boc-6″-_O_-triisopropylbenzenesulfonyl ABK (3) was obtained from penta-_N_-Boc ABK17 via selective
sulfonylation of the 6″-hydroxyl group with 2, 4, 6-triisopropylbenzenesulfonyl chloride (TIBS-Cl) at room temperature with 90% yield. In the 1H NMR spectrum of 3 in dimethyl sulfoxide
(DMSO)-_d_6, the H-6″ signals of penta-_N_-Boc ABK at 3.51 and 3.58 p.p.m. shifted to 4.11 and 4.28 p.p.m., respectively, confirming the selective sulfonylation of 6″-hydroxyl group in 3
(refer to experimental section). Introduction of the leaving groups by other reagents such as _p_-toluenesulfonyl chloride, methanesulfonyl chloride or I2 with triphenylphosphine resulted in
a decrease in regioselectivity. Treatment of 3 with NaN3 followed by reduction of the resulting azide group by the Staudinger reaction23 produced 6″-amine 4 in a high yield. Deprotection of
4 with aqueous trifluoroacetic acid provided 6″-amino-6″-deoxyarbekacin (1). Although the synthesis of 1 was described for a previous procedure,22 we have established a more convenient
method. On the other hand, _N_-alkanoylation of 4 with various carboxylic acids was effectively attained by using 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride
(DMT-MM)24 or the activated ester method25 to give 5A−Z with 41−95% yield. Removal of Boc groups afforded a variety of 6″-alkanoylamino-6″-deoxy derivatives (6A−Z) of ABK (refer to
Supplementary Information). The position of newly introduced acyl groups in 6A−Z was confirmed by HMBC correlations between the amide carbonyl and the hydrogens at C-6″. The structures of
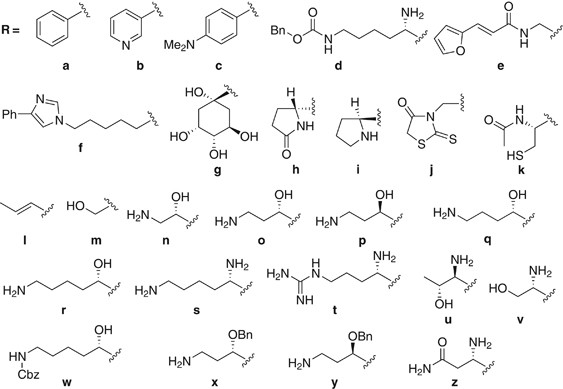
5A−Z and 6A−Z are shown in Figure 1. The antibacterial activities of 6″-amino-6″-deoxyarbekacin (1) and its 6″-_N_-alkanoyl derivatives 6A−Z are shown in Table 1. The derivatives (6A−J, W−Y)
containing the aromatic or aliphatic rings in the side-chains were less active than ABK. However, 6X and 6Y, possessing the terminal amino group, showed moderate antibacterial activity, and
this result suggested that the terminal amino group in the side-chain is important for the activity. In contrast, the derivatives (6K−V) containing the functionalized linear chain were
likely to display a relatively potent antibacterial activity. In particular, compounds 6N and 6O, with the (_S_)-3-amino-2-hydroxypropionyl and (_S_)-AHB residue, respectively, showed highly
efficacious antibacterial activity against ABK-resistant MRSA MS16526 (the resistant mechanism is a bifunctional enzyme AAC (6′)-APH (2″)). This result indicated that the antibacterial
activity is enhanced by the addition of the amino side-chain even in the case of the novel amino group at the C-6″ position. Improvement of antibacterial activity against ABK-resistant MRSA
is suspected to be a likely result of the inhibition of bifunctional enzyme AAC (6′)-APH (2″). In the AHB side-chain, a comparison between 6O and 6P indicated that the _S_-configuration is
preferable over the _R_-configuration. When the carbon-chain becomes longer than that of AHB, the activity tended to decrease, and a similar phenomenon was reported in the case of
1-_N_-alkanoyl derivatives of ABK.26 Compound 6S demonstrated that the replacement of the hydroxyl group by an amino group at the alpha position of the linear side-chain displays excellent
activity. Further, it was indicated in the compound 6T that the replacement of the terminal amino group by the guanidine group shows similar effects in terms of antimicrobial activity.
Interestingly, although 6N and 6V have the same functional groups in the side-chain, 6N was more active than 6V because of the difference in the position of the two functional groups. These
results indicated the importance of the presence and location of the basic functional groups for antibacterial activity. Moreover, we evaluated the antibacterial activity of 6O against
_Escherichia coli_ and _P. aeruginosa_ as Gram-negative bacteria and _Enterococcus faecalis_ as Gram-positive bacteria (Table 2). On the whole, 6O was equal or more active than ABK against
these bacteria, in particular, it exhibited prominent activity against _P. aeruginosa_. SYNTHESIS AND ANTIBACTERIAL ACTIVITY OF 4″-ALKANOYLAMINO-4″-EPI DERIVATIVES OF ABK We expected that
the introduction of the AHB residue into the 4″-epiamino group would potentiate the antibacterial activity, as inferred from the results of the SAR study on 6″-_N_-alkanoyl derivatives.
Synthesis of 4″-deoxy-4″-epiaminoarbekacin (2) and its 4″-_N_-alkanoyl derivatives is shown in Scheme 2. The reaction of 2″, 2‴-di-_O_-acetyl-3, 2′, 6′, 3″, 4‴-penta-_N_-Boc-6″-_O_-trityl
ABK17 (7) with methanesulfonyl chloride (MsCl) produced the 4″-_O_-mesyl derivative 8 in a high yield. Treatment of 8 with NaN3 followed by removal of the acetyl groups and the Staudinger
reaction23 of the azide group gave the 4″-epiamine 10. Deprotection of Boc and Tr groups under acidic conditions provided 2. On the other hand, _N_-alkanoylation of 10 with
(_S_)-2-benzyloxy-4-benzyloxycarbonylaminobutyric acid gave 11 in 86% yield. Removal of the Boc groups of 11 led to 12, and the deprotection of the Bn and Cbz groups provided the bis-AHB
derivative 13. The antibacterial activities of 4″-deoxy-4″-epiaminoarbekacin (2) and its 4″-_N_-alkanoyl derivatives (12 and 13) against _S. aureus_ are shown in Table 3. The
4″-deoxy-4″-epiamino derivative 2 showed moderate activity against Methicillin-sensitive _S. aureus_, although its activity against MRSA strains was remarkably lower than that of ABK as
reported.17 On the other hand, compound 12, which introduced the _N_,_O_-protected AHB residue into the _axial_ amino group at the C-4″ position was not active. In contrast, the bis-AHB
derivative 13 showed more potent antibacterial activity than that of 2 or 12. This suggested that the addition of the amino side-chain to the novel _axial_ amino group at the C-4″ position
also enhances the antibacterial activity. CONCLUSIONS We synthesized the 4″ or 6″- alkanoylamino derivatives of ABK _via_ the regioselective sulfonylation of the hydroxyl groups at the
corresponding positions. 6″-Alkanoylamino derivatives, which introduced the (_S_)-3-amino-2-hydroxypropionyl (6N) or AHB (6O) residue, showed potent antibacterial activity against _S.
aureus_ including ABK-resistant MRSA, although the 4″-alkanoylamino-4″-epi derivative 13 was less active than ABK. Furthermore, 6O was more active than ABK against Gram-negative bacteria
such as _E. coli_ and _P. aeruginosa_. This study can provide a basis for development of novel aminoglycoside antibiotics for clinically relevant bacteria that are recalcitrant to antibiotic
treatment. EXPERIMENTAL PROCEDURE GENERAL INFORMATION NMR spectra were recorded on a Bruker DPX 400 or AVANCE 500 spectrometer (Bruker, Billarica, MA, USA). Chemical shifts were measured
downfield from tetramethylsilane as an internal standard. Mass spectra were acquired on a Thermo Scientific LTQ XL spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) (ESI) or LTQ
Orbitrap mass spectrometer (HRMS). Elemental analysis was performed on a Perkin Elmer 2400 Series II CHNS/O Elemental Analyzer (Perkin Elmer, Waltham, MA, USA). TLC was conducted with silica
gel 60 F254 (Merck Millipore, Billerica, MA, USA) and visualized with ceric ammonium molybdate stain and/or UV. Silica gel column chromatography was carried out on Silica Gel 60 N (Kanto
Chemical, Tokyo, Japan). Resin column chromatography was carried out on Amberlite CG50 Type I (NH4+) (Dow Chemical, Midland, MI, USA). SYNTHESIS _Penta-N-Boc ABK._ To an aqueous solution
(170 ml) of ABK (10.0 g, 2.5 H2SO4 salt, 12.5 mmol), Et3N (28.5 ml, 204.7 mmol) and Boc2O (25.1 g, 115.2 mmol) in 1,4-dioxane (170 ml) were added, and the mixture was stirred at 60 °C for 3
h. The reaction mixture was quenched with concentrated aqueous NH3, and the quenched mixture was evaporated. The resulting residue was washed with H2O, and then concentrated _in vacuo_ to
provide penta-_N_-Boc ABK (13.4 g, 98%) as a colorless solid. 1H NMR (DMSO-_d__6_, 500 MHz, 343 K) δ 1.32 (m, 1H, H-4′_ax_), 1.36−1.40 (m, 45H, 5 X C(C_H_3)3), 1.50 (m, 1H, H-2_ax_), 1.61
(m, 1H, H-βa), 1.62 (m, 1H, H-3′_ax_), 1.64 (m, 1H, H-4′_eq_), 1.70 (m, 1H, H-3′_eq_), 1.84 (m, 1H, H-βb), 2.02 (m, 1H, H-2_eq_), 3.09 (m, 2H, H-γ), 3.14 (m, 2H, H-6′), 3.28 (m, 1H, H-4″),
3.34 (m, 1H, H-2″), 3.38 (m, 1H, H-3), 3.41 (m, 1H, H-2′), 3.44 (m, 1H, H-4), 3.49 (m, 1H, H-3″), 3.51 (m, 1H, H-6″a), 3.53 (m, 1H, H-5), 3.58 (m, 1H, H-6″b), 3.62 (m, 1H, H-6), 3.67 (m, 1H,
H-1), 3.77 (m, 1H, H-5″), 3.83 (m, 1H, H-α), 3.86 (m, 1H, H-5′), 3.98 (br, 1H, OH-2′), 4.05 (br, 1H, OH-6″), 4.51 (br, 1H, OH-4″), 4.78 (br, 1H, OH-5), 4.90 (br, 1H, OH-α), 4.92 (d, 1H,
_J_=3.1 Hz, H-1″) and 5.05 (d, 1H, _J_=2.9 Hz, H-1′); 13C NMR (DMSO-_d__6_, 125.8 MHz, 343 K) δ 23.8 (3′), 27.3 (4′), 28.2 (6C, C(_C_H3)3), 28.3 (6C, C(_C_H3)3), 29.0 (3C, C(_C_H3)3), 33.8
(2), 34.3 (β), 37.2 (γ), 44.6 (6′), 48.7 (1), 49.8 (3), 50.5 (2′), 55.8 (3″), 60.8 (6″), 66.9 (5′), 67.7 (4″), 69.5 (α), 70.2 (2″), 73.5 (5″), 75.4 (5), 77.5 (_C_(CH3)3), 77.6 _C_(CH3)3),
77.7 (_C_(CH3)3), 77.8 (2C, _C_(CH3)3), 81.2 (4), 81.8 (6), 98.2 (2C, 1′ and 1″), 154.8 (C=O), 154.9 (C=O), 155.5 (C=O), 155.7 (C=O), 156.5 (C=O) and 173.9 (C=O); ESI-MS calculated for
C47H84N6NaO20: 1075.56; found: 1075.60 (M+Na)+. _3, 2′, 6′, 3″, 4‴-Penta-N-tert-Boc-6″-O-(2, 4, 6-triisopropylbenzenesulfonyl)arbekacin (_ _3_). To a solution of penta-_N_-Boc ABK (17.4 g,
16.5 mmol) in pyridine (348 ml), 2,4,6-triisopropylbenzenesulfonyl chloride (25.0 g, 82.5 mmol) was added, and the mixture was stirred at room temperature for 3 days. The reaction mixture
was quenched with MeOH (35 ml), and the mixture was evaporated. The resulting residue was purified by silica gel column chromatography to provide 3 (19.5 g, 90%) as a colorless solid. 1H NMR
(DMSO-_d__6_, 500 MHz, 343 K) δ 1.18−1.23 (m, 18H, 3X CH(C_H_3)2), 1.35 (m, 1H, H-4′_ax_), 1.36−1.40 (m, 45H, 5X C(C_H_3)3), 1.52 (m, 1H, H-βa), 1.54 (m, 1H, H-2_ax_), 1.57 (m, 1H,
H-3′_ax_), 1.60 (m, 1H, H-4′_eq_), 1.66 (m, 1H, H-3′_eq_), 1.72 (m, 1H, H-βb), 1.80 (m, 1H, H-2_eq_), 3.03 (m, 2H, H-γ), 3.08 (m, 2H, H-6′), 3.10−3.13 (m, 3H, 3X C_H_(CH3)2), 3.29 (m, 1H,
H-2″), 3.32 (m, 1H, H-4″), 3.34 (m, 1H, H-3), 3.40 (m, 1H, H-2′), 3.44 (m, 1H, H-5), 3.48 (m, 1H, H-4), 3.54 (m, 1H, H-3″), 3.61 (m, 1H, H-6), 3.68 (m, 1H, H-1), 3.81 (m, 1H, H-α), 3.85 (m,
1H, H-5′), 4.09 (m, 1H, H-5″), 4.11 (m, 1H, H-6″a), 4.17 (br, 1H, OH-2′), 4.28 (m, 1H, H-6″b), 4.71 (br, 1H, OH-5), 4.80 (br, 1H, OH-4″), 4.97 (br, 1H, OH-α), 5.01 (d, 1H, _J_=3.1 Hz, H-1″),
5.08 (d, 1H, _J_=2.8 Hz, H-1′) and 7.11 (s, 2H, C6_H_2(_i_-Pr)3); 13C NMR (DMSO-_d__6_, 125.8 MHz, 343 K) δ 23.7 (3′), 24.4 (2C, CH(_C_H3)2), 24.7 (4C, CH(_C_H3)2), 27.3 (4′), 28.2 (6C,
C(_C_H3)3), 28.4 (6C, C(_C_H3)3), 29.0 (3C, C(_C_H3)3), 33.3 (3C, _C_H(CH3)2), 33.8 (2), 34.3 (β), 37.2 (γ), 44.6 (6′), 49.0 (1), 50.0 (3), 50.5 (2′), 55.8 (3″), 66.9 (5′), 67.0 (4″), 67.8
(6″), 69.5 (α), 69.7 (2″), 70.1 (5″), 75.5 (5), 80.4 (6), 81.7 (4), 98.1 (1″), 98.3 (1′), 121.0 (2C, _C_6H2(_i_-Pr)3), 122.3 (2C, _C_6H2(_i_-Pr)3), 123.6 (1C, _C_6H2(_i_-Pr)3), 129.2 (1C,
_C_6H2(_i_-Pr)3), 153.4 (C=O), 154.8 (C=O), 155.5 (C=O), 155.7 (C=O), 156.6 (C=O) and 173.9 (C=O); ESI-MS calculated for C62H106N6NaO22S: 1341.70; found: 1341.72 (M+Na)+.
_6″-Amino-6″-deoxy-3, 2′, 6′, 3″, 4‴-penta-N-tert-butoxycar-bonylarbekacin (_ _4_). To a solution of 3 (1.20 g, 0.91 mmol) in _N_,_N_-dimethylformamide (DMF) (24 ml), NaN3 (296 mg, 4.55
mmol) was added, and the mixture was stirred at 100 °C for 3 h. Concentration gave a residue, which was extracted with CHCl3. The organic solution was washed with water, dried over MgSO4 and
concentrated. The resulting residue was purified by silica gel column chromatography to provide the 6″- azide 3A (0.89 g, 91%) as a colorless solid. ESI-MS calculated for C47H83N9NaO19:
1100.57; found: 1100.56 (M+Na)+. To a solution of 3A (795 mg, 0.74 mmol) in tetrahydrofuran (THF)−H2O (5:1, 29 ml), PPh3 (290 mg, 1.11 mmol) was added and the mixture was stirred at 50 °C
for 4 h. Concentration gave a residue, which was purified by silica gel column chromatography to give 4 (644 mg, 89%) as a colorless solid. 1H NMR (DMSO-_d__6_, 500 MHz, 343 K) δ 1.32 (m,
1H, H-4′_ax_), 1.35−1.40 (m, 45H, 5 X C(C_H_3)3), 1.51 (m, 1H, H-2_ax_), 1.62 (m, 1H, H-βa), 1.63 (m, 1H, H-3′_ax_), 1.64 (m, 1H, H-4′_eq_), 1.69 (m, 1H, H-3′_eq_), 1.84 (m, 1H, H-βb), 2.02
(m, 1H, H-2_eq_), 3.07 (m, 2H, H-γ), 3.14 (m, 2H, H-6′), 3.27 (m, 1H, H-4″), 3.33 (m, 1H, H-2″), 3.38 (m, 1H, H-3), 3.42 (m, 1H, H-2′), 3.44 (m, 1H, H-4), 3.46 (m, 1H, H-6″a), 3.49 (m, 1H,
H-3″), 3.53 (m, 1H, H-5), 3.55 (m, 1H, H-6″b), 3.61 (m, 1H, H-6), 3.67 (m, 1H, H-1), 3.74 (m, 1H, H-5″), 3.85 (m, 1H, H-α), 3.86 (m, 1H, H-5′), 3.95 (br, 1H, OH-2′), 4.11 (br, 1H, OH-6″),
4.51 (br, 1H, OH-4″), 4.76 (br, 1H, OH-5), 4.82 (br, 1H, OH-α), 4.92 (d, 1H, _J_=3.2 Hz, H-1″) and 5.08 (d, 1H, _J_=2.9 Hz, H-1′); 13C NMR (DMSO-_d__6_, 125.8 MHz, 343 K) δ 23.7 (3′), 27.6
(4′), 28.2 (6C, C(_C_H3)3), 28.4 (6C, C(_C_H3)3), 29.0 (3C, C(_C_H3)3), 33.6 (2), 34.3 (β), 37.3 (γ), 44.5 (6′), 48.7 (1), 49.5 (3), 50.5 (2′), 55.7 (3″), 59.4 (6″), 66.7 (5′), 67.7 (4″),
69.5 (α), 70.1 (2″), 73.4 (5″), 75.8 (5), 77.5 (_C_(CH3)3), 77.6 _C_(CH3)3), 77.7 (_C_(CH3)3), 77.9 (2C, _C_(CH3)3), 81.2 (4), 81.5 (6), 98.2 (2C, 1′ and 1″), 154.9 (C=O), 155.0 (C=O), 155.5
(C=O), 155.8 (C=O), 156.5 (C=O) and 173.8 (C=O); ESI-MS calculated for C47H85N7NaO19: 1074.58; found: 1074.63 (M+Na)+. _6″-Amino-6″-deoxyarbekacin (_ _1_). A solution of 4 (650 mg, 0.6
mmol) in TFA−H2O (9:1, 13 ml) was kept at 0 °C for 2 h. Concentration gave a residue that was purified by resin column chromatography to provide 1 22 (358 mg, 94% as a carbonate) as a
colorless solid. _6″-N-Alkanoylamino-6″-deoxy-3, 2′, 6′, 3″, 4‴-penta-N-tert-butoxycarbonylarbekacin (_ _5A_ _−_ _Z_ _). Amidation by DMT-MM method_ (5A−L, S−V, X−Z). To a solution of 4
(50−300 mg, 0.05−0.29 mmol) in MeOH−THF−H2O (15:7.5:1, 17.5 ml), a variety of carboxylic acids (1.5 eq) and DMT-MM (2 eq) were added, and the mixture was stirred at room temperature for 1
day. Concentration gave a residue that was extracted with CHCl3. The organic solution was washed with saturated aqueous NaHCO3 and water, dried over MgSO4 and concentrated. The resulting
residue was purified by silica gel column chromatography to provide 5 (41−90%) as a colorless solid. _Amidation by activated ester method_ (5M−R,W). To a solution of 4 (1.0 g, 0.95 mmol) in
THF (20 ml), Et3N (0.33 ml, 2.4 mmol) and _N_-hydroxysuccinimide ester of the corresponding amino acid (5.71 mmol) were added, and the mixture was stirred at room temperature for 19 h. The
reaction mixture was quenched with 1M aqueous NH3, and the mixture was evaporated. The residue was extracted with CHCl3 and the organic solution was washed with saturated aqueous NaHCO3 and
water, dried over MgSO4 and concentrated _in vacuo_. The residue was purified by silica gel column chromatography to provide 5 (92−95%) as a colorless solid.
_6″-N-Alkanoylamino-6″-deoxyarbekacin (_ _6A_ _−_ _Z_). A solution of 5 (31−720 mg) in TFA−H2O (9:1, 20 v/w) was kept at 0 °C for 2 h. The solution was concentrated _in vacuo_, and then the
residue was purified by resin column chromatography to provide 6 (91−97% as a carbonate) as a colorless solid. _Compound _ _6O_. 1H NMR (26% ND3−D2O, 500 MHz): δ 1.40 (m, 1H, H-4′_ax_), 1.44
(m, 1H, H-2_ax_), 1.60 (m, 1H, H-3′_ax_), 1.65 (m, 1H, H-4′_eq_), 1.73 (m, 1H, H-3′_eq_), 1.79 (m, 1H, H-β′a), 1.82 (m, 1H, H-βa), 1.87 (m, 1H, H-β′b), 1.89 (m, 1H, H-βb), 1.93 (m, 1H,
H-2eq), 2.66 (m, 2H, H-6′), 2.72 (m, 2H, H-γ′), 2.74 (m, 2H, H-γ), 2.83 (m, 1H, H-2′), 2.85 (m, 1H, H-3), 2.96 (t, 1H, _J_=9.8 Hz, H-3″), 3.12 (t, 1H, _J_=9.8 Hz, H-4″), 3.31 (m, 1H, H-2″),
3.36 (m, 1H, H-4), 3.47 (t, 2H, _J_=4.7 Hz, H-6″), 3.66 (t, 1H, _J_=9.5 Hz, H-5), 3.73 (t, 1H, _J_=9.5 Hz, H-6), 3.83 (m, 1H, H-5′), 3.96 (m, 1H, H-1), 4.12 (dd, 1H, _J_=3.8 and 9.0 Hz,
H-α′), 4.15 (dd, 1H, _J_=3.8 and 9.2 Hz, H-α), 4.23 (m, 1H, H-5″), 5.04 (d, 1H, _J_=3.7 Hz, H-1″) and 5.18 (d, 1H, _J_=3.4 Hz, H-1′); 13C NMR (26% ND3−D2O, 125.8 MHz): δ 27.2 (3′), 28.1
(4′), 35.3 (2), 36.3 (β′), 37.1 (β), 38.2 (γ), 40.1 (γ′), 41.3 (6″), 45.9 (6′), 50.1 (3), 50.4 (2′), 50.2 (1), 55.8 (3″), 70.4 (α), 71.0 (α′), 71.2 (5″), 71.5 (5′), 72.1 (4″), 72.8 (2″),
75.2 (5), 81.2 (6), 87.9 (4), 99.3 (1″), 102.5 (1′), 171.0 (C=O) and 178.0 (C=O); ESI-HRMS calculated for C26H53N8O11: 653.3828; Found: 653.3825 (M+H)+; Analysis calculated for
C26H53N8O11·H2CO3·3H2O: C, 42.18; H, 7.87; N, 14.57. Found: C, 41.91; H, 7.98; N, 14.59. _2″, 2‴-Di-O-acetyl-3, 2′, 6′, 3″, 4‴-penta-N-tert-Boc 4″-O-mesyl-6″-O-tritylarbekacin (_ _8_). To a
solution of 7 17 (11.0 g, 7.97 mmol) in pyridine (220 ml) methanesulfonyl chloride (4.9 ml, 63.8 mmol) was added, and the mixture was stirred at room temperature for 3 h. The reaction
mixture was quenched with MeOH (22 ml) and was evaporated. The resulting residue was extracted with AcOEt, and the organic solution was washed with saturated aqueous NH4Cl and water, dried
over MgSO4 and concentrated. The residue was purified by silica gel column chromatography to provide 8 (11.0 g, 95%) as a colorless solid. ESI-MS calculated for C71H104N6NaO24S: 1479.67;
found: 1479.60 (M+Na)+. _2″,2‴-Di-O-acetyl-3, 2′, 6′, 3″, 4‴-penta-N-tert-Boc-4″-deoxy-4″-epiazide-6″-O-trityl- arbekacin (_ _9_). To a solution of 8 (12.0 g, 8.23 mmol) in DMF (360 ml) NaN3
(13.4 g, 206 mmol) was added, and the mixture was stirred at 100 °C for 1 day. Concentration gave a residue that was extracted with AcOEt. The organic layer was washed with water, dried
over MgSO4 and concentrated. The resulting residue was purified by silica gel column chromatography to provide 9 (2.0 g, 17%) as a colorless solid. In previous study,17 the yield of 9 was
58% from 7. Aiming at improvement of the yield, we used 4″-_O_-Ms derivative 8 as a starting material of 9, however, the result was a low yield because of elimination products
(2″,2‴-Di-_O_-acetyl-3, 2′, 6′, 3″, 4‴-penta-_N_-_tert_-Boc-4″-deoxy-4″,5″-ene -6″-O-tritylarbekacin and 2″,2‴-Di-_O_-acetyl-3, 2′, 6′, 3″, 4‴-penta-_N_-_tert_-Boc-4″-deoxy-3″,4″-ene
-6″-O-tritylarbekacin). ESI-MS calculated for C70H101N9NaO21: 1426.70; found: 1426.82 (M+Na)+. _3, 2′, 6′, 3″, 4‴-Penta-N-tert-Boc-4″-deoxy-4″-epiamino-6″-O-tritylarbekacin (_ _10_). A
solution of 9 (2.0 g, 1.42 mmol) in MeOH−aqueous concentrated NH3 (2:1, 90 ml) was stirred at room temperature for 2 h. Concentration gave a 2″, 2‴-dihydroxyl derivative 9A (1.9 g) as a
solid. ESI-MS calculated for C66H97N9NaO19: 1342.68; found: 1342.78 (M+Na)+. To a solution of 9A (1.9 g, 1.44 mmol) in THF−H2O (4:1, 30 ml) PPh3 (1.13 g, 4.31 mmol) was added, and the
mixture was stirred at 50 °C for 20 h. After concentration, the resulting residue was dissolved in pyridine (119 ml). To the solution concentrated aqueous NH3 (90 ml) was added, and the
mixture was stirred at room temperature for 3 days. The reaction mixture was concentrated, and then the residue was purified by silica gel column chromatography to provide 10 (900 mg, 49%
from 9) as a colorless solid. ESI-MS calculated for C66H99N7NaO19: 1316.69; found: 1316.82 (M+Na)+. _4″-Deoxy-4″-epiaminoarbekacin (_ _2_). A solution of 10 (50.1 mg, 0.44 mmol) in TFA−H2O
(9:1, 1.0 ml) was kept at 0 °C for 2 h. Concentration gave a residue, which was purified by resin column chromatography to provide 2 (20.6 mg, 87% as a carbonate) as a colorless solid. 1H
NMR (25% ND3-D2O, 400 MHz) δ 1.30 (m, 1H, H-4′_ax_), 1.45 (m, 1H, H-2_ax_), 1.56 (m, 1H, H-3′_ax_), 1.60 (m, 1H, H-4′_eq_), 1.75 (m, 1H, H-3′_eq_), 1.79 (m, 1H, H-βa), 1.86 (m, 1H, H-βb),
1.98 (m, 1H, H-2_eq_), 2.62 (m, 2H, H-6′), 2.77 (m, 2H, H-γ), 2.82 (m, 1H, H-2′), 2.93 (m, 1H, H-3), 2.99 (dd, 1H, _J_=3.7 and 7.0 Hz, H-3″), 3.07 (m, 1H, H-4″), 3.33 (t, 1H, _J_=9.1 Hz,
H-4), 3.57 (dd, 1H, _J_=4.0 and 7.0 Hz, H-2″), 3.66 (m, 1H, H-6″a), 3.71 (m, 1H, H-6″b), 3.73 (t, 1H, _J_=9.1 Hz, H-5), 3.76 (m, 1H, H-6), 3.82 (m, 1H, H-5′), 3.96 (m, 1H, H-1), 4.16 (m, 1H,
H-5″), 4.30 (m, 1H, H-α), 5.09 (d, 1H, _J_=4.0 Hz, H-1″) and 5.13 (d, 1H, _J_=3.5 Hz, H-1′); 13C NMR (25% ND3-D2O, 100.6 MHz) δ 26.9 (3′), 28.4 (4′), 35.2 (2), 37.2 (β), 38.2 (γ), 46.0
(6′), 50.1 (3), 50.4 (1), 50.8 (2′), 52.1 (3″), 52.2 (4″), 62.0 (6″), 69.9 (2″), 70.6 (5″), 71.5 (5′), 72.6 (α), 76.2 (5), 81.9 (6), 87.4 (4), 99.7 (1″), 102.4 (1′) and 177.4 (NH_C_O-1);
ESI-HRMS calculated for C22H46N7O9: 552.3358; found: 552.3357 (M+H)+. _4″-N-[(S)-2-Benzyloxy-4-N-benzyloxycarbonylaminobutylyl]-4″-deoxy-4″-epiaminoarbekacin (_ _12_). To a solution of 10
(350 mg, 0.27 mmol) in MeOH−THF−H2O (15:7.5:1, 17.5 ml) (_S_)-2-benzyloxy-4-benzyloxycarbonylaminobutyric acid (418 mg, 1.22 mmol) and DMT-MM (449 mg, 1.62 mmol) were added, and the mixture
was stirred at room temperature for 16 h. The reaction mixture was evaporated, then the residue was diluted with AcOEt, and the organic solution was washed with saturated aqueous NaHCO3 and
water, dried over MgSO4 and concentrated. The residue was purified by silica gel column chromatography to provide 11 (376 mg, 86%) as a colorless solid. ESI-MS calculated for C85H118N8NaO23:
1641.82; found: 1641.30 (M+Na)+. A solution of 11 (330 mg, 0.204 mmol) in TFA−H2O (9:1, 6.6 ml) was kept at 0 °C for 2 h. Concentration gave a residue, which was purified by resin column
chromatography to provide 12 (168 mg, 88% as a carbonate) as a colorless solid. 1H NMR (25% ND3-D2O, 400 MHz) δ 1.34 (m, 1H, H-4′_ax_), 1.48 (m, 1H, H-2_ax_), 1.55 (m, 1H, H-3′_ax_), 1.60
(m, 1H, H-4′_eq_), 1.72 (m, 1H, H-3′_eq_), 1.80 (m, 1H, H-βa), 1.82 (m, 1H, H-β′a), 1.85 (m, 1H, H-βb), 1.91 (m, 1H, H-β′b), 1.99 (m, 1H, H-2_eq_), 2.60 (m, 2H, H-6′), 2.77 (m, 2H, H-γ),
2.81 (m, 1H, H-2′), 2.93 (m, 1H, H-3), 3.10 (m, 1H, H-3″), 3.19 (m, 2H, H-γ′), 3.28 (m, 1H, H-4), 3.38 (m, 1H, H-2″), 3.46 (d, 2H, _J_=6.0 Hz, H-6″), 3.66 (m, 1H, H-5), 3.73 (m, 1H, H-6),
3.81 (m, 1H, H-5′), 3.98 (m, 1H, H-1), 4.08 (m, 1H, H-α′), 4.15 (m, 1H, H-4″), 4.23 (m, 1H, H-α), 4.40 (m, 1H, H-5″), 4.58 (m, 2H, C_H_2C6H5), 5.01 (d, 1H, _J_=4.0 Hz, H-1″), 5.09 (m, 2H,
C_H_2C6H5), 5.18 (d, 1H, _J_=3.6 Hz, H-1′) and 7.31−7.50 (m, 10H, 2X C6_H_5); 13C NMR (25% ND3-D2O, 100.6 MHz) δ 26.9 (3′), 28.4 (4′), 33.5 (β′), 35.2 (2), 37.2 (β), 37.7 (γ′), 38.2 (γ),
46.0 (6′), 50.0 (3), 50.3 (1), 50.8 (2′), 51.2 (3″), 51.3 (4″), 61.4 (6″), 67.6 (_C_H2C6H5), 70.6 (2″), 70.7 (5″), 71.0 (5′), 71.5 (α), 73.6 (_C_H2C6H5), 76.0 (5), 78.1 (α′), 81.8 (6), 87.4
(4), 99.5 (1″), 102.3 (1′), 128.6 (2C, _C_6H5), 129.2 (2C, _C_6H5), 129.3 (2C, _C_6H5), 129.4 (4C, _C_6H5), 129.6 (1C, _C_6H5), 129.8 (1C, _C_6H5), 158.9 (γ′-NH_C_O), 176.3 (NH_C_O-6″) and
177.4 (NH_C_O-1); ESI-HRMS calculated for C41H65N8O13: 877.4672; found: 877.4669 (M+H)+. _4″-N-[(S)-4-Amino-2-hydroxybutylyl]-4″-deoxy-4″-epi-aminoarbekacin (_ _13_). A solution of 12 (93.5
mg, 0.099 mmol) in 50% aqueous AcOH (5.6 ml) was hydrogenated in the presence of Pd/C for 4 h at room temperature. After filtration, the filtrate was concentrated, and the solid was purified
by resin column chromatography to provide 13 (55.7 mg, 78% as a carbonate) as a colorless solid. 1H NMR (25% ND3-D2O, 400 MHz) δ 1.36 (m, 1H, H-4′_ax_), 1.50 (m, 1H, H-2_ax_), 1.58 (m, 1H,
H-3′_ax_), 1.69 (m, 1H, H-4′_eq_), 1.76 (m, 1H, H-3′_eq_), 1.78 (m, 1H, H-βa), 1.81 (m, 1H, H-β′a), 1.86 (m, 1H, H-βb), 1.91 (m, 1H, H-β′b), 2.000 (m, 1H, H-2_eq_), 2.62 (m, 2H, H-6′), 2.75
(m, 1H, H-γ′a), 2.79 (m, 2H, H-γ), 2.81 (m, 1H, H-2′), 2.92 (m, 1H, H-3), 3.14 (m, 1H, H-3″), 3.18 (m, 1H, H-γ′b), 3.37 (m, 1H, H-4), 3.48 (m, 1H, H-2″), 3.56 (m, 2H, H-6″), 3.68 (m, 1H,
H-5), 3.78 (m, 1H, H-6), 3.83 (m, 1H, H-5′), 3.98 (m, 1H, H-1), 4.15 (dd, 1H, _J_=3.6 and 5.7 Hz, H-α), 4.27 (m, 1H, H-4″), 4.31 (m, 1H, H-α), 4.48 (m, 1H, H-5″), 5.13 (d, 1H, _J_=3.5 Hz,
H-1′) and 5.15 (d, 1H, _J_=3.9 Hz, H-1″); 13C NMR (25% ND3-D2O, 100.6 MHz) δ 26.9 (3′), 28.4 (4′), 35.2 (2), 37.1 (β′), 37.2 (β), 37.9 (γ′), 38.0 (γ), 46.0 (6′), 50.0 (3), 50.3 (1), 50.7
(2′), 50.7 (3″), 51.3 (4″), 61.2 (6″), 70.3 (2″), 70.5 (α), 70.7 (α′), 70.9 (5″), 71.4 (5′), 76.0 (5), 81.7 (6), 87.4 (4), 99.6 (1″), 102.3 (1′), 177.3 (NH_C_O-6″) and 178.3 (NH_C_O-1);
ESI-HRMS calculated for C26H53N8O11: 653.3835; found: 653.3839 (M+H)+. ANTIBACTERIAL ASSAY The MICs were examined by the serial agar dilution method using Mueller-Hinton agar (Becton,
Dickinson and Company, New Jersey, NJ, USA) for _S. aureus_, _E. coli_ and _P. aeruginosa_, and using Mueller-Hinton agar supplemented with 5% defibrinated sheep blood (Nippon Biotest
Laboratories Inc., Tokyo, Japan) under 5% CO2 for _E. faecalis_. The test suspension was prepared at approximately 104 CFU per 5 μl using a microplanter MIT-P inculum replicating apparatus.
The MIC was defined as the lowest concentration of antibiotic that inhibited development of visible growth on the agar after 18 h incubation at 37 °C for Gram-positive and Gram-negative
bacteria. REFERENCES * Moazed, D. & Noller, H. F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. _Nature_ 327, 389–394 (1987). Article CAS Google Scholar *
Vicens, Q. & Westhof, E. Crystal structure of Geneticin bound to a bacterial 16S ribosomal RNA A site oligonucleotide. _J. Mol. Biol._ 326, 1175–1188 (2003). Article CAS Google Scholar
* Kondo, J., Francois, B., Urzhumtsev, A. & Westhof, E. Crystal structure of the homo sapiens cytoplasmic ribosomal decoding site complexed with apramycin. _Angew. Chem. Int. Ed._ 45,
3310–3314 (2006). Article CAS Google Scholar * Perez-Fernandez, D. _et al_. C. 4'-O-substitutions determine selectivity of aminoglycoside antibiotics. _Nat. Commun._ 5, 3112 (2014).
Article Google Scholar * Magnet, S. & Blanchard, S. Molecular insights into aminoglycoside action and resistance. _J. Chem. Rev._ 105, 477–498 (2005). Article CAS Google Scholar *
Kondo, S., Iinuma, K., Yamamoto, H., Maeda, K. & Umezawa, H. Syntheses of 1-N-{(S)-4-amino-2-hydroxybutyryl}-kanamycin B and -3', 4'-dideoxykanamycin B active against
kanamycin-resistant bacteria. _J. Antibiot._ 26, 412–415 (1973). Article CAS Google Scholar * Deguchi, K., Yokota, N., Koguchi, M., Suzuki, Y. & Fukuyama, S. Antimicrobial activities
of arbekacin against methicillin-resistant Staphylococcus aureus isolated from patients of a pediatric ward. _Jpn. J. Antibiot._ 46, 794–800 (1993). CAS PubMed Google Scholar * Shigemura,
K. _et al_. Anti-MRSA drug use and antibiotic susceptibilities of MRSA at a university hospital in japan from 2007 to 2011. _J. Antibiot._ 66, 273–276 (2013). Article CAS Google Scholar
* Suga, T. _et al_. Aranorosin circumvents arbekacin-resistance in MRSA by inhibiting the bifunctional enzyme AAC(6′)/APH(2″). _J. Antibiot._ 65, 527–529 (2012). Article CAS Google Scholar
* Ishino, K., Ishikawa, J., Ikeda, Y. & Hotta, K. Characterization of a bifunctional aminoglycoside-modifying enzyme with novel substrate specificity and its gene from a clinical
isolate of methicillin-resistant Staphylococcus aureus with high arbekacin resistance. _J. Antibiot._ 57, 679–686 (2004). Article CAS Google Scholar * Chow, J. W. _et al_. Aminoglycoside
resistance genes aph(2")-Ib and aac(6′)-Im detected together in strains of both Escherichia coli and Enterococcus faecium. _Antimicrob. Agents Chemother._ 45, 2691–3694 (2001). Article
CAS Google Scholar * Aghazadeh, M. _et al_. Dissemination of aminoglycoside-modifying enzymes and 16S rRNA methylases among Acinetobacter baumannii and Pseudomonas aeruginosa isolates.
_Microbial Drug Resist._ 19, 282–288 (2013). Article CAS Google Scholar * Shaul, P. _et al_. Assessment of 6' and 6'''-N-acylation of aminoglycosides as a strategy to
overcome bacterial resistance. _Org. Biomol. Chem._ 9, 4057–4063 (2011). Article CAS Google Scholar * Zhang, J., Litke, A., Keller, K., Rai, R. & Chang, C. W. T. Synthesis of novel
aminoglycosides via allylic azide rearrangement for investigating the significance of 2'-amino group. _Bioorg. Med. Chem._ 18, 1396–1405 (2010). Article CAS Google Scholar * Chen, W.
_et al_. Synthesis, antiribosomal and antibacterial activity of 4'-O-glycopyranosyl paromomycin aminoglycoside antibiotics. _MedChemComm_ 5, 1179–1187 (2014). Article CAS Google
Scholar * Hiraiwa, Y. _et al_. Synthesis and antibacterial activity of 5-deoxy-5-episubstituted arbekacin derivatives. _Bioorg. Med. Chem. Lett_ 17, 3540–3543 (2007). Article CAS Google
Scholar * Hiraiwa, Y., Minowa, N., Usui, T., Akiyama, Y., Maebashi, K. & Ikeda, D. Effect of varying the 4″-position of arbekacin derivatives on antibacterial activity against MRSA and
Pseudomonas aeruginosa. _Bioorg. Med. Chem. Lett_ 17, 6369–6372 (2007). Article CAS Google Scholar * Kawaguchi, H., Naito, T., Nakagawa, S. & Fujisawa, K. BB-K8, a new semisynthetic
aminoglycoside antibiotic. _J. Antibiot._ 25, 695–708 (1972). Article CAS Google Scholar * Naito, T. _et al_. Aminoglycoside antibiotics. IX. Structure-activity relationship in 1-N-acyl
derivatives of kanamycin A (amikacin analogs). _J. Antibiot._ 27, 851–858 (1974). Article CAS Google Scholar * Takahashi, Y., Ueda, C., Tsuchiya, T. & Kobayashi, Y. Study on
fluorination-toxicity relationships. Syntheses of 1-N-[(2R,3R)- and (2R,3S)-4-amino-3-fluoro-2-hydroxybutanoyl] derivatives of kanamycins. _Carbohydr. Res._ 249, 57–76 (1993). Article CAS
Google Scholar * Kondo, S., Iinuma, K., Yamamoto, H., Ikeda, Y., Maeda, K. & Umezawa, H. Syntheses of (S)-4-amino-2-hydroxybutyryl derivatives of 3', 4'-dideoxykanamycin B and
their antibacterial activities. _J. Antibiot._ 26, 705–707 (1973). Article CAS Google Scholar * Minowa, N. _et al_. Novel aminoglycoside antibiotic effective against methicillin
resistant Staphylococcus aureus (MRSA). _PCT Int. Appl._ (2005) WO 2005070945. * Staudingar, H. & Meyer, J. Über neue organische phosphorverbindungen III. Phosphinmethylenderivate und
phosphinimine. _Helv. Chim. Acta_ 2, 635–646 (1919). Article Google Scholar * Kunishima, M., Kawachi, C., Hioki, K. & Terao, S. Formation of carboxamides by direct condensation of
carboxylic acids and amines in alcohols using a new alcohol- and water-soluble condensing agent: DMT-MM. _Tetrahedron_ 57, 1551–1558 (2001). Article CAS Google Scholar * Anderson, W. G.,
Zimmerman, E. J. & Callahan, M. F. The use of esters of N-hydroxysuccinimide in peptide synthesis. _J. Am. Chem. Soc._ 86, 1839–1842 (1964). Article CAS Google Scholar * Takahashi,
Y., Kohno, J. & Tsuchiya, T. Synthesis of 1-N-[(2S,4S)- and (2S,4R)-5-amino-4-fluoro-2-hydroxypentanoyl] dibekacins. _Carbohydr. Res._ 306, 349–360 (1998). Article CAS Google Scholar
Download references ACKNOWLEDGEMENTS We are grateful to Mr Yasuhiro Isogai and Ms Rina Shimonishi for assistance with the synthesis of derivatives. We also thank Dr Ryuichi Sawa and Dr
Kiyoko Iijima for the mass spectrometry experiments, Ms Yoshiko Koyama for the NMR spectrometry experiments. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of Microbial Chemistry
(BIKAKEN), Hiyoshi, Kawasaki, Japan Kazushige Sasaki, Yoshihiko Kobayashi, Takashi Kurihara, Yohei Yamashita, Yoshiaki Takahashi & Toshiaki Miyake * Institute of Microbial Chemistry
(BIKAKEN), Tokyo, Japan Yuzuru Akamatsu Authors * Kazushige Sasaki View author publications You can also search for this author inPubMed Google Scholar * Yoshihiko Kobayashi View author
publications You can also search for this author inPubMed Google Scholar * Takashi Kurihara View author publications You can also search for this author inPubMed Google Scholar * Yohei
Yamashita View author publications You can also search for this author inPubMed Google Scholar * Yoshiaki Takahashi View author publications You can also search for this author inPubMed
Google Scholar * Toshiaki Miyake View author publications You can also search for this author inPubMed Google Scholar * Yuzuru Akamatsu View author publications You can also search for this
author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Kazushige Sasaki. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on The Journal of Antibiotics
website SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (DOC 918 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sasaki, K., Kobayashi, Y.,
Kurihara, T. _et al._ Synthesis and antibacterial activity of 4″ or 6″-alkanoylamino derivatives of arbekacin. _J Antibiot_ 68, 741–747 (2015). https://doi.org/10.1038/ja.2015.61 Download
citation * Received: 09 March 2015 * Revised: 14 April 2015 * Accepted: 20 April 2015 * Published: 20 May 2015 * Issue Date: December 2015 * DOI: https://doi.org/10.1038/ja.2015.61 SHARE
THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative