Epstein-Barr virus and multiple sclerosis: interaction with HLA
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Epstein-Barr virus (EBV) infection, history of infectious mononucleosis (IM) and HLA-A and DRB1 have all been proposed as risk factors for multiple sclerosis (MS). Our aim was to analyse
possible interactions between antibodies against Epstein-Barr virus nuclear antigen 1 (EBNA1) or EBNA1 fragments, presence of DRB1*15 and absence of A*02. The study population includes newly
diagnosed cases and matched controls. Interaction on the additive scale was calculated using attributable proportion due to interaction (AP), which is the proportion of the incidence among
individuals exposed to two interacting factors that is attributable to the interaction per se. IM showed association with MS, odds ratio (OR)=1.89 (1.45–2.48% confidence interval (CI)), as
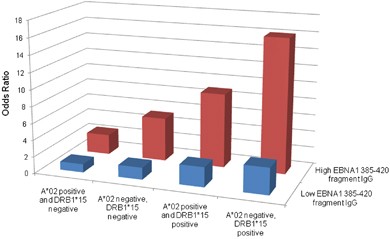
did raised EBNA1 IgG OR=1.74 (1.38–2.18 95%CI). All EBNA1 fragment IgGs were associated with MS risk. However, EBNA1 fragment 385–420 IgG levels were more strongly associated to MS than
total EBNA1 IgG, OR=3.60 (2.75–4.72 95%CI), and also interacted with both DRB1*15 and absence of A*02, AP 0.60 (0.45–0.76 95%CI) and AP 0.39 (0.18–0.61 95%CI), respectively. The observed
interaction between HLA class I and II genotype and reactivity to EBV-related epitopes suggest that the mechanism through which HLA genes influence the risk of MS may, at least in part,
involve the immune control of EBV infection.
The study was supported by grants from the Swedish Association for Persons with Neurological Disabilities, The Swedish Research Council, The Söderbergs Foundation, the AFA foundation, the
Swedish Foundation for Working Life and Social research and the FP6 program Neuropromise (LSHM-CT-2005-018637). We thank Nina Nordin and Karin Kai-Larsen for help with collecting data.
Author contributions: ES did HLA genotyping and the statistical analysis, provided figures and wrote the paper. PS provided reagents and performed the anti-EBV antibody measurements. ML did
HLA genotyping. AKH collected clinical information on the EIMS participants. FA contributed to writing of the paper. IK supervised HLA genotyping and the statistical analysis. JH, LA and TO
are responsible for the EIMS study and the design of the study. All authors contributed to the final paper.
I Kockum, L Alfredsson and T Olsson: These authors contributed equally to this paper
Department of Clinical Neuroscience, Neuroimmunology Unit, Center for Molecular Medicine L8:05, Karolinska Institutet, Stockholm, Sweden
Department of Clinical Neuroscience, Umeå University, Umeå, Sweden
Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden
Department of Cell Biology and Neuroscience, Istituto Superiore di Sanità, Rome, Italy
Department of Clinical Neuroscience, Neurogenetics group, Center for Molecular Medicine L8:00, Karolinska Institutet, Stockholm, Sweden
Ms Sundqvist has received research support from the Swedish Association for Persons with Neurological Disabilities. Professor Hillert has received unrestricted research support from
BiogenIdec, MerckSerono and Bayer Schering. Professor Alfredsson receives research support from the Swedish Medical Research Council (Dnr 521-2009-2596) and Swedish Council for Working life
and Social Research (Dnr 2009-0650). Professor Tomas Olsson has received grant support for MS research from unrestricted grant support from BiogenIdec, Bayer, SanofiAventis and Merck, and
also lecture fees and/or advisory board consultancies for the same companies. Other authors declare no conflict of interest.
Supplementary Information accompanies the paper on Genes and Immunity website
Anyone you share the following link with will be able to read this content: