Effects of ciprofloxacin on salt marsh sediment microbial communities
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Fluoroquinolones, a widely used class of antibiotics, are frequently detected in sediments and surface waters. Given their antimicrobial properties, the presence of these compounds
may alter the composition of microbial communities and promote antibiotic resistance in the environment. The purpose of this study was to measure sorption, and effects of ciprofloxacin on
microbial community composition, in sediment samples from three California salt marshes. Sediments were exposed to a ciprofloxacin concentration gradient (0–200 μg ml−1 ciprofloxacin) and
microbial community composition characterized using phospholipid fatty acid (PLFA) analysis. Sorption coefficients, expressed as log _K_d, were calculated from fits using the Freundlich
isotherm model. Ciprofloxacin strongly sorbed to all sediments and had log _K_d values, ranging from 2.9 to 4.3. Clay content was positively (_r_2=0.98) and pH negatively (_r_2=0.99)
correlated to _K_d values. Biomass, PLFA richness, sulfate reducer and Gram-negative bacteria markers increased with ciprofloxacin concentrations, while the 17 cy/precursor and
saturated/unsaturated biomarker ratios, indicators of starvation stress, decreased. The magnitude of the effect of ciprofloxacin on microbial communities was inversely correlated to the
degree of sorption to the sediments. Despite the fact that ciprofloxacin is a wide-spectrum antibiotic, its impact on sediment microbial communities was selective and appeared to favor
sulfate-reducing bacteria and Gram-negative bacteria. SIMILAR CONTENT BEING VIEWED BY OTHERS COMBINED EFFECTS OF MICROPOLLUTANTS AND THEIR DEGRADATION ON PROKARYOTIC COMMUNITIES AT THE
SEDIMENT–WATER INTERFACE Article Open access 22 July 2024 CLAY MINERALS AS A SOURCE OF CADMIUM TO ESTUARIES Article Open access 26 June 2020 UNDERSTANDING THE MECHANISMS BEHIND THE
ANTIBACTERIAL ACTIVITY OF MAGNESIUM HYDROXIDE NANOPARTICLES AGAINST SULFATE-REDUCING BACTERIA IN SEDIMENTS Article Open access 18 September 2024 INTRODUCTION There has been concern for
decades about potential ecological impacts of pharmaceuticals, but only recently have advances in analytical chemistry permitted detection of these chemicals at the concentrations typically
found in the environment (Halling-Sørensen et al., 1998; Zuccato et al., 2000; Bila and Dezotti, 2003; Boyd et al., 2003). Antibiotics reach the environment through intentional disposal of
surplus drugs to sewage, release to sewage through urine and feces, leaching from landfills and discharges from sewage treatment plants or confined animal farming operations (Daughton and
Ternes, 1999; Daughton, 2000). One frequently detected class of antibiotics in the environment is the fluoroquinolones, compounds inhibiting both Gram-positive (GP) and Gram-negative (GN)
bacteria, and commonly used to treat tuberculosis, digestive and urinary tract infections and anthrax. The mode of action of these compounds is to interfere with DNA synthesis by binding to
DNA gyrase and thus preventing replication (Fernandes, 1988; Hooper, 1999). Ciprofloxacin and levofloxacin account for 65% of the total fluoroquinolone use and represent 3.3 billion dollars
in global sales (Datamonitor Strategic Report, 2004). In a dose of ciprofloxacin ingested, an estimated 45%–62% is excreted unmetabolized in human urine and 15%–25% in feces (Golet et al.,
2003). Environmental risk studies have estimated environmental loadings of ciprofloxacin from European sewage treatment plants to be as high as 186.2 tones per year in 1999
(Halling-Sorensen, 2000). Monitoring surveys have detected antibiotics in aquatic ecosystems ranging from ng l−1 to mg l−1 concentrations (Giger et al., 2003). In the United States, 22
antibiotics and antimicrobial compounds were detected in 50% of the water samples from streams influenced by urbanized areas and livestock activities across the country, and 2.6% tested
positive for ciprofloxacin with a maximum concentration of 0.030 μg l−1 (Kolpin et al., 2002). Other studies have reported ciprofloxacin at concentrations of 118–400 ng l−1 in Canadian
wastewater treatment effluents (Miao et al., 2004), and 100–160 ng l−1 in secondary effluents from municipal wastewater treatment plants in Arizona, California and Georgia (Renew and Huang,
2004). Fluoroquinolones are strongly sorbed to organic matter (MacKay et al., 2004; MacKay and Figueroa, 2004) and clays (Nowara et al., 1997; Seremet and MacKay, 2003) with partition
coefficients ranging from log _K_d=2.45 to 2.69 for pure clays and log _K_oc up to 4.85 (Nowara et al., 1997). In one of few studies measuring ciprofloxacin associated with solid as well as
aqueous samples, sludge solids in a Swiss wastewater treatment plant contained an average of 2.2 mg l−1, more than three orders of magnitude higher than the mean concentration of 0.427 μg
l−1 in the raw sewage-filtered effluent (Golet et al., 2003). Several studies on ciprofloxacin sorption to clays and minerals have been published (Seremet and MacKay, 2003; Gu and
Karthikeyan, 2005), but there is no published data on sorption of ciprofloxacin to soil or sediments. However, based on the behavior of compounds similar in chemical structure, aqueous
samples may grossly underestimate the presence of ciprofloxacin in the environment. Salt marshes are destinations for many pollutants and nutrients, many of which are filtered out of surface
waters, before they enter the ocean. Because antibiotics are designed specifically to target microorganisms, the persistence of these compounds in sediments may negatively impact estuarine
microbial processes. Surprisingly, little research has addressed the effects of ciprofloxacin and other antibiotics on microbial communities in natural ecosystems. Ciprofloxacin has been
shown to negatively affect freshwater algae communities by decreasing species richness in microcosm studies at concentrations as low as 0.015 μg l−1 (Wilson et al., 2003). What are lacking
are controlled studies to determine what concentrations of ciprofloxacin cause changes in sediment microbial community structure and function. We hypothesized that microbial community
composition, biomass and richness are modified by ciprofloxacin at concentrations found in the environment, and that sorption plays an important role in controlling the magnitude of these
effects. For this purpose, sediments from three California salt marshes were used to determine sorption coefficients of ciprofloxacin and to determine the effects of the antibiotic on the
phospholipid fatty acid (PLFA) fingerprint of the sediment microbial community. This study is part of a larger project, the Pacific Estuarine Ecosystem Indicator Research (PEEIR) Consortium,
within the EPA's EaGLe's Program, that aims to develop indicators of toxicant-induced stress and bioavailability for wetland biota. MATERIALS AND METHODS SITE SELECTION AND
SEDIMENT SAMPLING Sediments were collected in the summer of 2003 from three salt marshes in the San Francisco Bay area: Stege Marsh (SM), Walker Creek (WC) and China Camp (CC). Both CC and
SM are located directly in the San Francisco Bay; WC is one of the main tributaries leading into Tomales Bay. Dominant plant species include _Spartina_ and _Salicornia_ in all the sites. SM
is highly contaminated with metals, pesticides and organic solvents (Hwang et al., 2006a, 2006b) and has been targeted for remediation by the Superfund program. WC has high levels of mercury
and CC is a relatively unpolluted site. More detailed descriptions of the sampling sites can be found elsewhere (Córdova-Kreylos et al., 2006; Hwang et al., 2006a, 2006b). Several cores
were collected from different locations within each salt marsh using a 2.5-cm diameter Teflon corer to a depth of 5 cm. The cores were pooled together and homogenized in sterile glass jars.
They were transported in ice and stored at 4 °C in a cold room until the experiments were started. For sorption experiments, samples were air-dried, sieved through a 2 mm mesh to ensure
uniform mass in the experiments and stored dry in glass jars. SEDIMENT CHARACTERIZATION Sediments were characterized for pH, cation exchange capacity, organic carbon content, total nitrogen
content and texture. Cation exchange capacity was determined by the compulsive exchange method and pH was measured using a gel pH electrode connected to a pH meter (Orion, Waltham, MA, USA)
as described in the Standard Methods of the Soil Science Society of America (SSSA) for soil analysis (Sparks, 1996). Texture was determined by particle size distribution analysis with laser
diffraction using a Beckman-Coulter LS-230 (Beckman-Coulter, Miami, FL, USA) with a 750 nm laser beam detection as described previously (Eshel et al., 2004). Carbon and nitrogen were
determined by micro-Dumas combustion using a C/N analyzer (Carlo Erba; Milan, Italy). SORPTION EXPERIMENTS Sorption experiments were performed according to EPA standard procedures as
described by Figueroa et al. (2004). A preliminary sorption experiment was performed for each sediment type to determine the optimum sediment/solution ratio and incubation time required to
reach the equilibrium concentrations in solution. To inhibit biodegradation, sodium azide was added at 1% of the sediment mass. Sorption curves were created with 12 ciprofloxacin
(Sigma-Aldrich, St Louis, MO, USA) concentrations ranging from 0.5 to 100 μg ml−1 in a 0.01 M CaCl2 solution. After equilibration the sediment was separated from the aqueous phase by
centrifugation at 8000 _g_ for 15 min. The aqueous solution was analyzed with a Perkin Elmer Series 2 liquid chromatograph (LC) connected to an ultraviolet diode array (DA) detector (SPD
M10A, Shimadzu). The LC-DA system was operated under the following conditions: column, Alltech Econosphere C18, 5 μm, 250 × 4 mm, eluent, phosphate buffer (30 mM KH2PO4, 10 mg l−1
tri-ethylamine, adjusted to pH 3±0.01)/acetonitrile, 70:30; flow rate, 1.5 ml min−1; injection, 20 μl; detection, ultraviolet 280 nm. Under these conditions, the retention time for
ciprofloxacin was 3.2 min and the detection limit, 0.5 μg ml−1. The amount of ciprofloxacin sorbed was calculated from the difference between the original and final aqueous concentrations.
The results were expressed as mass sorbed to the sediment (_C_s) in mg kg−1 and concentration remaining in solution (_C_e) in μg ml−1. The data obtained were fitted to the linear form of the
Freundlich equation (log _C_s=log _K_d+_n_ log _C_e), and log _K_d obtained from the slope of the linear regression of the points. Sorption coefficients were normalized for organic carbon
using the equation _K_oc=_K_d/_f_oc, where _f_oc is the organic carbon fraction in each sediment. MICROCOSM EXPOSURE EXPERIMENT Sediments were exposed to ciprofloxacin concentrations ranging
from 0.02 to 200 μg ml−1. The exposure was performed in microcosms made out of 60 ml glass serum bottles outfitted with thick butyl stoppers and aluminum seals. Each bottle contained 20 g
of sediment and 40 ml of mineral media (per liter: 5 g NaCl, 1.5 g KH2PO4, 5 g NH4Cl, 1 g MgCl2 and 1 g CaCl2) amended with the antibiotic and 20 mM Na2SO4. The only carbon source was
ciprofloxacin and the carbon present in the sediment slurries. The microcosms were flushed with nitrogen gas and the final CO2 concentration in the headspace was adjusted to 20%. The
microcosms were incubated in the dark for 30 days. Triplicate controls that were not autoclaved and contained no ciprofloxacin were also incubated. At the end of the incubation period, the
contents of the bottles were centrifuged at 3000 _g_ for 15 min and sediment collected for PLFA analysis. PLFA ANALYSIS AND NOMENCLATURE Sediment microbial community composition was
determined using PLFA analysis as described previously (Bossio and Scow, 1998). Briefly, frozen (−80 °C) sediment samples were freeze-dried and 8 g of dry sediment were used for the lipid
extraction. Lipids were extracted using a one-phase chloroform/methanol/phosphate-buffered solvent. Phospholipids were separated from nonpolar lipids and converted into fatty acid methyl
esters prior analysis. Quantification was performed using a Hewlett Packard 6890 Gas Chromatograph fitted with a 25 m Ultra 2 (5% phenyl)-methylopolysiloxane column (J&W Scientific,
Folsom, CA, USA). Identification was performed using bacterial fatty acid standards and software from the MIS Microbial Identification System (Microbial ID Inc., Newark, DE, USA). Lipids
were named following the accepted convention (for example, A:BωC) as described by Bossio and Scow (1998). Total microbial biomass was calculated by summing the mass of all detected fatty
acids (White et al., 1979; Frostegard et al., 1991; Bossio et al., 1998) and expressed as nanomoles of PLFA per gram of dry sediment. The number of different PLFAs detected in each sample
was used as a measure of richness. In addition, the following biomarkers and ratios were detected or calculated: sulfate-reducer bacteria (SRB) (br17:1 for _Desulfovibrio_, 10Me16:0 for
_Desulfobacter_ and 17:1 for _Desulfobulbus_); eukaryotes (polyunsaturated PLFAs); Gram-positives (branched PLFAs); Gram-negatives (monounsaturated PLFAs); 17 cy/precursor (17:0 cy/16:1ω7c)
and saturated/unsaturated (sat/unsat) PLFAs. STATISTICAL ANALYSIS Correspondence analysis was performed using the CANOCO software (Version 4.0, Microcomputer Power, Ithaca, NY, USA). Fatty
acid concentrations were expressed as nmol g−1 of dry sediment for the analysis. All PLFA detected and named were used for the multivariate analysis. Correspondence analysis plots
constructed in SigmaPlot V. 8.02 (SPSS Inc., 2002) were used to evaluate and compare PLFA fingerprints across microbial communities from different marshes and different ciprofloxacin
concentrations. Correspondence analysis was selected because the PLFA data set contains both nominal and zero values. Total PLFA biomass, richness and biomarkers for different ciprofloxacin
concentrations in each sediment were compared by one-way analysis of variance (or analysis of variance by ranks when data failed the normality test), and the Holm–Sidak multiple comparisons
test using SigmaStat (SPSS Inc., 2002). Differences were determined statistically significant if _P_<0.05. RESULTS CHARACTERIZATION OF TEST SEDIMENTS Sediment samples varied in pH, cation
exchange capacity, clay content, total nitrogen and organic carbon (Table 1). Organic carbon ranged from 1.3% to 2.8% with varying C/N ratios (8.86–14.05). SM had the highest clay content,
whereas both WC and CC sediments were high in sand content. Only SM, the most polluted site, had an acidic pH (5.11) and the highest cation exchange capacity. SORPTION EXPERIMENTS Sorption
data for all sediments showed good fit to the Freundlich sorption model (_r_2=0.91–0.96) (Table 2). The Freundlich model was thus used to evaluate relationships between sediment properties
and sorption. To facilitate comparisons between sediments, all fits were considered to approximate linearity (_n_∼1). Sorption was strongest in SM (log _K_d=4.27), followed by WC (log
_K_d=3.20) and CC (log _K_d=2.90). Because only three points were used for regression, only apparent correlations could be determined. The %OC was positively correlated with log _K_d
(_r_2=0.67), as was clay content (_r_2=0.98). Log _K_oc was positively correlated with clay content (_r_2=0.79). SM, the sediment with the highest clay content (12.8%), had the highest
sorption coefficient, followed by WC and CC sediments, containing 7.6% and 5.1% clay, respectively. The sorption coefficients and sediment pH values had a strong negative correlation
(_r_2=0.99). MICROBIAL COMMUNITY COMPOSITION A total of 79 different PLFAs were detected across all sediments, specifically 75, 58 and 55 lipids were detected in WC, CC and SM, respectively.
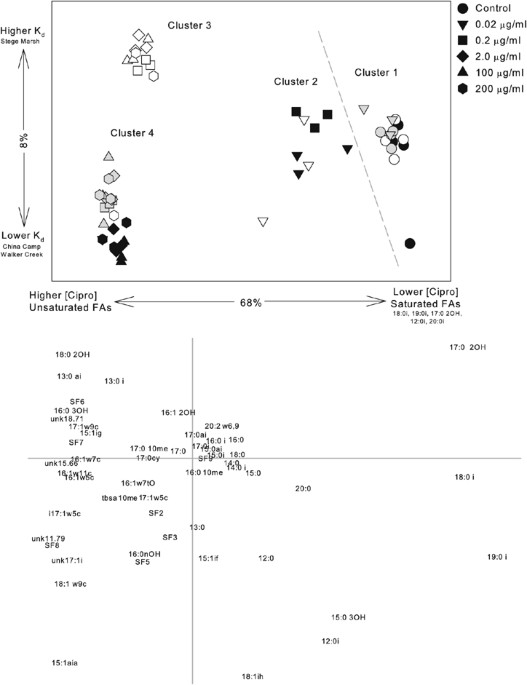
On the basis of a correspondence analysis of all sediments (Figure 1), untreated controls and lower ciprofloxacin concentration treatments grouped together on the right side of the first
axis in two clusters, whereas all other samples with higher ciprofloxacin concentrations grouped to the left (Figure 1). Overall, the first axis appeared to be related to ciprofloxacin
concentrations, and the second axis to _K_d. Saturated PLFA tended to be more abundant at lower ciprofloxacin concentrations and unsaturated PLFAs were associated with higher ciprofloxacin
concentrations (Figure 1, lipid plot). In correspondence analyses performed on each sediment independently, the variation explained on the first two axes decreased (from 85.2% in CC to 69.1%
in SM) with increasing sorption capacity of the sediments. In CC microcosms, the 0.02 μg ml−1 treatments grouped closely with the control samples. The higher concentration treatments formed
a tight cluster to the left side of the plot. For the WC sediments, the 0.02 and 0.2 μg ml−1 treatments grouped in the center of the first axis, and the higher treatments to the left. For
the SM sediments, the treatments were loosely clustered and less separated along the first axis. Associations were also observed in plots of individual sediments between saturated PLFAs and
controls and low ciprofloxacin concentrations, and between unsaturated PLFAs and higher ciprofloxacin concentrations (Figure 2). BIOMASS AND RICHNESS The total PLFA content for the controls
ranged from 8.4±0.3 to 11.6 nmol g−1 dry sediment (Table 3). In all three sediments, biomass increased with higher ciprofloxacin concentrations although the magnitude of increase varied by
sediment. CC had the largest net increase of biomass, with the highest ciprofloxacin treatment having triple the biomass of the control values (Table 3). Biomass differences between the
control and the 200 μg ml−1 treatments were significant in all sediments, except in SM. Phospholipid fatty acid richness, estimated from the number of peaks detected, also increased at
higher ciprofloxacin concentrations. The average total number of PLFAs detected in control samples of all sediments ranged from 25 to 31. Peak numbers increased by at least 50% in CC and WC
sediments when ciprofloxacin concentrations were 2 μg ml−1 or higher (Table 3). The most significant increase compared to controls was observed in WC's 2.0 μg ml−1 treatment, followed
by CC's 200 μg ml−1 treatment, whereas changes in SM were not statistically significant. BIOMARKERS Phospholipid fatty acid biomarkers representative of specific microbial groups, such
as those associated with SRB were dramatically affected by the addition of ciprofloxacin. All sediment controls had a similar SRB abundance and specific biomarkers for _Desulfobacter_ and
_Desulfobulbus_, but not _Desulfovibrio_, were present. Addition of 0.2, 2, 100 and 200 μg ml−1 of ciprofloxacin significantly increased SRB biomass in all sediments (Figure 3). Part of the
increase was due to enrichment of _Desulfovibrio_ biomarkers, which in the 200 μg ml−1 treatments accounted for approximately 10% in SM and 20% in CM and WC of the SRB biomass. In SM,
_Desulfobulbus_ biomarkers dominated at the highest ciprofloxacin concentrations (Figure 3). In WC and CC, _Desulfovibrio_ and _Desulfobulbus_ increased in abundance at higher ciprofloxacin
concentrations, but their relative abundance was very similar to _Desulfobacter_ biomarkers. The ratio of GN (monoenoic fatty acids) to GP biomarkers (branched fatty acids) indicated that GP
were more abundant in controls and the low ciprofloxacin concentration treatments. At higher ciprofloxacin concentrations, GN were the predominant group. Ratios of GN/GP ranged from 0.01 to
0.02 in all control sediments, but increased up to 1.4 at the highest ciprofloxacin concentrations in all sediments. Two biomarkers of stress were evaluated in all the microbial
communities: 17:0 cy per precursor and sat/unsat PLFAs. The ratio of 17:0 cy per precursor was <1 in all sediments treated with ciprofloxacin, with the exception of CC at 0.02 μg ml−1,
where the ratio was close to 3. All control microcosms had 17 cy per precursor ratios above 2, and as high as 4 for the control in CC sediment. Control microcosms had sat/unsat ratio above
10 and as high as 20 (SM), while all ciprofloxacin-treated microcosms had sat/unsat ratios below 5 (Figure 4). DISCUSSION The sorption coefficients obtained in this study (log
_K_d=2.90–4.27) were comparable to those obtained by other groups in batch experiments for ciprofloxacin and similar compounds. Log _K_d coefficients ranged from 2.69 to 3.75 for
enrofloxacin (with a similar chemical structure to ciprofloxacin) in a variety of soils (Seremet and MacKay, 2003). A log _K_d of 2.62 was reported for ciprofloxacin sorption in sewage
sludge with pH of 6.5 (Giger et al., 2003), and a log _K_d of 4.29 was reported for similar sludge with pH 7.5–8.4 (Golet et al., 2003). In our sediments, higher pH values were associated
with lower sorption coefficients (data not shown). Sorption is expected to be pH sensitive because ciprofloxacin speciation is pH dependent (Lin et al., 2004). A review reported log _K_oc
values for fluoroquinolones on sewage sludge and soils (loamy sand, clay and loam) ranging from 3.05 to 5.88 (Rao et al., 1993), similar to our obtained values of 4.51–5.82. In our
experiments, log _K_oc was positively correlated with clay content (_r_2=0.79), but not correlated to organic carbon content. This lack of correlation has been reported by other groups,
indicating the limitations of carbon-normalized coefficients in explaining sorption of antibiotics in soils (Tolls, 2001). Because ciprofloxacin is a broad-spectrum antibiotic, we expected
reduced biomass and numbers of PLFAs in the exposed sediment microbial communities. We also expected selective effects of the antibiotic; in clinical trials ciprofloxacin showed higher
bactericidal activity against GN than GP bacteria (Cunha et al., 1997; Dalhoff and Schmitz, 2003; Berlanga et al., 2004). In contrast, both biomass and PLFA numbers increased in
ciprofloxacin-treated microcosms, and GN biomarkers were higher than GP biomarkers in treated microcosm. The lack of antibiotic activity we observed in anaerobic sediment microcosms is
consistent with reports of delayed, diminished, or lack of antibiotic activity against infections involving anaerobic bacteria (Lewin et al., 1991; Zabinski et al., 1995; Morrissey and
George, 2001; Stein and Goldstein, 2006). All these studies have been performed on clinical isolates and pure cultures, but there is no reason to expect that soil microbes would behave
differently. Our results suggest that ciprofloxacin has a significant decrease or complete loss of antibiotic activity in anaerobic sediments. Substantial increases in microbial biomass
observed in CC and WC sediments, may be due to the use of ciprofloxacin as a carbon source by some of the sediment bacteria. However, confirmation of ciprofloxacin biodegradation would
require measurement of chemical disappearance, which was beyond the scope of this study. In both sediments, biomass appeared to have leveled off or slightly declined at the highest
ciprofloxacin concentrations. In contrast, microbial biomass was insensitive to ciprofloxacin concentration in SM, the sediment with the greatest sorption capacity. Another indicator of
higher carbon availability in ciprofloxacin-treated sediments is the 17 cy per precursor ratio. The 17 cy per precursor ratio was two to three times higher in controls than in treated
microcosms in all sediments. Increased ratios of 17 cy per precursor have been linked to starvation (Kieft et al., 1994, 1997; Bossio et al., 1998), providing additional support for the
hypothesis that more carbon was available to the enriched microbial populations (those showing up in PLFA analysis) (in the higher than in the lower ciprofloxacin treatments). Another
supporting result is the elevated sat/unsat ratios observed in control and 0.2 μg ml−1 CC treatment. Increased sat/unsat ratios have been observed in microbial communities under starvation
stress (Bossio et al., 1998), but also in those exposed to solvents (Ramos et al., 2002), and after entering stationary phase (Kieft et al., 1994, 1997). The SRB biomarkers used in this
study targeted _Desulfobacter_, _Desulfobulbus_ and _Desulfovibrio_, all are GN SRB grouped in the δ-Proteobacteria (Macalady et al., 2000; Rabus et al., 2006). Despite their grouping, these
genera differ in their metabolic versatility when it comes to carbon substrates. _Desulfovibrio_ are part of the _Desulfovibrionaceae_ family and are characterized by the incomplete
oxidation of carbon substrates with the production of acetate (Rabus et al., 2006). _Desulfovibrio_, originally considered metabolically specialized, has been recently shown to be able to
utilize H2, formate, lactate and crude oil components. _Desulfobulbus_, part of the _Desulfobulbaceae_, are complete acetate oxidizers with a very versatile carbon metabolism (Rabus et al.,
2006). _Desulfobacter_ belong to the _Desulfobacteriaceae_ family, are incomplete acetate oxidizers with a limited carbon metabolism that uses acetate as a carbon source almost exclusively
(Rabus et al., 2006). The differences in relative abundance of each biomarker group in the treated microcosms may be related to differences in carbon metabolic versatility. If this is
correct, _Desulfobacter_ biomarkers were constantly present at steady relative abundances both in controls and ciprofloxacin treatments reflecting the microorganisms’ ability to use acetate,
a common metabolite of many anaerobic metabolic pathways. _Desulfobulbus_’ metabolic versatility may have allowed it to more easily assimilate complex carbon substrates available, including
ciprofloxacin. _Desulfovibrio_ did not immediately appear to respond to higher carbon availability and this may be due to its lower abundance in the sediments. The dominating presence of
_Desulfobacter_ biomarkers in all controls, with a low abundance of _Desulfobulbus_ and no evidence of _Desulfovibrio_ is consistent with SRB distribution reported previously in salt
marshes. _Desulfobacteriaceae_ associated sequences accounted for over 80% of the recovered sequences, while less than 1% out of 1650 sequences were assigned to _Desulfovibrio_ in a salt
marsh study (Klepac-Ceraj et al., 2004). The dominance of _Desulfobacteriaceae_ in salt marsh communities has also been confirmed by other groups (Devereux et al., 1996; Rooney-Varga et al.,
1998). _Desulfovibrio_, _Desulfobacter_ and _Desulfobulbus_ accounted for up to 30% of the bacterial RNA present in sediment influences by _Spartina alterniflora_ in marsh sediments (Hines
et al., 1999), and of this, _Desulfobacteriaceae_ accounted for approximately 25% of total bacterial RNA with minimal contribution from _Desulfobulbus_ and _Desulfovibrio_ (1%–2% of total
bacterial RNA). Overall, the SRB biomarkers indicate a shift from an acetate-utilizing microbial community (dominated by _Desulfobacter_), to a community capable of using a more complex
carbon substrates. There are limited studies of the selective effects of ciprofloxacin on sulfate reducers. However, published reports indicate resistance in some clinical _Desulfovibrio_
(McDougall et al., 1997), and no change in gut SRB number in rats treated with ciprofloxacin (Ohge et al., 2003). Our results suggest that ciprofloxacin had no apparent negative effects on
SRB biomass or richness (as measured by PLFA) in anaerobic sediments. Changes in microbial community composition indicated that the magnitude of ciprofloxacin effects was inversely
correlated with the degree of sorption of ciprofloxacin to the sediments. Ciprofloxacin, and fluoroquinolones, in general have been shown to strongly sorb to soils and sediments,
particularly clays (Nowara et al., 1997; Giger et al., 2003; Cardoza et al., 2005), thus potentially reducing the bioavailability and antibiotic potency of ciprofloxacin (Halling-Sørensen et
al., 2003). These findings are consistent with our results, where the magnitude of the microbial community shift was smaller in SM, in which ciprofloxacin had the highest sorption
potential, than in other sediments. However, the differences between controls and treatments demonstrate that even with sorption, the antibiotic is capable of modifying the microbial
community. We have shown that ciprofloxacin is capable of modifying microbial community composition at concentrations as low as 20 μg ml−1 in anaerobic sediments. GN bacteria, including
_Desulfovibrio_, _Desulfobulbus_ and _Desulfobacter_, appeared more resilient to the effects of ciprofloxacin than did GP bacteria. Effects were evident despite the fact that the
concentration actually available to microorganisms was likely even lower due to substantial sorption to sediments (an estimated 80%–90% sorbed). Despite the fact that ciprofloxacin is a
wide-spectrum antibiotic, its impact on sediment microbial communities was selective and favored SRB. As human impacts on the environment increase with expanding populations and growing use
of medications, it is crucial to understand whether changes such as those observed in our study are evident in nature and what are the consequences for ecosystem microbial processes of these
changes. REFERENCES * Berlanga M, Montero MT, Hernandez-Borrell J, Vinas M . (2004). Influence of the cell wall on ciprofloxacin susceptibility in selected wild-type Gram-negative and
Gram-positive bacteria. _Int J Antimicrob Agents_ 23: 627–630. Article CAS Google Scholar * Bila DM, Dezotti M . (2003). Pharmaceutical drugs in the environment. _Quimica Nova_ 26:
523–530. Article CAS Google Scholar * Bossio DA, Scow KM . (1998). Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization
patterns. _Microb Ecol_ 35: 265–278. Article CAS Google Scholar * Bossio DA, Scow KM, Gunapala N, Graham KJ . (1998). Determinants of soil microbial communities: effects of agricultural
management, season, and soil type on phospholipid fatty acid profiles. _Microb Ecol_ 36: 1–12. Article CAS Google Scholar * Boyd GR, Reemtsma H, Grimm DA, Mitra S . (2003).
Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada. _Sci Total Environ_ 311: 135–149. Article CAS Google Scholar *
Cardoza LA, Knapp CW, Larive CK, Belden JB, Lydy M, Graham DW . (2005). Factors affecting the fate of ciprofloxacin in aquatic field systems. _Water Air Soil Pollution_ 161: 383–398. Article
CAS Google Scholar * Córdova-Kreylos AL, Cao YP, Green PG, Hwang HM, Kuivila KM, LaMontagne MG _et al_. (2006). Diversity, composition, and geographical distribution of microbial
communities in california salt marsh sediments. _Appl Environ Microbiol_ 72: 3357–3366. Article Google Scholar * Cunha BA, Qadri SMH, Ueno Y, Walters EA, Domenico P . (1997). Antibacterial
activity of trovafloxacin against nosocomial Gram-positive and Gram-negative isolates. _J Antimicrob Chemother_ 39: 29–34. Article CAS Google Scholar * Dalhoff A, Schmitz FJ . (2003).
_In vitro_ antibacterial activity and phamacodynamics of new quinolones. _Eur J Clin Microbiol Infect Dis_ 22: 203–221. CAS PubMed Google Scholar * Datamonitor (2004). Fluoroquinolones,
Established Products Drive Market Growth. [On-line] URL http://www.piribo.com/publications/prescription_drugs/DAT063.html. * Daughton CG . (2000). Pharmaceuticals in the environment:
Overarching issues and concerns. _Abs Papers ACS_ 219: U622. Google Scholar * Daughton CG, Ternes TA . (1999). Pharmaceuticals and personal care products in the environment: agents of
subtle change? _Environ Health Perspect_ 107: 907–938. Article CAS Google Scholar * Devereux R, Hines ME, Stahl DA . (1996). S cycling: Characterization of natural communities of
sulfate-reducing bacteria by 16S rRNA sequence comparisons. _Microb Ecol_ 32: 283–292. Article CAS Google Scholar * Eshel G, Levy GJ, Mingelgrin U, Singer MJ . (2004). Critical evaluation
of the use of laser diffraction for particle-size distribution analysis. _SSSA J_ 68: 736–743. Article CAS Google Scholar * Fernandes PB . (1988). Mode of action, and _in vitro_ and _in
vivo_ activities of the fluoroquinolones. _J Clin Pharmacol_ 28: 156–168. Article CAS Google Scholar * Figueroa RA, Leonard A, Mackay AA . (2004). Modeling tetracycline antibiotic
sorption to clays. _Environ Sci Technol_ 38: 476–483. Article CAS Google Scholar * Frostegård A, Tunlid A, Bååth E . (1991). Microbial biomass measured as total lipid phosphate in soils
of different organic content. _J Microbiol Methods_ 14: 151–163. Article Google Scholar * Giger W, Alder AC, Golet EM, Kohler HPE, McArdell CS, Molnar E _et al_. (2003). Occurrence and
fate of antibiotics as trace contaminants in wastewaters, sewage sludges, and surface waters. _Chimia_ 57: 485–491. Article CAS Google Scholar * Golet EM, Xifra I, Siegrist H, Alder AC,
Giger W . (2003). Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil. _Environ Sci Technol_ 37: 3243–3249. Article CAS Google Scholar * Gu C,
Karthikeyan KG . (2005). Sorption of the antimicrobial ciprofloxacin to aluminum and iron hydrous oxides. _Environ Sci Technol_ 39: 9166–9173. Article CAS Google Scholar *
Halling-Sorensen B . (2000). Algal toxicity of antibacterial agents used in intensive farming. _Chemosphere_ 40: 731–739. Article CAS Google Scholar * Halling-Sørensen B, Sengelov G,
Ingerslev F, Jensen LB . (2003). Reduced antimicrobial potencies of oxytetracycline, tylosin, sulfadiazin, streptomycin, ciprofloxacin, and olaquindox due to environmental processes. _Arch
Environ Contam Toxicol_ 44: 7–16. Article Google Scholar * Halling-Sørensen B, Nielsen SN, Lanzky PF, Ingerslev F, Lutzhoft HCH, Jorgensen SE . (1998). Occurrence, fate and effects of
pharmaceutical substances in the environment—A review. _Chemosphere_ 36: 357–394. Article Google Scholar * Hines ME, Evans RS, Genthner BRS, Willis SG, Friedman S, Rooney-Varga JN _et al_.
(1999). Molecular phylogenetic and biogeochemical studies of sulfate-reducing bacteria in the rhizosphere of _Spartina alterniflora_. _Appl Environ Microbiol_ 65: 2209–2216. CAS PubMed
PubMed Central Google Scholar * Hooper DC . (1999). Mode of action of fluoroquinolones. _Drugs_ 58: 6–10. Article CAS Google Scholar * Hwang HM, Green PG, Young TM . (2006a). Tidal salt
marsh sediment in California, USA. Part 1: occurrence and sources of organic contaminants. _Chemosphere_ 64: 1383–1392. Article CAS Google Scholar * Hwang HM, Green PG, Higashi RM, Young
TM . (2006b). Tidal salt marsh sediment in California, USA. Part 2: occurrence and anthropogenic input of trace metals. _Chemosphere_ 64: 1899–1909. Article CAS Google Scholar * Kieft
TL, Ringelberg DB, White DC . (1994). Changes in ester-linked phospholipid fatty-acid profiles of subsurface bacteria during starvation and desiccation in a porous-medium. _Appl Environ
Microbiol_ 60: 3292–3299. CAS PubMed PubMed Central Google Scholar * Kieft TL, Wilch E, Oconnor K, Ringelberg DB, White DC . (1997). Survival and phospholipid fatty acid profiles of
surface and subsurface bacteria in natural sediment microcosms. _Appl Environ Microbiol_ 63: 1531–1542. CAS PubMed PubMed Central Google Scholar * Klepac-Ceraj V, Bahr M, Crump BC, Teske
AP, Hobbie JE, Polz MF . (2004). High overall diversity and dominance of microdiverse relationships in salt marsh sulphate-reducing bacteria. _Environ Microbiol_ 6: 686–698. Article CAS
Google Scholar * Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB _et al_. (2002). Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams,
1999–2000: a national reconnaissance. _Environ Sci Technol_ 36: 1202–1211. Article CAS Google Scholar * Lewin CS, Morrissey I, Smith JT . (1991). The mode of action of quinolones—the
paradox in activity of low and high-concentrations and activity in the anaerobic environment. _Eur J Clin Microbiol Infect Dis_ 10: 240–248. Article CAS Google Scholar * Lin CE, Deng YJ,
Liao WS, Sun SW, Lin WY, Chen CC . (2004). Electrophoretic behavior and pK(a) determination of quinolones with a piperazinyl substituent by capillary zone electrophoresis. _J Chromatogr A_
1051: 283–290. Article CAS Google Scholar * Macalady JL, Mack EE, Nelson DC, Scow KM . (2000). Sediment microbial community structure and mercury methylation in mercury-polluted Clear
Lake, California. _Appl Environ Microbiol_ 66: 1479–1488. Article CAS Google Scholar * MacKay AA, Figueroa RA . (2004). Mechanisms of antibiotic sorption to agricultural soils. _Abs
Papers ACS_ 228: U641. Google Scholar * MacKay AA, Figueroa RA, Seremet DE, Vasudevan D . (2004). Sorption of antibiotics to soils. _Abs Papers ACS_ 228: U634. Google Scholar * McDougall
R, Robson J, Paterson D, Tee W . (1997). Bacteremia caused by a recently described novel _Desulfovibrio_ species. _J Clin Microbiol_ 35: 1805–1808. CAS PubMed PubMed Central Google
Scholar * Miao XS, Bishay F, Chen M, Metcalfe CD . (2004). Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. _Environ Sci Technol_ 38: 3533–3541.
Article CAS Google Scholar * Morrissey I, George JT . (2001). Measurement of the bactericidal activity of fluoroquinolones against _Streptococcus pneumoniae_ using the bactericidal index
method. _Int J Antimicrob Agents_ 17: 33–37. Article CAS Google Scholar * Nowara A, Burhenne J, Spiteller M . (1997). Binding of fluoroquinolone carboxylic acid derivatives to clay
minerals. _J Agric Food Chem_ 45: 1459–1463. Article CAS Google Scholar * Ohge H, Furne JK, Springfield J, Sueda T, Madoff RD, Levitt MD . (2003). The effect of antibiotics and bismuth on
fecal hydrogen sulfide and sulfate-reducing bacteria in the rat. _FEMS Microbiol Lett_ 228: 137–142. Article CAS Google Scholar * Rabus R, Hansen T, Widdel F . (2006). Dissimilatory
sulfate- and sulfur-reducing prokaryotes. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H and Stackebrandt E (eds). _The Prokaryotes: Ecophysiology and Biochemistry_. Springer New York:
New York, NY, USA. Google Scholar * Ramos JL, Duque E, Gallegos MT, Godoy P, Ramos-Gonzalez MI, Rojas A _et al_. (2002). Mechanisms of solvent tolerance in Gram-negative bacteria. _Annu Rev
Microbiol_ 56: 743–768. Article CAS Google Scholar * Rao PSC, Bellin CA, Brusseau ML . (1993). Coupling biodegradation of organic chemicals to sorption and transport in soils and
aquifers: paradigms and paradoxes. In: Chang FH (ed). _Sorption and Degradation of Pesticides and Organic Chemicals in Soil_. Soil Sci Soc Amer: Madison, WI, USA, pp 1–26. Google Scholar *
Renew JE, Huang CH . (2004). Simultaneous determination of fluoroquinolone, sulfonamide, and trimethoprim antibiotics in wastewater using tandem solid phase extraction and liquid
chromatography-electrospray mass spectrometry. _J Chromatogr A_ 1042: 113–121. Article CAS Google Scholar * Rooney-Varga JN, Genthner BRS, Devereux R, Willis SG, Friedman SD, Hines ME .
(1998). Phylogenetic and physiological diversity of sulphate-reducing bacteria isolated from a salt marsh sediment. _Syst Appl Microbiol_ 21: 557–568. Article CAS Google Scholar * Seremet
DE, MacKay AA . (2003). Sorption of ciprofloxacin and its substructures to pure clay. _Abs Papers ACS_ 226: U488. Google Scholar * Sparks DL (ed). (1996). _Methods of Soil Analysis—Part 3:
Chemical Methods. Soil Science Society of America Book Series, no. 5_. Wisconsin: Madison. Google Scholar * Stein GE, Goldstein EJC . (2006). Fluoroquinolones and anaerobes. _Clin Infect
Dis_ 42: 1598–1607. Article CAS Google Scholar * Tolls J . (2001). Sorption of veterinary pharmaceuticals in soils: a review. _Environ Sci Technol_ 35: 3397–3406. Article CAS Google
Scholar * White DC, Davis WM, Nickels JS, King JD, Bobbie RJ . (1979). Determination of the sedimentary microbial biomass by extractable lipid phosphate. _Oecologia_ 40: 51–62. Article CAS
Google Scholar * Wilson BA, Smith VH, Denoyelles F, Larive CK . (2003). Effects of three pharmaceutical and personal care products on natural freshwater algal assemblages. _Environ Sci
Technol_ 37: 1713–1719. Article CAS Google Scholar * Zabinski RA, Walker KJ, Larsson AJ, Moody JA, Kaatz GW, Rotschafer JC . (1995). Effect of aerobic and anaerobic environments on
antistaphylococcal activities of 5 fluoroquinolones. _Antimicrob Agents Chemother_ 39: 507–512. Article CAS Google Scholar * Zuccato E, Calamari D, Natangelo M, Fanelli R . (2000).
Presence of therapeutic drugs in the environment. _Lancet_ 355: 1789–1790. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This research was supported in part by the
CONACyT and UCMexus programs and UC Toxic Substances Research and Teaching Program. Additional support was provided by a grant from the US Environmental Protection Agency's Science to
Achieve Results (STAR) Estuarine and Great Lakes (EaGLe) Coastal Initiative through funding to the Pacific Estuarine Ecosystem Indicator Research (PEEIR) Consortium, US EPA Agreement
_EPA/R-82867601_ and by Grant number 5 P42 ES04699 from the National Institute of Environmental Health Sciences, NIH. Its contents are solely our responsibility and do not necessarily
represent the official views of the NIEHS, NIH. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Land, Air and Water Resources, University of California, Davis, CA, USA Ana Lucía
Córdova-Kreylos & Kate M Scow Authors * Ana Lucía Córdova-Kreylos View author publications You can also search for this author inPubMed Google Scholar * Kate M Scow View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Ana Lucía Córdova-Kreylos. RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Córdova-Kreylos, A., Scow, K. Effects of ciprofloxacin on salt marsh sediment microbial communities. _ISME J_ 1, 585–595 (2007).
https://doi.org/10.1038/ismej.2007.71 Download citation * Received: 11 May 2007 * Revised: 23 July 2007 * Accepted: 23 July 2007 * Published: 06 September 2007 * Issue Date: November 2007 *
DOI: https://doi.org/10.1038/ismej.2007.71 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * ciprofloxacin * salt marsh * PLFA * sorption * sulfate
reducers