Local adaptation for body color in drosophila americana
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Pigmentation is one of the most variable traits within and between _Drosophila_ species. Much of this diversity appears to be adaptive, with environmental factors often invoked as
selective forces. Here, we describe the geographic structure of pigmentation in _Drosophila americana_ and evaluate the hypothesis that it is a locally adapted trait. Body pigmentation was
quantified using digital images and spectrometry in up to 10 flies from each of 93 isofemale lines collected from 17 locations across the United States and found to correlate most strongly
with longitude. Sequence variation at putatively neutral loci showed no evidence of population structure and was inconsistent with an isolation-by-distance model, suggesting that the
pigmentation cline exists despite extensive gene flow throughout the species range, and is most likely the product of natural selection. In all other _Drosophila_ species examined to date,
dark pigmentation is associated with arid habitats; however, in _D. americana_, the darkest flies were collected from the most humid regions. To investigate this relationship further, we
examined desiccation resistance attributable to an allele that darkens pigmentation in _D. americana_. We found no significant effect of pigmentation on desiccation resistance in this
experiment, suggesting that pigmentation and desiccation resistance are not unequivocally linked in all _Drosophila_ species. SIMILAR CONTENT BEING VIEWED BY OTHERS DROSOPHILIDS WITH DARKER
CUTICLE HAVE HIGHER BODY TEMPERATURE UNDER LIGHT Article Open access 02 March 2023 RAPID SEASONAL CHANGES IN PHENOTYPES IN A WILD _DROSOPHILA_ POPULATION Article Open access 19 December 2023
ACCELERATION OF _DROSOPHILA SUBOBSCURA_ EVOLUTIONARY RESPONSE TO GLOBAL WARMING IN EUROPE Article 13 September 2024 INTRODUCTION Clinal variation, in which the average value of a trait
changes gradually over a geographic area, can be caused by either neutral or non-neutral evolutionary processes (reviewed by Kawecki and Ebert, 2004). For example, the neutral process of
genetic drift can generate a cline through spurious correlations between physical locations and segregating polymorphisms. Limited migration between populations (especially when migration
rates are correlated with geographic distance) promotes cline formation. Alternatively, natural selection can generate a cline when graded selection favors different genotypes in different
geographic regions. In these cases, the balance between selection and gene flow results in a cline, with gene flow acting as a homogenizing force among populations and opposing phenotypic
divergence. Phenotypic plasticity can also create clines in the wild; however, phenotypic differences among populations for plastic traits disappear when individuals are reared in a common
environment (for example, Maherali et al., 2002). That is, clines generated directly by the environment do not necessarily involve genetic differentiation. In animals, clinal variation is
often observed for body color. For example, in humans, skin color is darkest at the equator, with decreasing melanin in populations located toward the poles (Jablonski and Chaplin, 2000); in
deer mice, coat color varies across Florida and Alabama, with the lightest phenotypes found closest to the Gulf of Mexico (Mullen and Hoekstra, 2008); and in the flat periwinkle snail,
shell color varies in the Gulf of Maine, with the darkest shells found in the most northern, coolest waters (Phifer-Rixey et al., 2008). Each of these clines appears to be adaptive, with
selection pressures, including ultraviolet penetration, camouflage and thermoregulation, respectively. In _Drosophila_, pigmentation clines have been reported for _Drosophila melanogaster_
(for example, David et al., 1985; Pool and Aquadro, 2007; Parkash et al., 2008), _D. simulans_ (Capy et al., 1988), the _D. dunni_ species subgroup (Hollocher et al., 2000; Brisson et al.,
2005) and other _Drosophila_ species (reviewed by Rajpurohit et al., 2008). These clines correlate with both geographic (that is, latitude, altitude) and climatic (that is, temperature,
humidity) factors. Laboratory studies in _D. melanogaster_ and _D. polymorpha_ show differences in desiccation resistance between color morphs (Kalmus, 1941; Brisson et al., 2005; Rajpurohit
et al., 2008; Parkash et al., 2009a, 2009b), whereas studies in other insects show an effect of pigmentation on thermoregulation (for example, Watt, 1969; Brakefield and Willmer, 1985).
_Drosophila_ pigmentation is also known to be a plastic trait affected by environmental factors, such as food and temperature (for example, Gibert et al., 2007). This study examines the
geographic distribution of body color in _D. americana_, a member of the _virilis_ species group. The ancestor of _D. americana_ colonized North America at least three million years ago and
the species appears to have maintained a relatively stable, large effective population size since that time: patterns of codon usage in _D. americana_ are more consistent with a theoretical
population genetic ‘equilibrium’ than they are in the more commonly studied _D. melanogaster_ (Maside et al., 2004; Haddrill et al., 2005). Consistent with this observation, previous studies
of _D. americana_ suggest extensive gene flow among populations (McAllister, 2002, 2003; Vieira et al., 2003; McAllister and Evans, 2006; Schäfer et al., 2006; Morales-Hojas et al., 2008).
Despite these signs of genetic homogeneity, however, ‘a yellowish western group and a blackish eastern group’ have been reported within this species (Throckmorton, 1982, p 239). These
‘western forms’ were collected primarily from Kansas, Nebraska, South Dakota and Montana (Hsu, 1951). _D. novamexicana_, the closest relative of _D. americana_ (Caletka and McAllister, 2004;
Morales-Hojas et al., 2008), has even lighter and more yellow pigmentation than the western strains of _D. americana_, and has been collected from Arizona, Colorado, Utah and New Mexico
(Throckmorton, 1982, p 239), suggesting a trans-species pigmentation cline that extends longitudinally across the United States. Here, we provide the first quantitative description of the
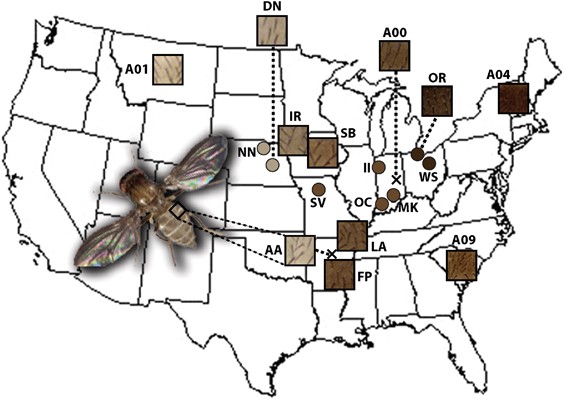
pigmentation cline in _D. americana_ by measuring body color in 93 isofemale lines collected from 17 sites that span much of the latitudinal and longitudinal ranges of _D. americana_. Two
different methods for quantifying pigmentation were used, one provides visual documentation and the other allows high-throughput scoring of live flies. Pigmentation differences among lines
and among collection sites are shown to correlate with the longitude, which in turn correlates with the relative humidity. Patterns of sequence variation suggest extensive gene flow
throughout the species range (consistent with previous studies) and reject an isolation-by-distance model of cline formation. We explore the hypothesis that differences in relative humidity
among collection sites promote cline formation by testing for an effect of _D. americana_ pigmentation alleles (that have been introgressed into _D. novamexicana_) on desiccation resistance.
In contrast to studies of other Drosophila species (for example, Brisson et al., 2005; Parkash et al., 2009a, 2009b), we find no effect of pigmentation on desiccation resistance. We
conclude by comparing these results with pigmentation clines observed in other _Drosophila_ species. MATERIALS AND METHODS FLY STRAINS Two different strategies were used to measure
pigmentation in _D. americana_. In ‘dataset A’, 13 isofemale lines, derived from 11 broadly distributed geographic locations in the central and eastern United States, were analyzed to
provide a species-wide assessment of variability (Supplementary Table 1). Four of these lines, which were obtained from the Drosophila Species Stock Center (Tucson, AZ, USA), were
established ∼50 years ago from a single female fly captured at different collection sites. The remaining nine lines were established from female flies collected between 1999 and 2003 at
seven other locations. With the exception of one site (Duncan, NE, USA), each of the collection sites included in dataset A is represented by only a single isofemale line. These lines
capture the breadth of pigment variation over the geographic range of the species. ‘Dataset B’ contains a deeper sampling of fewer sites (that is, 80 isofemale lines from eight different
locations), with lines established from flies collected in June and September of 2007 (Supplementary Table 1). Collection sites in dataset B form a coarse longitudinal transect extending
between 82° 98′W longitude and bounded by 38° 43′N latitude. Isofemale lines from localities near the eastern and western extremes of the transect (OR and DN, respectively) are included in
both datasets; however, different isofemale lines from these collection sites are used in datasets A and B. All fly stocks were maintained on standard yeast–glucose medium at 20–22 °C.
Before pigmentation scoring, three male and three female flies were placed into a vial and their offspring raised at 20 °C. (Controlling the number of parents in each vial resulted in a
similar larval density among genotypes.) Flies were collected within 3 days of eclosion and aged 1 week to allow body color to stabilize. All isofemale lines within dataset A or dataset B
were reared simultaneously, under identical conditions (that is, light, humidity, temperature, batch of media) to minimize the effect of environmental differences among genotypes.
QUANTIFYING PIGMENTATION For dataset A, dorsal abdominal pigmentation of each isofemale line was measured in five male and five female flies (aged 7–10 days) that had been placed in a 10:1
ethanol:glycerol mixture and stored at room temperature for 1 h to 1 month. The storage time of each individual was variable within each line and did not differ systematically among lines.
We found that abdominal pigmentation is visually stable over this time window under these conditions. The dorsal abdominal cuticle was dissected from each fly, all underlying tissue was
removed and the single layer of adult cuticle was mounted in Hoyer's solution. All mounted cuticles were imaged using a Scion 1394 (Frederick, MD, USA) camera under constant lighting
conditions. Body color was quantified for each fly by using Image J (NIH, Bethesda, MD, USA) to calculate the average median pixel intensity of 20 randomly selected (and non-overlapping)
regions in gray-scale images of the dorsal abdominal cuticle from segments A3, A4 and A5, using a measurement scale that ranged from 0 (black) to 255 (white) (Supplementary Figure 1). The
mean and median coefficients of variation for individual flies were both 6%. A subset of samples was also analyzed using color images and found to provide similar discrimination among
phenotypes to their gray-scale counterparts. For dataset B, dorsal abdominal pigmentation of each isofemale line was measured in five male and five female flies, aged 7–10 days, using a
custom-built R-series Fiber Optic Reflection Probe with a 50-μm diameter fiber, an LS-1 Tungsten Halogen Light Source and a USB4000 Spectrometer (Ocean Optics Inc., Dunedin, FL, USA). The
reflection probe contained six fiber optic wires that transmitted light to the fly cuticle and a seventh, central, fiber optic wire that transmitted light reflected off the sample to the
spectrometer. The tip of the probe was encased by a custom-built shield constructed by the instrument shop in the chemistry department at the University of Michigan, following the blueprint
described at http://www.lifesci.ucsb.edu/~endler/OceanOpticsList.pdf. This probe shield ensures a constant distance (∼1 cm) and angle (45°) between the fly cuticle and probe among
measurements (Uy and Endler, 2004). The diameter of the probe tip (∼0.7 mm) is approximately half of the anteroposterior length of one _D. americana_ dorsal abdominal segment (that is,
tergite). After calibrating the SpectraSuite Spectroscopy Operating Software (Ocean Optics) with a WS-1 Diffuse Reflection Standard (Ocean Optics), the spectral reflectance of visible light
(ranging from 0 to 100%) was recorded from five non-overlapping regions of dorsal abdominal cuticle (all located within segments A3, A4 and A5) from each fly. All measurements were collected
over 2 consecutive days, with the isofemale lines scored in random order. Reference spectra taken from four dissected and mounted _D. americana_ abdominal cuticles with varying pigmentation
intensities were found to be similar on both days. Light from 610 to 660 nm wavelengths provided the greatest discrimination among the lightest and darkest control cuticles (Supplementary
Figure 2), and custom Perl scripts were used to calculate the average reflectance of light in this range from each reflectance spectrum. In general, replicate measurements from the same fly
were similar (mean and median coefficients of variation were 16 and 13%, respectively); however, extreme outliers were occasionally observed, which most likely resulted from the misalignment
of the probe tip with the fly cuticle. To reduce the impact of these outliers, the median value from each fly (rather than the mean) was used for analysis. DNA SEQUENCE VARIATION AND
POPULATION GENETIC ANALYSIS Genomic DNA was extracted from a single male fly from each isofemale line using the ‘squish prep’ protocol (Gloor et al., 1993). For dataset A, regions from the
following genes were amplified and sequenced in all lines except FP, which died before molecular analysis: _cytochrome b_ (_cytB_, mitochondrial, 619 bp), _transformer_ (_tra_, nuclear, 839
bp), _bazooka_ (_baz_, nuclear, 575 bp), _l(1)G0007_ (nuclear, 513 bp). In dataset B, the 839-bp region from the _tra_ gene was successfully amplified and sequenced in 34 of the 80 isofemale
lines, including at least three lines from each collection site. Sequences of primers used for both amplification and sequencing are available upon request. Sequences were assembled and
aligned using CodonCode Aligner (Dedham, MA, USA) and manually validated by PJW for dataset A and by DCY for dataset B. They are available through Genbank with the following accession
numbers: GU299293–GU299340 (dataset A) and GU248275–GU248308 (dataset B). Seven of the 34 _tra_ sequences from dataset B (WS07.14, DN07.52 × 41, NN07.08, II07.10, OR07.10, SC07.18, MK07.24)
were heterozygous at 1–7 sites and were resolved into two haplotypes (both of which were included in the sequence analysis), using the PHASEv2.1 algorithm (Stephens and Donnelly, 2003)
implemented in DnaSP v5.10.00 (Librado and Rozas, 2009). The following measures of genetic variability were calculated for each gene region using DnaSP v5.10.00 (Librado and Rozas, 2009):
the number of segregating sites (_S_), haplotype diversity (_H_d), nucleotide diversity per site (_π_) and theta per site based on _S_(_θ_). For _tra_ sequences of lines included in dataset
B, we also calculated Fst and Kst (Hudson et al., 1992) and assessed their significance using DnaSP and Arlequin v3.11 (Excoffier et al., 2005), respectively. DnaSP was also used to
calculate the test statistics Tajima's _D_ and Fu and Li's _D_*, and their statistical significance was determined using the distributions provided in the original descriptions of
these statistics (Tajima, 1989; Fu and Li, 1993), as well as using 10 000 coalescent simulations based on summary statistics of the observed samples. Pair-wise genetic distances among all
strains were calculated for each gene using the Tamura–Nei distance model of nucleotide substitutions (Tamura and Nei, 1993), as implemented in MEGA v4.0.2 (Kumar et al., 2004). Sites with
missing data or gaps were excluded from all analyses. STATISTICAL ANALYSES Pigmentation was analyzed primarily using PROC MIXED in SAS v9.1 (Cary, NC, USA), with all models described below
fitted using restricted maximum likelihood. For dataset A, pigmentation measurements were fitted to the following model to test for effects of line and sex: where _Y__ijkl_ is the mean
pigmentation intensity for cuticle region _l_, from individual _k_, of sex _j_, from line _i_. _L_ and _S_ are fixed effects of isofemale line and sex, respectively; _I_ is the random effect
of individual within each sex × line combination; and _e__ijkl_ is the residual error. _Y__ijkl_ was weighted by the area of the cuticle region analyzed, with larger regions weighted more
heavily than smaller regions. For each line (_L__i_) and each sex within each line (_SL__ij_), the least-squares mean and 95% confidence interval were calculated. Least-squares means were
compared among lines using the Tukey's honestly significant difference _post hoc_ test. For dataset B, pigmentation measurements, consisting of a single (median) pigmentation score per
fly, were fitted to the following model: where _Y__ijkl_ is the pigmentation score for individual _l_, from isofemale line _k_, of sex _j_, from geographic population _i_. _P_ and _S_ are
fixed effects of population and sex, respectively; _L_ is the random effect of line within each population by sex combination; and _e__ijkl_ is the error among pigmentation measures from
individuals derived from the same isofemale line. For each population (_P__i_) and each sex within each population (_SP__ij_), the least-squares mean and 95% confidence interval were
calculated. Least-squares means were compared among populations using Tukey's honestly significant difference _post hoc_ test. To test for geographic trends in pigmentation, we fitted
both datasets to the following model: where _Y__jkl_ is the least-squares mean pigmentation intensity for each line _l_ collected from latitude _j_ and longitude _k_. _T_ and _G_ represent
the continuous covariates of latitude and longitude, respectively. For dataset A, only the intermediate of the three lines from Duncan, NE was used, to avoid over-weighting data from this
location. Male and female flies were analyzed separately for each dataset, because a significant effect of sex was detected (see Results section). To test for evidence of
isolation-by-distance model, we used a Mantel test to compare genetic and geographic distances among lines in dataset A and populations in dataset B. This test was conducted using the
web-based Isolation-by-distance Web Service software v3.15 (Jensen et al., 2005), available at http://ibdws.sdsu.edu/. Geographic distances for this test were measured in kilometers and
calculated based on longitude and latitude of collection sites using the web-based software developed by Dr John Byers (US Arid-Land Agricultural Research Center, USDA-ARS,
http://www.chemical-ecology.net/java/lat-long.htm). This analysis was also performed using geographic distances measured in degrees longitude. Genetic distances were calculated as described
in the DNA sequence variation section above. Mantel tests were performed using both the raw genetic distances and the logarithm of genetic distances. Significance was assessed using 1000
permutations of the genetic and geographic distances, conducted by the Isolation-by-Distance Web Service software. DESICCATION RESISTANCE Interspecific introgression lines were used to test
specifically whether alleles that affect pigmentation have a corresponding effect on desiccation resistance. As described in Wittkopp et al. (2009), lines were constructed by crossing _D.
americana_ female flies to male flies of their lightly pigmented sister species, _D. novamexicana_, and backcrossing the resulting F1 hybrid female flies to _D. novamexicana_ male flies.
Backcrossing was continued for 10 consecutive generations, with a single female heterozygous fly for the _D. americana_ and _D. novamexicana_ alleles of pigmentation genes _tan_ and _ebony_
genes mated to a _D. novamexicana_ male fly in each generation. The introduction of either the _tan_ or _ebony_ genomic region from _D. americana_ into _D. novamexicana_ was sufficient to
cause a visible darkening of pigmentation, with flies carrying _D. americana_ alleles for both quantitative trait loci (QTLs) being visibly darker than those carrying _D. americana_ alleles
for either QTL region alone (Wittkopp et al., 2009). Using these introgression lines, we constructed sex-specific pairs of genotypes with significant differences in pigmentation. The two
male genotypes were both hemizygous for the _D. americana tan_ QTL allele, but differed by the presence or absence of the _D. americana ebony_ QTL allele, resulting in ‘dark’ and ‘light’
pigmentation phenotypes, respectively. Similarly, the two female genotypes were both heterozygous for the _D. americana tan_ QTL allele, but differed by the presence or absence of the _D.
americana ebony_ QTL allele, again, resulting in ‘dark’ and ‘light’ pigmentation phenotypes, respectively. Desiccation resistance was measured by placing 7- to 10-day-old virgin male and
female flies into 5-ml Polystyrene round-bottom vials with mesh caps (BD Falcon, Bedford, MA, USA), which were stored in a 5.7-l plastic snap-lid container (Rubbermaid) with 200 g of
Drierite (8 mesh), sealed with parafilm, and stored at 20 °C. A control container was prepared in the same manner, with the substitution of a moist paper towel for the Drierite. Wired
indoor/outdoor hygrometers (RadioShack) were used to monitor the relative humidity in each container: the desiccant container maintained a relative humidity level of <20% (the minimum
detectable with the hygrometer) throughout the experiment, whereas the control container maintained an average of 85% relative humidity. Each container held 10 replicate vials of female
flies and eight replicate vials of male flies, with each vial containing three ‘light’ and three ‘dark’ flies of the same sex. Beginning 15 h after placing the vials in the box, the number
of dead flies (assessed by lack of visible movement when the vial was tapped) and the pigmentation of each dead fly (light or dark) were recorded every hour until all flies in the
desiccation group died (50 h). Survival curves were compared using a non-parametric log-rank test, which compares the observed numbers of deaths at each time point between samples. RESULTS
To characterize the geographic distribution of body color in _D. americana_, we examined two distinct sets of isofemale lines. The first (‘dataset A’), which contained a single isofemale
line from each of 11 populations that span the known east–west range of _D. americana_ (Throckmorton, 1982), was used to provide an overview of pigmentation differences across the species’
range. The second (‘dataset B’), which contained multiple isofemale lines derived from each of eight populations representing a coarse longitudinal transect through the central region of the
species range, was used to assess body color variation within and between collection sites. The geographically extreme populations from dataset A were not included in dataset B because only
a single isofemale line was available from these sites. Figure 1 and Supplementary Table 1 describe the collection sites and individual isofemale lines in more detail. QUANTITATIVE METRICS
FOR ADULT BODY COLOR IN _DROSOPHILA_ _Drosophila_ pigmentation is typically analyzed using a subjective and arbitrary scoring scale based on visual assessments of pigmentation (for example,
Hollocher et al., 2000; David et al., 2002; Wittkopp et al., 2003b; Brisson et al., 2005). Although these measurements are generally consistent for a single observer under controlled
lighting conditions, discriminating among subtle gradations of body color is challenging for even the most experienced researcher. A preliminary visual assessment of pigmentation among
isofemale lines of _D. americana_ revealed obvious differences between the lightest and darkest lines, with subtle variation in intermediate body colors that we were unable to reliably and
consistently classify by eye. Therefore, we concluded that an objective and quantitative method of pigmentation scoring was essential for describing the geographic distribution of body color
in _D. americana_. Two quantitative methods for scoring _Drosophila_ pigmentation were developed and used in this study. The first method, which was applied to the 13 isofemale lines in
dataset A, involved dissection of dorsal abdominal cuticles from preserved flies (five males and five females per line) followed by imaging and computational analysis of digital images from
each individual cuticle. This method produced semipermanent samples and pictures of isolated body cuticles (Figure 1); however, the dissection, mounting and imaging was labor-intensive and
time-consuming, making it impractical for analyzing multiple individuals from each of the 80 isofemale lines included in dataset B. To overcome this technical hurdle, we adapted a custom
spectrometry system that allowed us to measure rapidly the pigmentation of live (but anesthetized) flies. Using this method, we quantified the pigmentation of 800 flies (five males and five
females from each of 80 lines) for dataset B in only 2 days. THE DISTRIBUTION OF BODY COLOR VARIATION WITHIN _D. AMERICANA_ To test for differences in pigmentation between sexes and among
collection sites, measurements from datasets A and B were fitted separately to linear mixed models (see Materials and methods). Significant differences in pigmentation were observed among
collection sites in both dataset A (F=12.46, _P_<0.0001) and dataset B (F=36.01, _P_<0.0001). _Post hoc_ analysis of these data identified four statistically distinct pigmentation
groups in dataset A and two statistically distinct groups in dataset B (Table 1); all locations within one group showed statistically significant differences in pigmentation from locations
in all other groups. _D. americana_ female flies were found to be slightly, but significantly, lighter in color than _D. americana_ male flies in both datasets (dataset A: F=4.32,
_P_=0.0405; dataset B: F=10.83, _P_<0.0001), although this sexual dimorphism is not visually apparent under a dissecting microscope and has not been recognized previously. Male flies were
5.5% darker than female flies in dataset A and 12.5% darker than female flies in dataset B, which may be partially due to colored tissues underlying the cuticle in dataset B that were
removed for dataset A. To examine the geographic distribution of different pigmentation phenotypes, we fitted the pigmentation measures to a linear model that included the latitude and
longitude of the collection site as covariates. A highly significant effect of longitude was observed for both datasets, whereas the effect of latitude showed no significant effect in either
case (Table 2). Manual inspection of the geographic distribution of pigmentation phenotypes suggests that the longitudinal gradient may actually be nonlinear, with the largest change in
pigmentation occurring near 90° west longitude; however, nonlinear models fit to our data with SAS v9.1 (proc NLIN) and Cfit (Gay et al., 2008) failed to converge. CLINAL VARIATION IS
INCONSISTENT WITH A NEUTRAL ISOLATION-BY-DISTANCE MODEL The observed longitudinal gradient of pigmentation in _D. americana_ may be caused by local adaptation or genetic drift with
geographically limited migration (isolation-by-distance). These two different evolutionary processes can be distinguished by comparing the spatial distribution of pigmentation with the
spatial distribution of genetic variation. Specifically, clines resulting from isolation-by-distance are expected to show a positive correlation between genetic and geographic distances at
neutral loci, whereas clines resulting from natural selection despite ongoing gene flow are not. To distinguish between these hypotheses, we surveyed sequence variation among isofemale lines
in both datasets. For dataset A, regions from the _cytB_, _baz_, _l(1)G0007_ and _tra_ genes were sequenced in 12 of the 13 isofemale lines; no sequences were obtained from the FP isofemale
line because the stock died before molecular genetic analysis. According to Flybase (Drysdale and Consortium, 2008), none of the loci surveyed affects pigmentation. Neutrality tests based
on Tajima's _D_ and Fu and Li's _D_*, both of which compare the observed distribution of polymorphism with a distribution expected under a neutral model, were consistent with
neutrality (Table 3), suggesting that variation of the sequenced loci should reflect gene flow among the populations sampled. A region of sequence from the _tra_ gene obtained from 34
isofemale lines from dataset B, including at least three lines from each collection site, was also consistent with neutrality (Table 3). Furthermore, pair-wise Fst and Kst for sequences from
dataset B showed no significant differences between populations after correcting for multiple tests (Table 4), and there was also no evidence of population subdivision when all populations
in dataset B were considered together (Kst=0.018, _P_=0.20). Finally, Fst and Kst were also not significant for either dataset when sequences were compared between ‘light’ and ‘dark’
pigmentation classes (Table 5). Sequences from both dataset A and dataset B were used separately to test a model of isolation-by-distance by using a Mantel test to compare pair-wise genetic
and geographic distances. In dataset A, we found no significant relationship between genetic and geographic distances, regardless of whether geographic distance was measured in kilometers
(Table 6) or degrees longitude (data not shown). Similarly, sequences from dataset B were also inconsistent with an isolation-by-distance model, regardless of whether the pair-wise
Tamura–Nei genetic distance among isofemale lines or Fst between populations was used to estimate genetic distance or whether kilometers (Table 6) or degrees longitude (data not shown) was
used to measure geographic distance. Lacking clear evidence for genetic differentiation among collection sites, which is consistent with previous studies that also failed to find evidence of
population structure in _D. americana_ using different samples (McAllister, 2003; Vieira et al., 2003; Maside et al., 2004; McAllister and Evans, 2006; Schäfer et al., 2006; Morales-Hojas
et al., 2008), as well as the rejection of an isolation-by-distance model by both datasets, we conclude that the observed clinal variation for pigmentation in _D. americana_ is unlikely to
be the product of genetic drift in distinct populations, but rather is more likely maintained across the species range by natural selection for locally adaptive phenotypes. DIFFERENTIAL
SELECTION FOR DESICCATION RESISTANCE UNLIKELY TO EXPLAIN THE PIGMENTATION CLINE As described above, we found that pigmentation in _D. americana_ correlates much more strongly with longitude
than with latitude (Figures 2a and b and Supplementary Figure 3). Further analysis showed that pigmentation in dataset B also correlates significantly with altitude (Figure 2c), although
this is not surprising given that longitude and altitude are themselves correlated for the collection sites examined (_R_2=0.52). In other _Drosophila_ species, latitude and altitude are the
primary correlates with pigmentation clines (see Discussion). Differences in temperature and relative humidity among collection sites, which presumably affect thermal and desiccation
tolerances, respectively, are the most commonly invoked selective agents for the formation and maintenance of pigmentation clines in _Drosophila_ (reviewed by True, 2003, Wittkopp et al.,
2003a and Rajpurohit et al., 2008), and among all collection sites examined in this study, relative humidity correlates more strongly with longitude (_R_2=0.37) than with latitude
(_R_2=0.08), whereas the opposite is true of temperature—it correlates more strongly with latitude (_R_2=0.96) than longitude (_R_2=0.37). Despite these correlations, no significant direct
correlation was found between pigmentation and temperature or relative humidity in either dataset (Figures 2d and e). Associations between pigmentation and humidity have been reported in at
least seven _Drosophila_ species (Brisson et al., 2005; Rajpurohit et al., 2008; Parkash et al., 2008, 2009b). In all cases, darker flies were collected from less humid environments.
Interestingly, _D. americana_ appears to show the opposite pattern: lighter flies were collected from less humid environments (Figure 2e), suggesting that distinct selective mechanisms may
be operating in _D. americana_. To test the effect of pigmentation on desiccation resistance as specifically as possible, we compared desiccation resistance between sex-specific pairs of
introgression lines that differed dramatically for pigmentation, but minimally for genotype. This experimental strategy minimizes the possibility that correlated variation with no effect on
pigmentation causes differences in desiccation resistance through other physiological mechanisms. As described in the Materials and methods, the introgression lines used for this analysis
contained genetic material from both _D. americana_ and its closest relative, _D. novamexicana_ (Wittkopp et al., 2009), with the dark and light genotypes examined differing only by the
presence or absence, respectively, of the _D. americana_ allele of _ebony_ and surrounding genes. We measured desiccation resistance in each of these pigmentation classes using the same
desiccation tolerance assay that was used to show differences in desiccation resistance between pigmentation classes of other _Drosophila_ species (Brisson et al., 2005; Rajpurohit et al.,
2008; Parkash et al., 2008, 2009a, 2009b). Surprisingly, we found no significant difference in desiccation resistance between light and dark flies of either sex (Figure 3). That is, flies of
the same sex had similar survival times (as measured by a log rank test) under desiccating conditions, regardless of whether they had light or dark pigmentation (males: _χ_2=0.3, degrees of
freedom=1, _P_=0.58; females: _χ_2=1.4, degrees of freedom=1, _P_=0.2). Significant differences in survival time were observed between the sexes, however. Male flies survived longer for
both light (_χ_2=21.2, degrees of freedom=1, _P_=4 × 10−6) and dark (_χ_2=27.4, degrees of freedom=1, _P_=2 × 10−7) phenotypes, which may be caused by sexual dimorphism and/or differences in
their X-chromosome genotypes. Sexual dimorphism for desiccation resistance has been reported in other _Drosophila_ species as well, although female flies typically survive longer than male
flies under desiccating conditions (Brisson et al., 2005; Matzkin et al., 2007). DISCUSSION Pigmentation is one of the most variable traits in the genus _Drosophila_: differences in body
color are common among individuals within a population, among populations of the same species and among closely related species. This study uses two objective methods of scoring
pigmentation, one of which allows for high-throughput analysis, to provide a quantitative description of body color variation among geographic isolates of _D. americana._ A longitudinal
gradient for pigmentation is described, with the lightest body color found in the western extent of the species range. The findings are consistent with previous references to variable
pigmentation between western and eastern flies, which were previously recognized based solely on anecdotal observations. Moreover, this study revealed the existence of a slight sexual
dimorphism characterized by more lightly pigmented female flies. Patterns of _D. americana_ sequence variation (observed in this and previous studies) indicate extensive gene flow among
populations and are inconsistent with the differentially pigmented forms being established by a neutral isolation-by-distance model of evolution. In contrast, Hsu (1952) identified several
chromosomal inversions that differ in frequency between western and eastern populations. An inversion located distally on chromosome 2 contains the _ebony_ locus that contributes to
pigmentation differences (Wittkopp et al., 2009). Thus, the recognition of geographically distinct populations on the basis of chromosomes and pigmentation is not entirely independent. The
distinction between western and eastern populations is, however, not reflected in patterns of sequence variation throughout the genome. The presence of a pigmentation cline in _D.
americana_, despite the homogenizing effects of gene flow, suggests that pigmentation differences observed among collection sites are adaptive and the product of natural selection. (However,
it is also possible that genes affecting pigmentation are linked to genes affecting another trait that is locally adaptive, and pigmentation correlates with longitude as a result of linkage
and clinal selection pressures for this other trait.) Differences in relative humidity exist across the species range that might favor different pigmentation phenotypes in different
locations; however, laboratory assays failed to show any significant difference in desiccation resistance between flies with light and dark pigmentation. Below, we compare these results with
pigmentation clines in other _Drosophila_ species. THE LONGITUDINAL PIGMENTATION CLINE OF _D. AMERICANA_ IS ATYPICAL FOR _DROSOPHILA_ In _D. melanogaster_ populations from multiple
continents, thoracic pigmentation correlates with latitude: flies from higher latitudes have darker pigmentation (Munjal et al., 1997; Parkash and Munjal, 1999; Parkash et al., 2008). Darker
thoracic pigmentation is also characteristic of high-altitude populations from India, which persist in an environment with lower relative humidity than low altitude populations (Parkash and
Munjal, 1999; Parkash et al., 2008). A similar relationship between thoracic pigmentation and relative humidity was observed for seasonal pigmentation variation of _D. melanogaster_ in
montane regions of India (Parkash et al., 2009a). In sub-Saharan Africa, abdominal pigmentation of _D. melanogaster_ correlates with latitude, but correlates even more strongly with altitude
(Pool and Aquadro, 2007). _D. simulans_, a close relative of _D. melanogaster_, has much less variation for body color, yet still shows a weak correlation with latitude for thoracic
pigmentation (Capy et al., 1988). In the _dunni_ species subgroup, a latitudinal cline exists for abdominal pigmentation that includes multiple species and extends from Puerto Rico through
the Lesser Antilles islands in the Caribbean (Heed and Krishnamurthy, 1959; Hollocher et al., 2000). In contrast to studies of _D. melanogaster_, in which the darkest phenotypes are found at
the highest latitudes, the darkest phenotypes in the _dunni_ species group are found closest to the equator. Genetic analysis indicates that pigmentation differences among species in the
_dunni_ group are more likely to have been established by natural selection than through patterns of common ancestry among species (Hollocher et al., 2000). Considering that all of the
previously described pigmentation clines in _Drosophila_ correlate with latitude, the absence of a latitudinal cline and the discovery of a longitudinal pigmentation cline in _D. americana_
are surprising. In _D. melanogaster_, the correlation between pigmentation and latitude appears to be explained largely by differences in altitude; however, we found that this is unlikely to
be the case for _D. americana._ Among the North American sites sampled for this work, altitude shows a similar correlation with both latitude (_R_2=0.49) and longitude (_R_2=0.52), and in
dataset A, which contains more comprehensive sampling of variation within _D. americana_ than dataset B, pigmentation does not correlate significantly with altitude (Figure 2c). (Note that a
significant correlation with altitude was observed for dataset B, however.) _D. AMERICANA_ SHOWS UNIQUE RELATIONSHIPS AMONG PIGMENTATION, RELATIVE HUMIDITY AND DESICCATION RESISTANCE
Relative humidity (or aridity) is one of the most frequently invoked environmental correlates with pigmentation in _Drosophila_, and differences in desiccation resistance between light and
dark pigmentation morphs have been reported for multiple species (reviewed by Rajpurohit et al., 2008, True, 2003 and Wittkopp and Beldade, 2009). For example, in _D. melanogaster_, a
laboratory assay showed that darker flies collected from natural populations survived longer under desiccating conditions (for example, Parkash et al., 2008), with a similar pattern observed
among seasonal morphs (Parkash et al., 2009a). _D. polymorpha_, a close relative of the _dunni_ species group that does not show an obvious pigmentation cline, is enriched for darker
phenotypes in warm, arid open areas in comparison with cooler, more humid covered forests (Brisson et al., 2005). These darker forms of _D. polymorpha_ were found to survive longer than
their lighter counterparts under desiccating conditions in the laboratory. Indeed, darker body pigmentation has been shown to increase desiccation resistance in _D. nepalensis_, _D.
takahashii_, _D. ananassae_, _D. jambulina_ and _D. immigrans_ (Rajpurohit et al., 2008; Parkash et al., 2008, 2009b). This increase in desiccation resistance appears to be caused by a
slower rate of water loss in individuals with greater melanization (Brisson et al., 2005; Rajpurohit et al., 2008). In light of these data, the presence of darker _D. americana_ in more
humid areas is surprising and suggests that the primary selective force promoting the pigmentation cline in _D. americana_ might be different from that in other species. It is also possible
that pigmentation has a different effect on desiccation resistance in different species. Consistent with this latter possibility, we observed no significant difference in desiccation
resistance between light and dark forms of _D. americana_/_D. novamexicana_ introgression lines. Our experiment used virtually the same assay for desiccation resistance as previous studies
(that is, survival time in a desiccating environment); however, its design differed from previous work in two important ways. First, we compared defined genotypes derived from introgression
lines rather than natural isolates or individuals from a segregating F2 (or other recombinant) population. This allowed us to analyze flies that were genetically homogeneous within a
pigmentation class and differed for only a single region of the genome between pigmentation classes, which greatly reduces the possibility that genetic variation affecting traits other than
pigmentation contributes to differences in desiccation resistance. Second, we tested the effects of _D. americana_ pigmentation alleles on desiccation resistance in the genetic background of
its sister species, _D. novamexicana_. The _D. novamexicana_ background and/or interactions between the two different species alleles might have altered the relationship between
pigmentation and desiccation resistance; however, we see no reason to suspect that this is the case. The light pigmentation of _D. novamexicana_ appears to be an extension of the _D.
americana_ longitudinal gradient (Throckmorton, 1982) and the two species retain many shared ancestral polymorphisms (Hilton and Hey, 1996; Morales-Hojas et al., 2008; Wittkopp et al.,
2009). In summary, we conclude that our data do not support the hypothesis that differences in relative humidity among collection sites cause selection for differences in desiccation
resistance that are mediated by differences in body color. That said, our data are also insufficient to disprove such a hypothesis. The standard laboratory assay for desiccation tolerance is
extremely crude: variation in relative humidity among wild populations is much less extreme than the difference between high and low humidity chambers set up in the laboratory and the
phenotypes affected by humidity levels in the wild are likely much more subtle than death. Parkash et al. (2009a) have recently shown that desiccation stress alters mate choice, copulation
duration and fecundity of _D. jambulina_, and we suspect that desiccation effects such as these have a much larger impact on fitness in the wild. ACCESSION CODES ACCESSIONS GENBANK/EMBL/DDBJ
* GU248275–GU248308 (dataset B) * GU299293–GU299340 (dataset A) REFERENCES * Brakefield PM, Willmer PG (1985). The basis of thermal melanism in the ladybird _Adalia bipunctata_: differences
in reflectance and thermal properties between the morphs. _Heredity_ 54: 9–14. Article Google Scholar * Brisson JA, De Toni DC, Duncan I, Templeton AR (2005). Abdominal pigmentation
variation in _Drosophila polymorpha_: geographic variation in the trait, and underlying phylogeography. _Evolution_ 59: 1046–1059. Article CAS PubMed Google Scholar * Caletka BC,
McAllister BF (2004). A genealogical view of chromosomal evolution and species delimitation in the _Drosophila virilis_ species subgroup. _Mol Phylogenet Evol_ 33: 664–670. Article CAS
PubMed Google Scholar * Capy P, David JR, Robertson A (1988). Thoracic trident pigmentation in natural populations of _Drosophila simulans_: a comparison with _D. melanogaster_. _Heredity_
61: 263–268. Article Google Scholar * David JR, Capy P, Payant V, Tsakas S (1985). Thoracic trident pigmentation in _Drosophila melanogaster_: differentiation of geographical populations.
_Génét Sél Evol_ 17: 211–223. Article CAS PubMed PubMed Central Google Scholar * David JR, Gibert P, Petavy G, Moreteau B (2002). Variable modes of inheritance of morphometrical traits
in hybrids between _Drosophila melanogaster_ and _Drosophila simulans_. _Proc Biol Sci_ 269: 127–135. Article CAS PubMed PubMed Central Google Scholar * Drysdale R, Consortium F
(2008). FlyBase: a database for the _Drosophila_ research community. _Methods Mol Biol_ 420: 45–59. Article CAS PubMed Google Scholar * Excoffier L, Laval G, Schneider S (2005). Arlequin
ver. 3.0: an integrated software package for population genetics data analysis. _Evol Bioinform Online_ 1: 47–50. Article CAS Google Scholar * Fu YX, Li WH (1993). Statistical tests of
neutrality of mutations. _Genetics_ 133: 693–709. CAS PubMed PubMed Central Google Scholar * Gay L, Crochet PA, Bell DA, Lenormand T (2008). Comparing genetic and phenotypic clines in
hybrid zones: a window on tension zone models. _Evolution_ 62: 2789–2806. Article PubMed Google Scholar * Gibert JM, Peronnet F, Schlötterer C (2007). Phenotypic plasticity in
_Drosophila_ pigmentation caused by temperature sensitivity of a chromatin regulator network. _PLoS Genet_ 3: e30. Article PubMed PubMed Central Google Scholar * Gloor GB, Preston CR,
Johnson-Schlitz DM, Nassif NA, Phillis RW, Benz WK _et al_. (1993). Type I repressors of P element mobility. _Genetics_ 135: 81–95. CAS PubMed PubMed Central Google Scholar * Haddrill
PR, Thornton KR, Charlesworth B, Andolfatto P (2005). Multilocus patterns of nucleotide variability and the demographic and selection history of _Drosophila melanogaster_ populations.
_Genome Res_ 15: 790–799. Article CAS PubMed PubMed Central Google Scholar * Heed WB, Krishnamurthy NB (1959). Genetic studies on the _cardini_ group of _Drosophila_ in the West Indies.
_Univ Texas Publ_ 5914: 155–179. Google Scholar * Hilton H, Hey J (1996). DNA sequence variation at the period locus reveals the history of species and speciation events in the _Drosophila
virilis_ group. _Genetics_ 144: 1015–1025. CAS PubMed PubMed Central Google Scholar * Hollocher H, Hatcher JL, Dyreson EG (2000). Evolution of abdominal pigmentation differences across
species in the _Drosophila dunni_ subgroup. _Evolution_ 54: 2046–2056. Article CAS PubMed Google Scholar * Hsu TC (1951). Chromosomal variation and evolution in the _virilis_ group of
_Drosophila_. _Thesis_, University of Texas, Austin, TX. * Hsu TC (1952). Chromosomal variation and evolution in the _virilis_ group of _Drosophila_. _Univ Texas Publ_ 5204: 35–72. Google
Scholar * Hudson RR, Slatkin M, Maddison WP (1992). Estimation of levels of gene flow from DNA sequence data. _Genetics_ 132: 583–589. CAS PubMed PubMed Central Google Scholar *
Jablonski NG, Chaplin G (2000). The evolution of human skin coloration. _J Hum Evol_ 39: 57–106. Article CAS PubMed Google Scholar * Jensen JL, Bohonak AJ, Kelley ST (2005). Isolation by
distance, web service. _BMC Genet_ 6: 13. Article PubMed PubMed Central Google Scholar * Kalmus H (1941). The resistance to desiccation of _Drosophila_ mutants affecting body colour.
_Proc Biol Sci_ 130: 185–201. Google Scholar * Kawecki TJ, Ebert D (2004). Conceptual issues in local adaptation. _Ecol Lett_ 7: 1225–1241. Article Google Scholar * Kumar S, Tamura K, Nei
M (2004). MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. _Brief Bioinform_ 5: 150–163. Article CAS PubMed Google Scholar * Librado P,
Rozas J (2009). DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. _Bioinformatics_ 25: 1451–1452. Article CAS PubMed Google Scholar * Maherali H, Williams BL,
Paige KN, Delucia EH (2002). Hydraulic differentiation of _Ponderosa_ pine populations along a climate gradient is not associated with ecotypic divergence. _Funct Ecol_ 16: 510–521. Article
Google Scholar * Maside X, Lee AW, Charlesworth B (2004). Selection on codon usage in _Drosophila americana_. _Curr Biol_ 14: 150–154. Article CAS PubMed Google Scholar * Matzkin LM,
Watts TD, Markow TA (2007). Desiccation resistance in four _Drosophila_ species: sex and population effects. _Fly (Austin)_ 1: 268–273. Article Google Scholar * McAllister BF (2002).
Chromosomal and allelic variation in _Drosophila americana_: selective maintenance of a chromosomal cline. _Genome_ 45: 13–21. Article PubMed Google Scholar * McAllister BF (2003).
Sequence differentiation associated with an inversion on the neo-X chromosome of _Drosophila americana_. _Genetics_ 165: 1317–1328. CAS PubMed PubMed Central Google Scholar * McAllister
BF, Evans AL (2006). Increased nucleotide diversity with transient Y linkage in _Drosophila americana_. _PLoS ONE_ 1: e112. Article PubMed PubMed Central Google Scholar * Morales-Hojas
R, Vieira CP, Vieira J (2008). Inferring the evolutionary history of _Drosophila americana_ and _Drosophila novamexicana_ using a multilocus approach and the influence of chromosomal
rearrangements in single gene analyses. _Mol Ecol_ 17: 2910–2926. Article CAS PubMed Google Scholar * Mullen LM, Hoekstra HE (2008). Natural selection along an environmental gradient: a
classic cline in mouse pigmentation. _Evolution_ 62: 1555–1570. Article CAS PubMed Google Scholar * Munjal AK, Karan D, Gibert P, Moreteau B, Parkash R, David JR (1997). Thoracic trident
pigmentation in _Drosophila melanogaster_: latitudinal and altitudinal clines in Indian populations. _Genet Sel Evol_ 29: 601–610. Article PubMed Central Google Scholar * Parkash R,
Munjal AK (1999). Phenotypic variability of thoracic pigmentation in Indian populations of _Drosophila melanogaster_. _J Zool Syst Evol Res_ 37: 133–140. Article Google Scholar * Parkash
R, Rajpurohit S, Ramniwas S (2008). Changes in body melanisation and desiccation resistance in highland vs lowland populations of _D. melanogaster_. _J Insect Physiol_ 54: 1050–1056. Article
CAS PubMed Google Scholar * Parkash R, Sharma V, Kalra B (2009a). Impact of body melanisation on desiccation resistance in montane populations of _D. melanogaster_: analysis of seasonal
variation. _J Insect Physiol_ 55: 898–908. Article CAS PubMed Google Scholar * Parkash R, Singh S, Ramniwas S (2009b). Seasonal changes in humidity level in the tropics impact body
color polymorphism and desiccation resistance in _Drosophila jambulina_—evidence for melanism–desiccation hypothesis. _J Insect Physiol_ 55: 358–368. Article CAS PubMed Google Scholar *
Phifer-Rixey M, Heckman M, Trussell GC, Schmidt PS (2008). Maintenance of clinal variation for shell colour phenotype in the flat periwinkle _Littorina obtusata_. _J Evol Biol_ 21: 966–978.
Article CAS PubMed Google Scholar * Pool JE, Aquadro CF (2007). The genetic basis of adaptive pigmentation variation in _Drosophila melanogaster_. _Mol Ecol_ 16: 2844–2851. Article
PubMed PubMed Central Google Scholar * Rajpurohit S, Parkash R, Ramniwas S (2008). Body melanization and its adaptive role in thermoregulation and tolerance against desiccating conditions
in drosophilids. _Entomol Res_ 38: 49–60. Article Google Scholar * Schäfer MA, Orsini L, McAllister BF, Schlötterer C (2006). Patterns of microsatellite variation through a transition
zone of a chromosomal cline in _Drosophila americana_. _Heredity_ 97: 291–295. Article PubMed Google Scholar * Stephens M, Donnelly P (2003). A comparison of bayesian methods for
haplotype reconstruction. _Am J Hum Genet_ 73: 1162–1169. Article CAS PubMed PubMed Central Google Scholar * Tajima F (1989). Statistical method for testing the neutral mutation
hypothesis by DNA polymorphism. _Genetics_ 123: 585–595. CAS PubMed PubMed Central Google Scholar * Tamura K, Nei M (1993). Estimation of the number of nucleotide substitutions in the
control region of mitochondrial DNA in humans and chimpanzees. _Mol Biol Evol_ 10: 512–526. CAS PubMed Google Scholar * Throckmorton LH (1982). The _virilis_ species group. In: Ashburner
M, Carson HL, Thompson JN (eds). _The Genetics and Biology of Drosophila_ Vol. 3b. Academic Press: London. pp 227–296. Google Scholar * True JR (2003). Insect melanism: the molecules
matter. _Trends Ecol Evol_ 18: 640–647. Article Google Scholar * Uy JAC, Endler JA (2004). Modification of visual background increases the conspicuousness of golden-collared manakin
displays. _Behav Ecol_ 15: 1003–1010. Article Google Scholar * Vieira CP, Coelho PA, Vieira J (2003). Inferences on the evolutionary history of the _Drosophila americana_ polymorphic X/4
fusion from patterns of polymorphism at the X-linked _paralytic_ and _elav_ genes. _Genetics_ 164: 1459–1469. CAS PubMed PubMed Central Google Scholar * Watt WB (1969). Adaptive
significance of pigment polymorphisms in Colias butterflies, II. Thermoregulation and photoperiodically controlled melanin variation in _Colias eurytheme_. _Proc Natl Acad Sci USA_ 63:
767–774. Article CAS PubMed PubMed Central Google Scholar * Wittkopp PJ, Beldade P (2009). Development and evolution of insect pigmentation: genetic mechanisms and the potential
consequences of pleiotropy. _Semin Cell Dev Biol_ 20: 65–71. Article CAS PubMed Google Scholar * Wittkopp PJ, Carroll SB, Kopp A (2003a). Evolution in black and white: genetic control of
pigment patterns in _Drosophila_. _Trends Genet_ 19: 495–504. Article CAS PubMed Google Scholar * Wittkopp PJ, Stewart EE, Arnold LL, Neidert AH, Haerum BK, Thompson EM _et al_. (2009).
Intraspecific polymorphism to interspecific divergence: genetics of pigmentation in _Drosophila_. _Science_ 326: 540–544. Article CAS PubMed Google Scholar * Wittkopp PJ, Williams BL,
Selegue JE, Carroll SB (2003b). _Drosophila_ pigmentation evolution: divergent genotypes underlying convergent phenotypes. _Proc Natl Acad Sci USA_ 100: 1808–1813. Article CAS PubMed
PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank Emma Stewart, Belinda Haerum, Adam Neidert, Gizem Kalay and Monica Woll for technical assistance; Paulina Mena
and the Tucson Drosophila Species Stock Center for providing strains of _D. americana_ and rearing advice; officials of the US Fish and Wildlife Service for permission to collect within the
National Wildlife Refuge system; Lacey Knowles, Jonathan Gruber, Gizem Kalay, Joseph Coolon and anonymous reviewers for helpful comments on the manuscript; and the Margaret and Herman Sokol
Endowment and National Science Foundation (DEB-0640485, DEB-0420399) for funding. PJW is an Alfred P Sloan Research Fellow. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Ecology and Evolutionary Biology, Ann Arbor, MI, USA P J Wittkopp, G Smith-Winberry & A M Cooley * Department of Molecular, Cellular, and Developmental Biology, University of Michigan,
Ann Arbor, MI, USA P J Wittkopp, L L Arnold, E M Thompson, D C Yuan & Q Song * Department of Biology, University of Iowa, Iowa City, IA, USA B F McAllister Authors * P J Wittkopp View
author publications You can also search for this author inPubMed Google Scholar * G Smith-Winberry View author publications You can also search for this author inPubMed Google Scholar * L L
Arnold View author publications You can also search for this author inPubMed Google Scholar * E M Thompson View author publications You can also search for this author inPubMed Google
Scholar * A M Cooley View author publications You can also search for this author inPubMed Google Scholar * D C Yuan View author publications You can also search for this author inPubMed
Google Scholar * Q Song View author publications You can also search for this author inPubMed Google Scholar * B F McAllister View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to P J Wittkopp. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION
Supplementary Information accompanies the paper on Heredity website SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (PDF 852 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Wittkopp, P., Smith-Winberry, G., Arnold, L. _et al._ Local adaptation for body color in _Drosophila americana_. _Heredity_ 106, 592–602 (2011).
https://doi.org/10.1038/hdy.2010.90 Download citation * Received: 13 April 2010 * Revised: 03 June 2010 * Accepted: 08 June 2010 * Published: 07 July 2010 * Issue Date: April 2011 * DOI:
https://doi.org/10.1038/hdy.2010.90 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * selection * cline * pigmentation * evolution * variation