Mhc associations with clinical and autoantibody manifestations in european sle
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Systemic lupus erythematosus (SLE) is a clinically heterogeneous disease affecting multiple organ systems and characterized by autoantibody formation to nuclear components. Although
genetic variation within the major histocompatibility complex (MHC) is associated with SLE, its role in the development of clinical manifestations and autoantibody production is not well
defined. We conducted a meta-analysis of four independent European SLE case collections for associations between SLE sub-phenotypes and MHC single-nucleotide polymorphism genotypes, human
leukocyte antigen (HLA) alleles and variant HLA amino acids. Of the 11 American College of Rheumatology criteria and 7 autoantibody sub-phenotypes examined, anti-Ro/SSA and anti-La/SSB
antibody subsets exhibited the highest number and most statistically significant associations. HLA-DRB1*03:01 was significantly associated with both sub-phenotypes. We found evidence of
associations independent of MHC class II variants in the anti-Ro subset alone. Conditional analyses showed that anti-Ro and anti-La subsets are independently associated with HLA-DRB1*0301,
and that the HLA-DRB1*03:01 association with SLE is largely but not completely driven by the association of this allele with these sub-phenotypes. Our results provide strong evidence for a
multilevel risk model for HLA-DRB1*03:01 in SLE, where the association with anti-Ro and anti-La antibody-positive SLE is much stronger than SLE without these autoantibodies. SIMILAR CONTENT
BEING VIEWED BY OTHERS SYSTEMIC LUPUS ERYTHEMATOSUS GENETICS: INSIGHTS INTO PATHOGENESIS AND IMPLICATIONS FOR THERAPY Article 04 September 2024 A SYSTEMATIC REVIEW AND META-ANALYSIS OF HLA
CLASS II ASSOCIATIONS IN PATIENTS WITH IGG4 AUTOIMMUNITY Article Open access 02 June 2022 ELF1 SERVES AS A POTENTIAL BIOMARKER FOR THE DISEASE ACTIVITY AND RENAL INVOLVEMENT IN SYSTEMIC
LUPUS ERYTHEMATOSUS Article Open access 04 November 2024 INTRODUCTION Systemic lupus erythematosus (SLE; OMIM 152700) is a complex autoimmune disease that can affect multiple organ systems.
Processes involving both the innate and adaptive immune systems contribute to its development.1 The disease is clinically heterogeneous, and affected individuals only need 4 out of 11 of the
American College of Rheumatology (ACR) criteria to be classified as having SLE. Although patients may differ in their clinical manifestations, patients do share a propensity to develop
autoantibodies directed against nucleic acids and associated nuclear and cellular proteins. There is overwhelming evidence of a genetic component to SLE risk with higher concordance rates
observed between monozygotic twins (20–40%) compared with dizygotic twins (2–5%).2 The familial aggregation for SLE (sibling risk ratio, _λ_s=8–29)2, 3 is higher than other autoimmune
diseases, and the estimate of heritability is approximately 66%.4 Genetic association studies of SLE have been successful in identifying multiple loci.5, 6, 7, 8, 9, 10, 11 However,
relatively few studies have investigated the genetic association with specific SLE sub-phenotypes.12, 13, 14, 15 These studies focused mainly on major histocompatibility complex (MHC) class
II genes, and found evidence that class II alleles such as _HLA-DRB1*03:01_ are associated with auto-antibody production.13 Our study substantially expands this work by not only analysing
imputed classical human leukocyte antigen (HLA) alleles, but also examining variant HLA amino-acid positions in conjunction with single-nucleotide polymorphism (SNP) genotypes across the
extended MHC region (chromosome 6: 26–34 Mb). Our aim was to discover genetic loci within the MHC region that are associated with specific clinical and/or immunological manifestations within
SLE cases and hence to find evidence of genetic variants that may drive specific forms of the disease. For complex heterogeneous diseases such as SLE, comprehensive sub-phenotype studies
are critical in order to understand how previously identified genetic associations contribute to disease pathogenesis and specific disease manifestations. RESULTS STUDY SAMPLE For this
study, we collected genetic and sub-phenotype data from 3070 SLE cases of European descent characterized in four genetic association studies of SLE. These SLE cases were previously examined
in a large meta-analysis that examined the association between MHC genetic variation and SLE susceptibility.16 Table 1 describes the genotyping platform, number of genotyped MHC SNPs, and
sample size of each case collection in the study. Given the strong genetic associations observed with anti-Ro/SSA and anti-La/SSB autoantibody production described below, Table 1 also
provides the frequency of these antibodies for each case collection. Genetic (SNP) imputation was performed previously16 for each case collection, resulting in a total of 7119 SNPs common
between the four collections. In addition, classical HLA class I and II alleles as well as their corresponding variant amino acids (AAs, see Materials and Methods) were imputed and analysed.
SELECTION OF SUB-PHENOTYPES FOR ANALYSIS We examined the 11 ACR classification criteria17 and 7 SLE-related autoantibodies (anti-double-stranded DNA, anti-Sm, anti-RNP, anti-Ro/SSA,
anti-La/SSB, anti-cardiolipin IgG and anti-cardiolipin IgM) as candidate sub-phenotypes for this study. Single-marker associations for each candidate sub-phenotype with all variants were
assessed using logistic regression adjusted for population substructure and case collection (Supplementary Table 1). We analysed 7656 variants in total (7119 SNPs, 199 HLA alleles and 338
HLA amino-acid positions (see methods)). The specific sub-phenotypes comprising anti-Ro and anti-La antibodies demonstrated by far the most associations: 1635 and 1828 variants,
respectively, at _P_<0.00001. For all other sub-phenotypes, there were fewer than 30 variants that were significant at this level. Thus, we targeted anti-Ro and anti-La antibody subsets
for detailed investigation as they have the strongest evidence for a genetic aetiology. ANTI-RO ANTIBODY SUB-PHENOTYPE STEPWISE CONDITIONAL ANALYSIS The most associated marker (in terms of
_P_-value as a single marker) was the class III SNP rs3129962 in _BTNL2_ (_P_=9.47 × 10−27; odds ratio (OR)=2.44, 95% confidence interval (CI)=2.08–2.94; Table 2A). This marker is in linkage
disequilibrium (LD) with _HLA-DRB1*03:01_ (_R_2=0.84, _D_′=0.99). When conditioning on this SNP as a covariate in forward stepwise regression, the next most associated marker was the class
II SNP, rs9271731, between _HLA-DRB1_ and _HLA-DQA1_ (_P_=9.56 × 10−07; OR=1.54, 95% CI=1.30–1.85). This SNP is in LD with _HLA-DRB1*15:01_ (_R_2=0.72, _D_′=1). When using rs9271731 as an
additional covariate, one further association signal was detected at the class II SNP, rs3957146, between _HLA-DQB1_ and _HLA-DQA2 (P_=5.70 × 10−06; OR=0.52, 95% CI=0.39–0.69). Of note, the
effect sizes (ORs) and _P_-values that we present here are estimated from the multivariate models returned by stepwise regression (columns 2–3 in Table 2). The association results for a
given variant from single marker analyses can be seen in the last two columns of Table 2. The most associated amino acid (AA) was at position 77 in HLA-DRB1 with the common AA threonine
having a protective effect (_P_=2.72 × 10−13; OR=0.49; 95% CI=0.41–0.60). _HLA-DRB1*03:01_ and _HLA-DRB1*03:02_ encode the single alternative AA, asparagine, (_R_2=1). _HLA-DRB1*03:02_ is
not significantly associated with this sub-phenotype (_P_=0.37) possibly because of this allele being rare (frequency of 0.01% in our data). We cannot be certain that this lack of
association applies to the general population and this needs to be investigated to address this uncertainty. All other _HLA-DRB1_ alleles code for threonine. The single marker _P_-value for
this AA was very close to that of the most strongly associated SNP (see last column in Table 2). Therefore, we ran a stepwise regression starting from this marker. When conditioning on this
AA, the next most associated marker was the class II SNP, rs9271731, between _HLA-DRB1_ and _HLA-DQA1_ (_P_=4.5 × 10−08; OR=1.63, 95% CI=1.37–1.95). When using rs9271731 as an additional
covariate, one further association signal was detected at the class III SNP, rs3130781, in _DPCR1 (P_=1.76 × 10−05; OR=1.44, 95% CI=1.22–1.71). The SNP rs3130781 is in LD with
_HLA-DRB1*03:01_ (_R_2=0.29, _D_′=0.64) and _HLA-B*08:01_ (_R_2=0.29, _D_′=0.72). One final association signal was detected at _HLA-DQB1*03:02_ (_P_=2.49 × 10−05; OR=0.56, 95% CI=0.42–0.73).
The results from this analysis can be seen in Table 2B. Owing to the correlation between the most associated SNPs with known associated _HLA-DRB1_ alleles (rs3129962 tags
_HLA-DRB1*03:01_/Thr77 in DRB1 (_R_2=0.84); rs9271731 tags _HLA-DRB1*15:01_), we performed stepwise regression conditioning on these HLA alleles as covariates. When conditioning on
_HLA-DRB1*03:01_ and _HLA-DRB1*15:01_, the next most associated marker (rs9275582) was in class II between _HLA-DQB1-HLA-DQA2_ (_P_=2.99 × 10−06; OR=0.61; 95% CI=0.5–0.76). The most
significant HLA allele was _HLA-DQB1*03:02_, which is in LD with rs9275582 (_R_2=0.29, _D_′=0.80). These two sets of results can be seen in Tables 2C and 2D. We note that _HLA-DQB1*03:02_ is
in LD (_R_2=0.58) with rs3957146 (the third associated SNP in the first stepwise regression presented in Table 2). A simple stepwise regression analysis including only AA variants indicated
associations with Thr77, Leu67 and Gln96 in _HLA-DRB1_ (Table 2E). The _HLA-DRB1_ AA glutamine at position 96 is in LD with _HLA-DRB1*15:01_ (_R_2=0.82, _D_′=1.00). MODEL CHOICE USING THE
BAYESIAN INFORMATION CRITERION (BIC) Owing to the extended LD, an analysis of the MHC using stepwise regression to find evidence for multiple independently associated variants can lead to
many models depending on the first marker conditioned on (used as a covariate for further association analysis). This was discussed previously16 and here we also used the BIC as an aid to
model choice; the lower the BIC, the better fit the model is to the data (see methods). In our analysis of sub-phenotype data, there was not much difference between models A, C, D and E in
Table 2 in terms of the BIC, which represents the relative belief in a model given the data. However, model B, which began the forward stepwise regression with threonine at position 77 in
_HLA-DRB1,_ had the lowest BIC. This model does have one more term than the other four models. Our extended model search (see methods) did not result in a model with a lower BIC. HAPLOTYPE
ANALYSIS There are two main extended MHC haplotypes associated with SLE in northern Europeans that contain the class II alleles _HLA-DRB1*03:01_ and _HLA-DRB1*15:01_.18 These extended
haplotypes are comprised of the following HLA alleles: _HLA-A*03:01_—_HLA-B*07:02_—_HLA-C*07:02—_ _HLA-DRB1*15:01_ _—HLA-DQA1*01:02_—_HLA-DQB1*06:02_ and
_HLA-A*01:01_—_HLA-B*08:01_—_HLA-C*07:01—_ _HLA-DRB1*03:01_—_HLA-DQA1*05:01_—_HLA-DQB1*02:01_. We tested for association of these extended haplotypes with anti-Ro antibody status with the
hypothesis that the association signals at _HLA-DRB1*03:01 and HLA-DRB1*15:01_ are independent of these haplotypes. We observed significant effects for both haplotypes (_HLA-DRB1*03:01:
P_=1.02 × 10−12, OR=2.17; _HLA-DRB1*15:01: P_=0.02, OR=1.71). We found evidence that _HLA-DRB1*03:01_ is associated independently of the _HLA-B*08:01-DRB1*03:01_ haplotypic background
(_P_=3.05 × 10−07), whereas we fail to find evidence that _HLA-DRB1*15:01_ (_P_=0.17) is independent of the _HLA-B*07:02-DRB1*15:01_ haplotype. ANTI-LA ANTIBODY SUBPHENOTYPE STEPWISE
CONDITIONAL ANALYSIS The most strongly associated marker with the anti-La autoantibody sub-phenotype was the SNP rs2894254, in the class III region (_P_=3.40 × 10−30; OR=3.38, 95%
CI=2.74–4.16). This SNP is in LD (_R_2=0.84, _D_′=0.99) with _HLA-DRB1*03:01_. We do not find further associations when conditioning on this SNP as a covariate. However, if we condition on
_HLA-DRB1*03:01_, we find a further association with rs9268832, located between _HLA-DRA_ and _HLA-DRB5_ in class II (_P_=6.53 × 10−06; OR=1.64; 95% CI=1.32–2.04). Results from these two
models can be seen in Table 3. The _HLA-DRB1_ AA threonine at position 77 was observed to have a protective effect, consistent with the anti-Ro analyses. However, this AA was not the most
associated marker (_P_=2.4 × 10−28). Conditioning on Thr77, we find an additional association with rs2227139, located in _HLA-DRA_ in class II (_P_=6.47 × 10−06; OR=1.64; 95% CI=1.32–2.04).
The SNP, rs2227139, is in LD with rs9268832 (_R_2=0.91, _D_′=0.96). MODEL CHOICE USING THE BIC As with the analysis of anti-Ro, we used the BIC as an aid to model comparison. The model
including AA variation has the lowest BIC (model C in Table 3) but is only slightly lower than the model conditioning on _HLA-DRB1*03:01_. Therefore, we cannot choose between the AA and the
HLA allele as the best explanation for the data; however, conditional on either of these we find an independent association in class II. Both of these models have a lower BIC than model A,
which only has the single most associated SNP (rs2894254). These data therefore favour two independent associations in class II, one of which is most likely _HLA-DRB1*03:01_ or the
_HLA-DRB1_ AA threonine at position 77. Our extended model search (see methods) returned the same models as in Table 3. HAPLOTYPE ANALYSIS We observed significant effects for the
_HLA-DRB1*03:01_ haplotype but not the _HLA-DRB1*15:01_ haplotype with anti-La antibody status (_HLA-DRB1*03:01: P_=1.19 × 10−16, OR=3.12; _HLA-DRB1*15:01: P_=0.63). We found evidence that
_HLA-DRB1*03:01_ is associated independently of the _HLA-B*08:01-DRB1*03:01_ haplotype (_P_=6.42 × 10−13). INDEPENDENCE OF ANTI-RO AND ANTI-LA AUTOANTIBODY ASSOCIATIONS WITH _HLA-DRB1*03:01_
Thus far, we have observed strong evidence of association between _HLA-DRB1*03:01_ and both anti-Ro and anti-La autoantibody subsets. As these two phenotypes are correlated (_R_2=0.27), we
performed conditional analyses to determine whether the associations for each sub-phenotype were independent of each other. We performed logistic regression analysis with each sub-phenotype
as an outcome and the other sub-phenotype as a covariate. Table 4 displays the sample sizes and _HLA-DRB1*03:01_ frequencies for these case only analyses. When conditioning on anti-La as a
covariate, _HLA-DRB1*03:01_ continues to be strongly associated with anti-Ro antibody status (_P_=1.23 × 10−07, OR=1.60 95% CI=1.02–2.54). Also, when conditioning on anti-Ro,
_HLA-DRB1*03:01_ continues to be strongly associated with anti-La antibody status (_P_=1.66 × 10−12, OR=2.57 95% CI=1.98–3.34). To assess the robustness of these conditional regression
results, we examined the anti-Ro association in only anti-La-negative cases and found that _HLA-DRB1*03:01_ was still strongly associated with anti-Ro (_P_=6.79 × 10−07, OR=1.58 95%
CI=1.32–1.89). In anti-La antibody-positive SLE cases, _HLA-DRB1*03:01_ is weakly associated with anti-Ro (_P_=0.055, OR=2.37 95% CI=0.98–5.74). We performed the same analyses for the
anti-La antibody subset, stratifying on the anti-Ro phenotype. In anti-Ro-positive SLE cases, _HLA-DRB1*03:01_ is strongly associated with anti-La (_P_=6.18 × 10−12, OR=2.81 95%
CI=2.09–3.77). Among anti-Ro-negative SLE cases, _HLA-DRB1*03:01_ is weakly associated with anti-La (_P_=0.06, OR=1.96 95% CI=0.97–3.73). Therefore, we conclude that the association signal
for _HLA-DRB1*03:01_ with anti-La is not due to this sub-phenotype’s correlation with anti-Ro, and vice-versa. THE _HLA-DRB1*03:01_ ASSOCIATION WITH SLE SUSCEPTIBILITY IS INDEPENDENT OF THE
ASSOCIATION WITH ANTI-RO AND ANTI-LA ANTIBODY SUBSETS We have provided strong evidence for the association between _HLA-DRB1*03:01_ and both anti-Ro and anti-La antibody subsets. This
_HLA-DRB1_ allele has been consistently and strongly associated with SLE susceptibility in European populations,16 and this is confirmed in our current data (_P_=3.38 × 10−49; OR=1.86 95%
CI=1.71–2.02). However, the association of _HLA-DRB1*03:01_ with anti-Ro/anti-La antibody subsets and SLE susceptibility may not be independent—the _DRB1*03:01_ association with SLE may be
purely secondary to its association with anti-Ro and anti-La antibody status. If the association between _HLA-DRB1*03:01_ and SLE status is not driven entirely by sub-phenotype then one
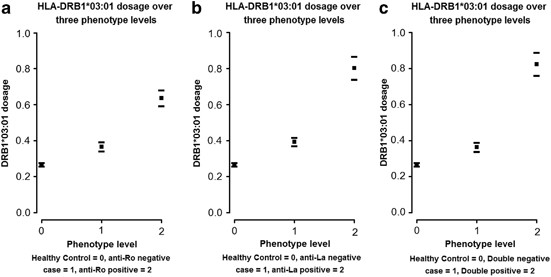
could hypothesize a three-level model of disease type (unaffected; sub-phenotype-negative case; sub-phenotype-positive case) based on increasing _HLA-DRB1*03:01_ frequency. Figure 1 plots
the change in _HLA-DRB1*03:01_ dosage over levels of disease; the average dosage appears to increase over all three levels. Therefore, we examined (see methods) the hypothesis that the
_HLA-DRB1*03:01_ association with anti-Ro and anti-La antibody sub-phenotypes explains the association of _DRB1*03:01_ with SLE in general. We also tested whether the risk was additive over
the three levels of disease. ANTI-RO ANTIBODY SUB-PHENOTYPE We found a significant difference in _HLA-DRB1*03:01_ dosage between healthy controls and anti-Ro antibody negative cases (_P_=
1.97 × 10−14). The estimated change in dosage was 0.1 (95% CI=0.08–0.13), equivalent to a change in allele frequency of 0.05 (95% CI=0.04–0.06). We also found a significant increase in
dosage between anti-Ro-negative cases and anti-Ro positive cases (_P_=2.97 × 10−33). The estimated change in dosage (see Table 5) is 0.27 (95% CI=0.22–0.31), equivalent to a change in
frequency of 0.13 (95% CI=0.11–0.16). We found evidence against the hypothesis that the increase in dosage is additive over the three disease levels (_P_=0.008). Our final test against the
additive model implies that the difference in _HLA-DRB1*03:01_ dosage between anti-Ro(−)/anti-Ro(+) status (increase of 0.27) in the cases is more than double that of the difference between
cases and healthy controls (increase of 0.10). ANTI-LA ANTIBODY SUBPHENOTYPE We found a significant difference in _HLA-DRB1*03:01_ dosage between healthy controls and anti-La-negative cases
(_P_=3.57 × 10−25). The estimated change (see Table 5) in dosage is 0.13 (95% CI=0.11–0.15), equivalent to a change in frequency of 0.06 (95% CI=0.05–0.08). We also found a significant
increase in dosage between anti-La-negative and anti-La-positive cases (_P_=2.45 × 10−39). The estimated change in dosage (see Table 5) is 0.41 (95% CI=0.35–0.47), equivalent to a change in
frequency of 0.21 (95% CI=0.18–0.24). We found evidence against the hypothesis that the increase in dosage is additive over the three disease levels (_P_=1.5 × 10−04). Table 5 displays the
effect sizes and _P_-values for this analysis. Our final test against the additive model implies that the difference in _HLA-DRB1*03:01_ dosage between anti-La(−)/anti-La(+) status (increase
of 0.41) in the cases is more than triple that of the difference between cases and healthy controls (increase of 0.13). DOUBLE POSITIVE AND DOUBLE NEGATIVE ANTI-RO AND ANTI-LA ANTIBODY
SUB-PHENOTYPES Our study was large enough to determine whether the frequency of _HLA-DRB1*03:01_ differs between SLE cases who are double negative for anti-Ro and anti-La antibodies
(_N_=1781) and healthy controls (_N_=9782). It is known that these antibodies are present in approximately 2% of the healthy population; however, we do not have this phenotype data for the
controls. The following results therefore assume that all controls are negative for antinuclear antibodies. We found a significant association of _HLA-DRB1*03:01_ with the double negative
SLE cases/healthy controls status (OR=1.49, 95% CI=1.35–1.65; _P_=2.23 × 10−14). Further analysis demonstrated a stronger association with the double positive (_n_=259)/double negative SLE
case status (OR=3.71, 95% CI=2.97–4.64; _P_=2.00 × 10−16). To test whether these two odds ratios differ, we ran the same analysis for a three-stage risk model as we did for anti-Ro and
anti-La antibody subsets separately (see above, Table 5 results). We found very strong evidence against the hypothesis that the increase in dosage is additive over the three disease levels
(_P_=6.65 × 10−06). The non-additive effect leads to a very large odds ratio between double positive SLE cases and healthy controls, which we found to be 5.27 (95% CI=4.31–6.44; _P_=3.14 ×
10−59; Figure 1). DISCUSSION Our results confirm, in the largest SLE sub-phenotype genetic association study to date, that the often replicated genetic association at _HLA-DRB1*03:01_ does
not just influence SLE susceptibility but is also associated with anti-Ro and anti-La autoantibody production. For the first time, we have shown that _HLA-DRB1*03:01_ is associated with SLE
_per se_, independent of anti-Ro and anti-La antibody subsets. These data implicate _HLA-DRB1*03:01_ and variants in LD with it in the predisposition to anti-Ro and anti-La autoantibody
production as well as processes outside of this manifestation. We do not find conclusive evidence that variant HLA AAs explain the majority of the MHC association signal in anti-Ro and
anti-La autoantibody subsets in SLE. This is largely due to the confounding effects of extended LD displayed by the associated DRB1*03:01 and to a lesser extent, the DRB1*15:01 haplotypes in
our study cohorts. These results contrast with those of a recent study in anti-CCP-positive rheumatoid arthritis, where five HLA AA variants were suggested to largely explain the MHC
association with disease status.19 In this case, the disease-associated variants generally reside on a diversity of haplotypes. Studies in other autoimmune/inflammatory diseases have either
not shown robust association signals with variant HLA AA data or like the present study have shown association with AAs in strong LD with previously associated HLA alleles. It may be that
HLA amino association signals are more complex than the single-variant testing method we and others have used. Limitations of the present study include the heterogeneity in autoantibody
testing procedures and sub-phenotype data collection between the four studies. As a result, data were tabulated and analysed in an essentially binary format (that is, individual cases were
classified as positive, negative or missing for each trait), to allow meta-analysis. However, in so doing, a degree of noise is inevitable, which would reduce our power to detect true
association signals particularly in the less common sub-phenotypes. We were also limited by the imputation required to analyse a consistent set of SNPs across studies and the reliance on HLA
imputation. In addition, we are constrained in our conclusions on differences in results for anti-Ro and anti-La antibody subsets given the much smaller sample size available for the
anti-La phenotype. Thus, we have confined some of our analyses to the most robust association; that of _HLA-DRB1*03:01_ with both anti-Ro and anti-La antibody sub-phenotypes. We must also
allow for the possibility that associations with _HLA-DRB1*03:01_ could exist with other SLE subsets that overlap with anti-Ro/La, but have not been detected in our study. This highlights
the need for extension of this work to other cohorts with sub-phenotype data in order to increase sample size and power across as wide a range of phenotypes as possible. In both anti-Ro and
anti-La sub-phenotypes, we find evidence of secondary independent associations in the class II region of the MHC after conditioning on _HLA-DRB1*03:01_, and we find additional signals in
class II and class III for anti-Ro. We have shown that the association of _HLA-DRB1*03:01_ with anti-Ro antibody status is independent of the association with anti-La and vice-versa. We have
also shown that the association between SLE case/healthy control and _HLA-DRB1*03:01_ is not purely due to the association with anti-Ro and anti-La antibody sub-phenotypes. This implies a
three-level model of risk for increasing dosage of _HLA-DRB1*03:01,_ where the frequency of this allele is higher in anti-Ro-negative cases than in healthy controls and higher still in
anti-Ro-positive cases than anti-Ro-negative cases. The same is true for anti-La. In fact, we find very strong evidence that the _HLA-DRB1*03:01_ risk of anti-Ro/anti-La double positive
within SLE patients is much greater than the risk of anti-Ro/anti-La double negative (other lupus phenotypes without these anti-bodies present) in the general population. We can conclude
that the association of _HLA-DRB1*03:01_ with SLE is driven to a large extent but not entirely by anti-Ro and anti-La auto-antibody sub-phenotypes. Although we do find evidence of an
independent class III association with anti-Ro, there is some uncertainty. We find a significant association with the class III SNP rs3130781 conditional on the AA Thr77-DRB1. However, when
conditioning on the markers in model C in Table 2 (_HLA-DRB1*03:01_+_HLA-DRB1*15:01_+rs9275582; BIC=2829.8) in a forward stepwise regression, the association with rs3130781 is not
significant (_P_=4.2 × 10−05). This is also the case for model D in Table 2 (_HLA-DRB1*03:01_+_HLA-DRB1*15:01_+_HLA-DQB*03:02;_ BIC=2829.6). So conditional on _HLA-DRB1*03:01_ and
_HLA-DRB1*15:01,_ we find an independent association in class II but not class III. However, we did consider conditioning on _HLA-DRB1*03:01_ alone, where a stepwise regression returned a
class II SNP (rs9271731; _R_2 with HLA-DRB1*15:01=0.72) and the class III SNP rs3130781. This model has a BIC=2929.00. Hence, there is uncertainty as to whether there is an independent class
III effect when conditioning on _HLA-DRB1*03:01_; all three models fit the data equally well (not much difference in the BIC). Nevertheless, the best model in Table 2 does suggest that
there is an independent class III effect conditional on the class II AA Thr77-DRB1. This model has a much lower BIC than any others. There is some evidence, therefore, of a class III
association with anti-Ro; however, we believe that more data, and ideally across diverse populations (to help remove effects due to LD), are required to be more definitive about this. The
results of the present study while enlightening are confounded by the strong and extended LD present on the principally associated _HLA-DRB1*03:01_ and _HLA-DRB1*15:01_ haplotypes.
Complementary studies in accurately phenotyped southern European and non-European SLE cohorts, which show haplotypic diversity at the MHC, will allow refinement of the sub-phenotype
association signals found in the predominantly northern European populations studied thus far.20 These efforts may still yield association intervals that harbour several genes/variants.
Therefore, future work will inevitably require re-sequencing, transcriptomic and epigenetic studies in order to tease out these complex association signals. MATERIALS AND METHODS STUDY
DESIGN This study is a meta-analysis of four studies taken from work described in a previous paper.16 We only included four of the six previous studies in this work as sub-phenotype data
were not available from the other two studies (named ‘Affy500K’ and ‘Affy100K’ in the previous paper). We refer to the previous meta-analysis of SLE case–control data as the ‘parent study’
in this work. The number of SLE cases and controls in this paper for the four included studies are the same as in the parent study, and quality control (QC) procedures for these data are
described in full in the previous paper, including tests for relatedness and adjustments for population structure. We include some QC descriptions below for clarity in this paper. QC AND
IMPUTATION SNPS We only analysed SNPs that passed QC in our previous paper,16 which utilized these data: 90% genotyping for all subjects and SNPs, minor allele frequency >0.01 and
Hardy–Weinberg equilibrium (false discovery rate of 0.05). HLA IMPUTATION We imputed HLA genotypes using HLA*IMP V2.21 Only genotyped SNPs in each case collection were used for this
imputation. We used posterior probabilities of HLA genotypes, rather than most likely genotypes, in order to allow for uncertainty in imputation. From these probabilities, we calculated
dosages for each allele (expected number of alleles 0<_x_<2). We had HLA-DRB1 typed data in two studies: the ‘Illumina Combined MHC panel’ study (_N_=1608) and the ‘Illumina Custom
panel’ study (_N_=605). This allowed for assessment of accuracy, which for the two main reported positive associations in this paper were as follows: for _HLA-DRB1*03:01,_ we achieved
sensitivity of 0.992/0.999 and specificity of 0.995/0.993 for the Illumina Combined MHC panel and Illumina Custom panel’ respectively. For _HLA-DRB1*15:01,_ we achieved sensitivity of
0.980/0.992 and specificity of 0.996/0.997. AA TRANSLATION AA sequences for each HLA allele were extracted from the European Bioinformatics Institute HLA database
(http://www.ebi.ac.uk/ipd/imgt/hla/). HLA allele dosages were converted to AA dosages at each position; the dosage for a particular amino acid ‘A’ at position ‘p’ would be the sum of HLA
alleles’ dosage that coded for amino acid ‘A’ at position ‘p’. The total dosage for each position is therefore equal to 2 and this total is split between each possible AA at the position. We
had data at 338 AA positions that had variable AAs (HLA-A=67, HLA-B=75, HLA-C=71, HLA-DPB1=21, HLA-DQA1=41, HLA-DQB1=61, HLA-DRB1=52). Owing to multiple possible AAs at each position, we
actually had 1255 possible position/AA variants in total. ADJUSTMENT FOR POPULATION STRUCTURE We analysed the data with the statistical computing language R22 using logistic regression. All
analyses were adjusted for ancestry utilizing the first principal component (PC) or percentage of northern European ancestry, as previously described16 and included a covariate for project.
As the PCs were computed specifically for each case collection, we also included interaction terms between projects and ancestry to allow for different effect sizes in the adjustment for
population structure. SINGLE-MARKER ANALYSIS OF CANDIDATE SUB-PHENOTYPES AND ANALYSIS OF SLE AS A SIMPLE DISEASE OUTCOME We examined the 11 ACR criteria17 and presence of 7 SLE-related
auto-antibodies (anti-Ro/SSA, anti-La/SSB, anti-double-stranded DNA, anti-RNP, anti-Sm and anticardiolipin IgG and IgM) as candidate sub-phenotypes for detailed analysis. To determine which
sub-phenotypes were most strongly influenced by genetic variation in the MHC, we tested each sub-phenotype for association with all variants (SNPs, HLA alleles and HLA AAs) in single-variant
association tests using logistic regression adjusted for population substructure and case collection. We also tested the association between markers and SLE as a simple disease outcome for
the four studies considered here. Results for association with _HLA-DRB1*03:01_ are discussed in the beginning of the section titled ‘The _HLA-DRB1*03:01_ association with SLE susceptibility
is independent of the association with anti-Ro and anti-La antibody subsets’. CONDITIONAL ASSOCIATION ANALYSIS OF ANTI-RO AND ANTI-LA Owing to numerous single-marker associations within the
extended LD of the MHC, we used conditional analyses to narrow these associations to those with the best evidence for strength and independence. All analyses utilized logistic regression
with ancestry and project covariates (see above) and were halted when the evidence for association with a new term was _P_>3 × 10−05. We performed classic forward stepwise regression,
conditioning on the top variant to find the second variant, and so on. A simple forward stepwise approach can lead to over-fitting (selecting many correlated markers) and the results may be
misleading because of selected markers potentially tagging two or more independently associated markers.16 Therefore, we also performed a model search using the BIC16 as the inclusion metric
in a stepwise regression using the R22 ‘step()’ function, first starting with no prior model (other than covariates above) and also starting from _HLA-DRB1*03:01_ and _HLA-DRB1*15:01_ as
initial model terms. Although BIC optimization was used to select model terms, we terminated the selection when it would result in a term with _P_>3 × 10–5. The BIC23, 24 is a penalized
likelihood model choice criterion similar to the Akaike Information Criterion24 except there is a stronger penalty for additional model parameters that increases with sample size. The BIC is
therefore more conservative and favours smaller models than the Akaike Information Criterion. As with the Akaike Information Criterion, the smaller the BIC the better the model is judged to
fit the data. HAPLOTYPE ANALYSIS OF ANTI-RO AND ANTI-LA Given the high degree of correlation between the associated variants identified from the model searches described above, we conducted
a haplotype analysis of these variants using PLINK25 using the best-guess genotypes estimated from HLA*IMP2. We used PLINK to phase haplotypes and perform multivariate logistic regression
where terms are haplotypes rather than individual variants, optionally controlling for individual variants or haplotypes. MULTIPLE TESTING In the MHC, a Bonferroni adjustment for multiple
testing is inappropriate because of the extensive LD and hence correlated variants. In order to determine the number of independent variants, we performed a PC analysis of all SNPs. In our
data, we found that 374 PCs had eigenvalues >1 and these PCs explained 96% of the variance. Thus, we used a multiple-testing threshold of _P_<0.01/374=3 × 10−5. TESTING FOR
INDEPENDENCE BETWEEN THE SLE ASSOCIATION AND SUB-PHENOTYPE ASSOCIATION WITH _HLA-DRB1*03:01_ We fitted a linear regression model with dosage for _HLA-DRB1*03:01_ as the outcome and both
case/control status and sub-phenotype status as explanatory variables. We therefore tested each effect conditional on the other. A significant association for case/control status conditional
on sub-phenotype implies that we reject the hypothesis that sub-phenotype is solely driving the case/control association. This is equivalent to setting the three-level status as a factor in
the regression in terms of model fit. But rather than obtaining an estimate of dosage change between healthy controls and sub-phenotype positive as we would in a three-level factor (where
the baseline is healthy control), we get an estimate of change between sub-phenotype positive and sub-phenotype negative. In both models, we also get an estimate of change between healthy
controls and sub-phenotype negative. Furthermore, we tested the hypothesis that the increase in dosage is additive over the three disease levels (Healthy-Control Case sub-phenotype
negative/Case sub-phenotype positive). This is achieved by fitting a model with an additive effect for dosage over the three phenotype levels. This additive model is nested within our model
used to test independence of sub-phenotype association with SLE-case/healthy control, so we performed a likelihood ratio test. A rejection of this additive model, in favour of the
three-level factor model (described in the previous paragraph), is evidence that the change in dosage over sub-phenotype within cases is different than the change in dosage between healthy
controls and SLE without the sub-phenotype. REFERENCES * Guerra SG, Vyse TJ, Graham DSC . The genetics of lupus: a functional perspective. _Arthritis Res Ther_ 2012; 14: 211. Article Google
Scholar * Deapen D, Escalante A, Weinrib L, Horwitz D, Bachman B, Royburman P _et al_. A revised estimate of twin concordance in systemic lupus-erythematosus. _Arthritis Rheum_ 1992; 35:
311–318. Article CAS Google Scholar * Alarcon-Segovia D, Alarcon-Riquelme ME, Cardiel MH, Caeiro F, Massardo L, Villa AR _et al_. Familial aggregation of systemic lupus erythematosus,
rheumatoid arthritis, and other autoimmune diseases in 1177 lupus patients from the GLADEL cohort. _Arthritis Rheum_ 2005; 52: 1138–1147. Article Google Scholar * Lawrence JS, Martins CL,
Drake GLA . Family survey of lupus-erythematosus.1. Heritability. _J Rheumatol_ 1987; 14: 913–921. CAS PubMed Google Scholar * Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO,
Kimberly RP, Moser KL _et al_. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. _Nat Genet_
2008; 40: 204–210. Article CAS Google Scholar * Hom G, Graham RR, Modrek B, Taylor KE, Ortmann W, Garnier S _et al_. Association of systemic lupus erythematosus with C8orf13-BLK and
ITGAM-ITGAX. _New Engl J Med_ 2008; 358: 900–909. Article CAS Google Scholar * Yang W, Shen N, Ye DQ, Liu Q, Zhang Y, Qian XX _et al_. Genome-wide association study in Asian populations
identifies variants in ETS1 and WDFY4 associated with systemic lupus erythematosus. _PLoS Genetics_ 2010; 6: e1000841. Article Google Scholar * Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z
_et al_. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. _Nat Genetics_ 2009; 41: 1234–1237. Article CAS
Google Scholar * Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X _et al_. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for
systemic lupus erythematosus. _Nat Genetics_ 2009; 41: 1228–1233. Article CAS Google Scholar * Graham DSC, Morris DL, Bhangale TR, Criswell LA, Syvanen AC, Ronnblom L _et al_. Association
of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with Systemic Lupus Erythematosus. _PLoS Genetics_ 2011; 7: e1002341. Article Google Scholar * Li R, Yang W, Zhang J, Hirankarn N, Pan HF, Mok CC _et
al_. Association of CD247 with systemic lupus erythematosus in Asian populations. _Lupus_ 2012; 21: 75–83. Article CAS Google Scholar * Taylor KE, Chung SA, Graham RR, Ortmann WA, Lee
AT, Langefeld CD _et al_. Risk alleles for systemic lupus erythematosus in a large case-control collection and associations with clinical subphenotypes. _PLoS Genetics_ 2011; 7: e1001311.
Article CAS Google Scholar * Hamilton RG, Harley JB, Bias WB, Roebber M, Reichlin M, Hochberg MC _et al_. 2 Ro (Ss-a) autoantibody responses in systemic lupus-erythematosus—correlation of
Hla-Dr/Dq specificities with quantitative expression of Ro (Ss-a) autoantibody. _Arthritis Rheum_ 1988; 31: 496–505. Article CAS Google Scholar * Arnett FC, Hamilton RG, Reveille JD,
Bias WB, Harley JB, Reichlin M . Genetic studies of Ro (SS-A) and La (SS-B) autoantibodies in families with systemic lupus erythematosus and primary Sjogren’s syndrome. _Arthritis Rheum_
1989; 32: 413–419. Article CAS Google Scholar * Harley JB, Sestak AL, Willis LG, Fu SM, Hansen JA, Reichlin M . A model for disease heterogeneity in systemic lupus erythematosus.
Relationships between histocompatibility antigens, autoantibodies, and lymphopenia or renal disease. _Arthritis Rheum_ 1989; 32: 826–836. CAS PubMed Google Scholar * Morris DL, Taylor KE,
Fernando MM, Nititham J, Alarcon-Riquelme ME, Barcellos LF _et al_. Unraveling multiple MHC gene associations with systemic lupus erythematosus: model choice indicates a role for HLA
alleles and non-HLA genes in Europeans. _Am J Hum Genet_ 2012; 91: 778–793. Article CAS Google Scholar * Hochberg MC . Updating the American College of Rheumatology revised criteria for
the classification of systemic lupus erythematosus. _Arthritis Rheum_ 1997; 40: 1725–1725. Article CAS Google Scholar * Fernando MM, Stevens CR, Sabeti PC, Walsh EC, McWhinnie AJ, Shah A
_et al_. Identification of two independent risk factors for lupus within the MHC in United Kingdom families. _PLoS Genetics_ 2007; 3: e192. Article Google Scholar * Raychaudhuri S, Sandor
C, Stahl EA, Freudenberg J, Lee H-S, Jia X _et al_. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. _Nat Genetics_
2012; 44: 291–296. Article CAS Google Scholar * Fernando MMA, Freudenberg J, Lee A, Morris DL, Boteva L, Rhodes B _et al_. Transancestral mapping of the MHC region in systemic lupus
erythematosus identifies new independent and interacting loci at MSH5, HLA-DPB1 and HLA-G. _Ann Rheum Dis_ 2012; 71: 777–784. Article CAS Google Scholar * Dilthey A, Leslie S, Moutsianas
L, Shen JD, Cox C, Nelson MR _et al_. Multi-population classical HLA type imputation. _Plos Comput Biol_ 2013; 9: e1002877. Article CAS Google Scholar * R Core Team . _R: A Language and
Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna, Austria, 2013. Google Scholar * Andel J, Perez MG, Negrao AI . Estimating the dimension of a
linear-model. _Kybernetika_ 1981; 17: 514–525. Google Scholar * Lazic SE . Model based inference in the life sciences: a primer on evidence. _J Roy Stat Soc a Sta_ 2011; 174: 506–506.
Article Google Scholar * Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D _et al_. PLINK: a tool set for whole-genome association and population-based linkage analyses.
_Am J Hum Genet_ 2007; 81: 559–575. Article CAS Google Scholar * Barcellos LF, May SL, Ramsay PP, Quach HL, Lane JA, Nititham J _et al_. High-density SNP screening of the major
histocompatibility complex in systemic lupus erythematosus demonstrates strong evidence for independent susceptibility regions. _PLoS Genetics_ 2009; 5: e1000696. Article Google Scholar *
Rioux JD, Goyette P, Vyse TJ, Hammarstrom L, Fernando MM, Green T _et al_. Mapping of multiple susceptibility variants within the MHC region for 7 immune-mediated diseases. _Proc Nat Acad
Sci USA_ 2009; 106: 18680–18685. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank the original study participants and their families for their contributions to
this research, along with clinical colleagues who facilitated data collection. We thank Alexander Dilthey for his advice during the HLA imputation. We also thank the investigators of IMAGEN
(John D Rioux, Philippe Goyette, Timothy J Vyse, Lennart Hammarström, Michelle MA Fernando, Todd Green, Philip L De Jager, Sylvain Foisy, Joanne Wang, Paul IW de Bakker, Stephen Leslie,
Gilean McVean, Leonid Padyukov, Lars Alfredsson, Vito Annese, David A Hafler, Qiang Pan-Hammarström, Ritva Matell, Stephen J Sawcer, Alastair D Compston, Bruce AC Cree, Daniel B Mirel, Mark
J Daly, Tim W Behrens, Lars Klareskog, Peter K Gregersen, Jorge R Oksenberg and Stephen L Hauser). A full list of the investigators who contributed to the generation of the Wellcome Trust
Case-Control Consortium data is available from the WTCCC website (see Web Resources). This study was founded by Swedish Research Council, Instituto de Salud Carlos III (PI12/02558) partly
financed by FEDER funds of the EU, and the BIOLUPUS RNP funded by the European Science Foundation to MEA-R; American College of Rheumatology Rheumatology Research Foundation Physician
Scientist Development Award and National Institutes of Health, National Center for Advancing Translational Sciences through UCSF-CTSI Grant KL2TR000143 to SAC. Arthritis Research UK funded a
Clinician Scientist Fellowship for MMAF (ref 18239) and the Arthritis Research UK funded DLM under (ref 17761/PI TJV). MEA-R was funded by the Swedish Research Council and Instituto de
Salud Carlos III grant number PS09/00129 cofinanced through FEDER funds of the European Union and the Consejería de Salud de Andalucía PI0012. The IMAGEN consortium was supported by Grant
AI067152 from the National Institutes of Allergy and Infectious Diseases. Funding for the Wellcome Trust Case-Control Consortium project was provided by the Wellcome Trust under award 076113
and 085475. Cord blood samples were collected by V L Nimgaonkar’s group at the University of Pittsburgh, as part of a multi-institutional collaborative research project with J Smoller, MD
DSc and P Sklar, MD PhD (Massachusetts General Hospital; grant MH 63420). Support for the Illumina MHC Panel study was provided by the NIH (AR052300, AR02175, AR22804, AR62277, AR42460,
AI024717, AI083194, AR62277, AI082714, AI53747, AI31584, DE15223, RR20143, PR094002, AI62629, AR48940, AR19084, AR043274, AI063274, AI40076, AR052125, HG006828, AR048929, and AR049084),
research grants from the US Department of Veterans Affairs, US Department of Defense (PR094002), American College of Rheumatology, Alliance for Lupus Research, Rheuminations, the Lupus
Foundation of Minnesota and the Mary Kirkland Center for Lupus Research. This study was performed in part in the General Clinical Research Center, Moffitt Hospital, University of California
San Francisco, with funds provided by the National Center for Research Resources, 5 M01 RR-00079, US Public Health Service. Web Resources: The URLs for data presented herein are as follows:
A full list of the investigators who contributed to the generation of the WTCCC data is available from http://www.wtccc.org.uk. Online Mendelian in MAN (OMIM): http://www.omim.org AUTHOR
INFORMATION Author notes * M M A Fernando and K E Taylor: These Authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of Medical & Molecular Genetics, King’s
College London School of Medicine, Guy’s Hospital, London, UK D L Morris, M M A Fernando & T J Vyse * Department of Medicine, Rosalind Russell Medical Research Center for Arthritis,
University of California San Francisco, San Francisco, CA, USA K E Taylor, S A Chung, J Nititham & L A Criswell * Department of Human DNA Variability, GENYO, Centro de Genómica e
Investigación Oncológica Pfizer-Universidad de Granada—Junta de Andalucía, Granada, Spain M E Alarcón-Riquelme * Arthritis and Clinical Immunology Program, Oklahoma Medical Research
Foundation, Oklahoma, OK, USA M E Alarcón-Riquelme & P M Gaffney * Division of Epidemiology, Genetic Epidemiology and Genomics Laboratory, School of Public Health, University of
California, Berkeley, CA, USA L F Barcellos * Immunology Biomarkers Group, Genentech, South San Francisco, CA, USA T W Behrens, R R Graham & G Hom * Department of Neurology, Yale School
of Medicine, Connecticut, CT, USA C Cotsapas * Rheumatology Service, Hospital Provincial de Rosario, Sanatorio Parque, Rosario, Argentina B A Pons-Estel * The Robert S. Boas Center for
Genomics and Human Genetics, Feinstein Institute for Medical Research, North Shore LIJ Health System, Manhasset, NY, USA P K Gregersen * Cincinnati Children’s Hospital Medical Center and US
Department of Veterans Affairs Medical Center, Cincinnati, OH, USA J B Harley * Department of Neurology, University of California San Francisco, San Francisco, CA, USA S L Hauser *
Department of Biostatistical Sciences, Wake Forest University Health Sciences, Wake Forest, NC, USA C D Langefeld * Children’s Hospital Oakland Research Institute, Oakland, CA, USA J A Noble
* Department of Medicine, Université de Montréal and Research Center, Montreal Heart Institute, Montreal, QC, Canada J D Rioux * University of California Davis, Davis, CA, USA M F Seldin
Authors * D L Morris View author publications You can also search for this author inPubMed Google Scholar * M M A Fernando View author publications You can also search for this author
inPubMed Google Scholar * K E Taylor View author publications You can also search for this author inPubMed Google Scholar * S A Chung View author publications You can also search for this
author inPubMed Google Scholar * J Nititham View author publications You can also search for this author inPubMed Google Scholar * M E Alarcón-Riquelme View author publications You can also
search for this author inPubMed Google Scholar * L F Barcellos View author publications You can also search for this author inPubMed Google Scholar * T W Behrens View author publications You
can also search for this author inPubMed Google Scholar * C Cotsapas View author publications You can also search for this author inPubMed Google Scholar * P M Gaffney View author
publications You can also search for this author inPubMed Google Scholar * R R Graham View author publications You can also search for this author inPubMed Google Scholar * B A Pons-Estel
View author publications You can also search for this author inPubMed Google Scholar * P K Gregersen View author publications You can also search for this author inPubMed Google Scholar * J
B Harley View author publications You can also search for this author inPubMed Google Scholar * S L Hauser View author publications You can also search for this author inPubMed Google
Scholar * G Hom View author publications You can also search for this author inPubMed Google Scholar * C D Langefeld View author publications You can also search for this author inPubMed
Google Scholar * J A Noble View author publications You can also search for this author inPubMed Google Scholar * J D Rioux View author publications You can also search for this author
inPubMed Google Scholar * M F Seldin View author publications You can also search for this author inPubMed Google Scholar * T J Vyse View author publications You can also search for this
author inPubMed Google Scholar * L A Criswell View author publications You can also search for this author inPubMed Google Scholar CONSORTIA SYSTEMIC LUPUS ERYTHEMATOSUS GENETICS CONSORTIUM
* John B Harley * , Marta E Alarcón-Riquelme * , Lindsey A Criswell * , Patrick M Gaffney * , Chaim O Jacob * , Robert P Kimberly * , Kathy L M Sivils * , Betty P Tsao * , Timothy J Vyse *
& Carl D Langefeld CORRESPONDING AUTHOR Correspondence to D L Morris. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION MEMBERS
OF THE SYSTEMIC LUPUS ERYTHEMATOSUS GENETICS CONSORTIUM John B Harley, Marta E Alarcón-Riquelme, Lindsey A Criswell, Patrick M Gaffney, Chaim O Jacob, Robert P Kimberly, Kathy L M Sivils,
Betty P Tsao, Timothy J Vyse and Carl D Langefeld. Supplementary Information accompanies this paper on Genes and Immunity website SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (DOC 67
KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Morris, D., Fernando, M., Taylor, K. _et al._ MHC associations with clinical
and autoantibody manifestations in European SLE. _Genes Immun_ 15, 210–217 (2014). https://doi.org/10.1038/gene.2014.6 Download citation * Received: 15 October 2013 * Revised: 07 January
2014 * Accepted: 10 January 2014 * Published: 06 March 2014 * Issue Date: June 2014 * DOI: https://doi.org/10.1038/gene.2014.6 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative KEYWORDS * Sub-phenotype analysis * MHC * meta-analysis * genetics * systemic lupus erythematosus * Europeans