Enabling t cell therapy for established solid tumors using genetic-enhancing modifiers
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
T cell therapies have produced remarkably effective clinical responses for certain blood cancers, illustrating their power and promise. However, despite extensive research and thousands of
clinical trials worldwide, T cell therapies remain ineffective against most cancer types. More importantly, most patients treated with CD19 or B cell maturation antigen (BCMA) chimeric
antigen receptor (CAR)-T cells will eventually relapse despite having initial complete responses. This suggests there is much to be learned to achieve durable, long-term responses even for
successful CAR-T therapies. DISCOVERY OF T CELL-INTRINSIC GENETIC-ENHANCING MODIFIERS Achelois BioPharma has focused on discovering T cell-intrinsic genetic-enhancing modifiers (GEMs) since
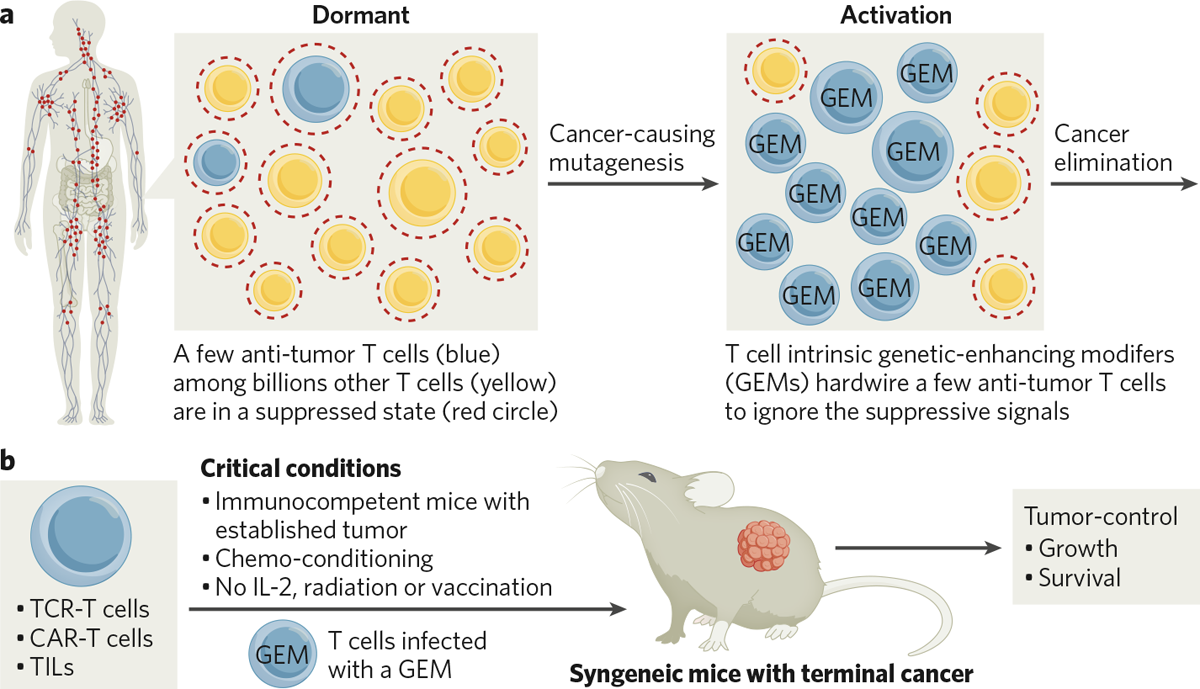
the company was founded in 2015. Achelois scientists postulated that during normal immune responses, GEMs reprogram a select few activated antigen-specific T cells to effectively eliminate
cancer and virus-infected cells, while billions of irrelevant ones remain suppressed and dormant (Fig. 1a). Thus, GEMs may be used to reprogram tumor-specific T cells, including
tumor-infiltrated lymphocyte (TILs), CAR-T cells, and T cell receptor (TCR)-T cells, to overcome immune suppression and control and eliminate established solid tumors. To identify and verify
GEMs, the Achelois team designed an in vivo adoptive cell therapy-based screen that models terminal cancer patients. Since tolerant, suppressive barriers are only present in immunocompetent
hosts, mice with intact immune systems and established tumors were used in the screen. These mice were conditioned with chemotherapy and then treated using tumor-specific T cells modified
with GEMs (Fig. 1b). In contrast, many clinical-stage T cell therapeutics, which were only verified in immune-deficient preclinical models, struggled to show potency in immunocompetent
patients. Achelois used this in vivo phenotypic screen to systematically test more than 200 proteins that have critical T cell-intrinsic functions, such as activating and inhibitory immune
checkpoints, or molecules that play a part in various inflammatory processes, including viral infection, chronic inflammation, or autoimmune disease. Candidate GEMs were introduced through
retroviral infection into a model TCR-T cell targeting mouse melanoma antigen GP100, and these were used to treat mice bearing established B16F0 melanoma tumors (Fig. 1b). Selected GEMs
enabled the elimination or control of established melanoma tumors by TCR-T cells. GEM T CELL THERAPY The GEMs discovered from the phenotypic screen are highly potent. Treatment with some of
the GEM-TCR-T cells alone melted away large, established melanoma tumors in mice. In comparison, blocking some well-known inhibitory checkpoints in TCR-T cells had modest and transient
effects on tumor control. Moreover, such robust control of late-stage solid tumors has not yet been accomplished in previous studies, even with the help of radiation, IL-2, and vaccination.
Verified GEMs range from cell surface receptors to intracellular signaling molecules, and control diverse biological processes in tumor-specific T cells, including cytolytic activities,
proliferation, and memory signaling. Most importantly, Achelois showed that GEMs enhance the function of TCR-T cells, CAR-T cells and tumor infiltrating lymphocytes (TILs) tested, suggesting
that GEMs are intrinsic programs of activated, antigen-specific T cells. GEMs function like essential software instructions, enabling T cell therapies to work in immunocompetent patients
for a broad set of cancer indications. Achelois believes GEMs can bridge the gap between the robust cytolytic activity of highly potent cells seen preclinically and the minimal activity in
immunocompetent patients across thousands of T-cell therapy trials. Next-generation T cell therapies incorporating GEMs may overcome tumor tolerance suppression barriers and establish
long-lasting antitumor immunity. Achelois has developed a strong pipeline of GEM-T cell therapies, including GEM-enhanced CD19 and BCMA CAR-T cell therapies for patients relapsed from
first-generation CAR-T treatments. The company has observed impressive clinical results in patients who have relapsed after first-generation CD19 CAR-T treatment. Achelois is also developing
GEM-enhanced TCR-T cell or TIL therapies for solid tumor indications and has built good manufacturing practice (GMP) facilities in the United States as it prepares to file investigational
new drug applications to the US Food and Drug Administration to support trials for GEM-T cell therapies for various indications. READY TO PARTNER Achelois welcomes opportunities to partner
with companies seeking to boost the efficacy of their T cell therapy assets. The company is also open to flexibly collaborating to discover new GEMs, licensing out GEMs for partners to
enhance their own T cell therapy pipeline, or co-developing GEM-T therapies using GEMs and Achelois’ manufacturing facility and processes.